Submitted:
30 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Functionalized Olefinic Substrates
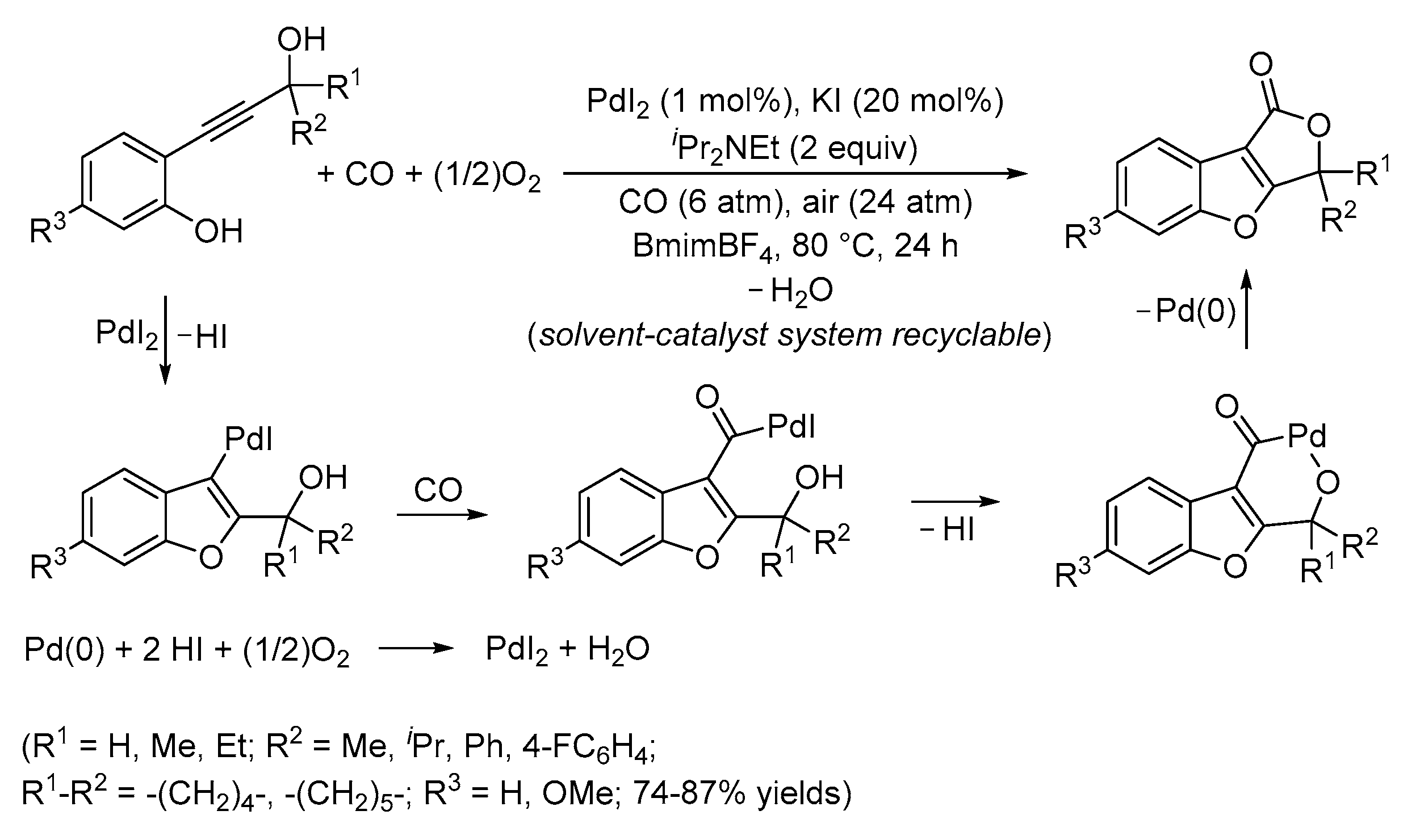
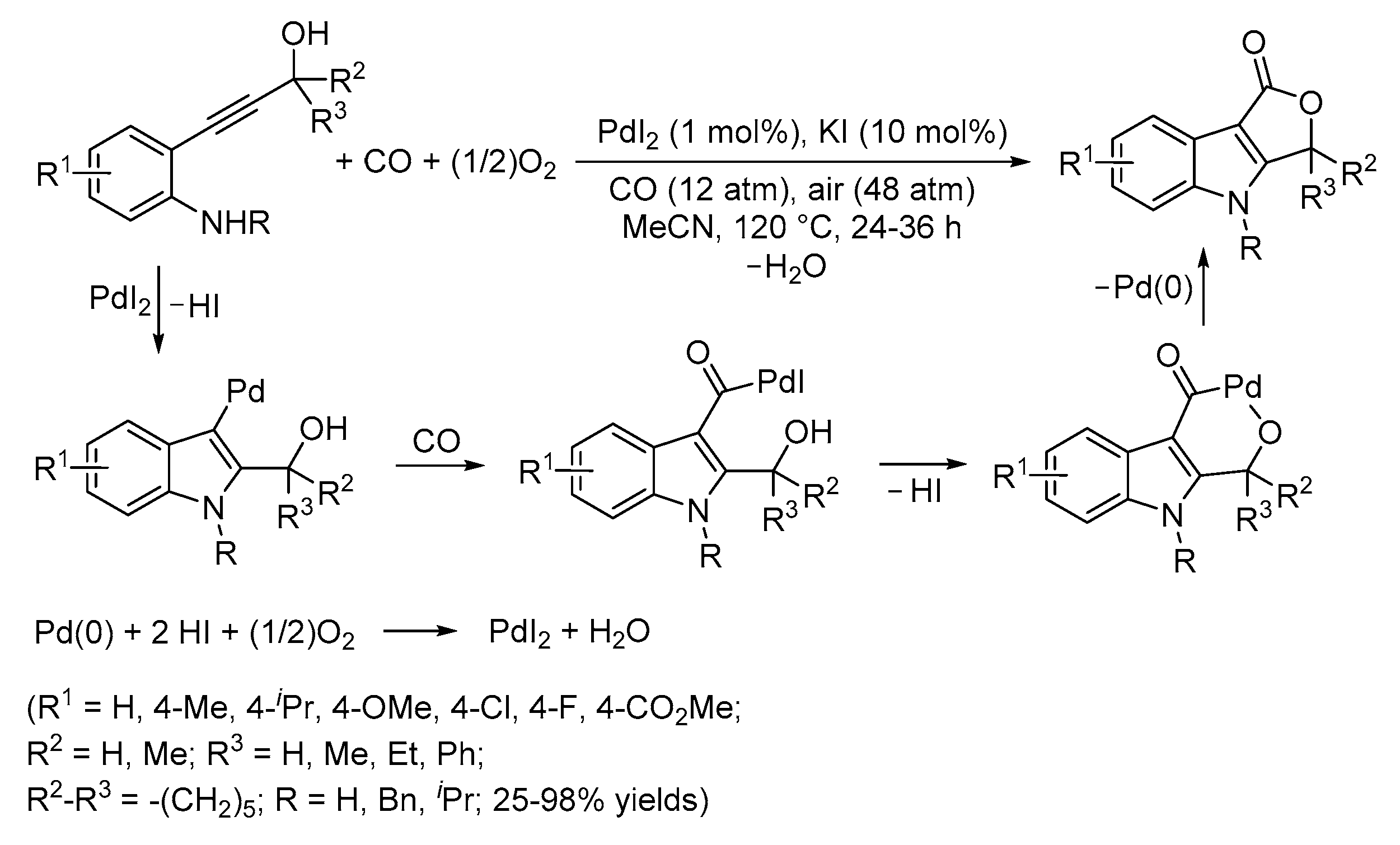
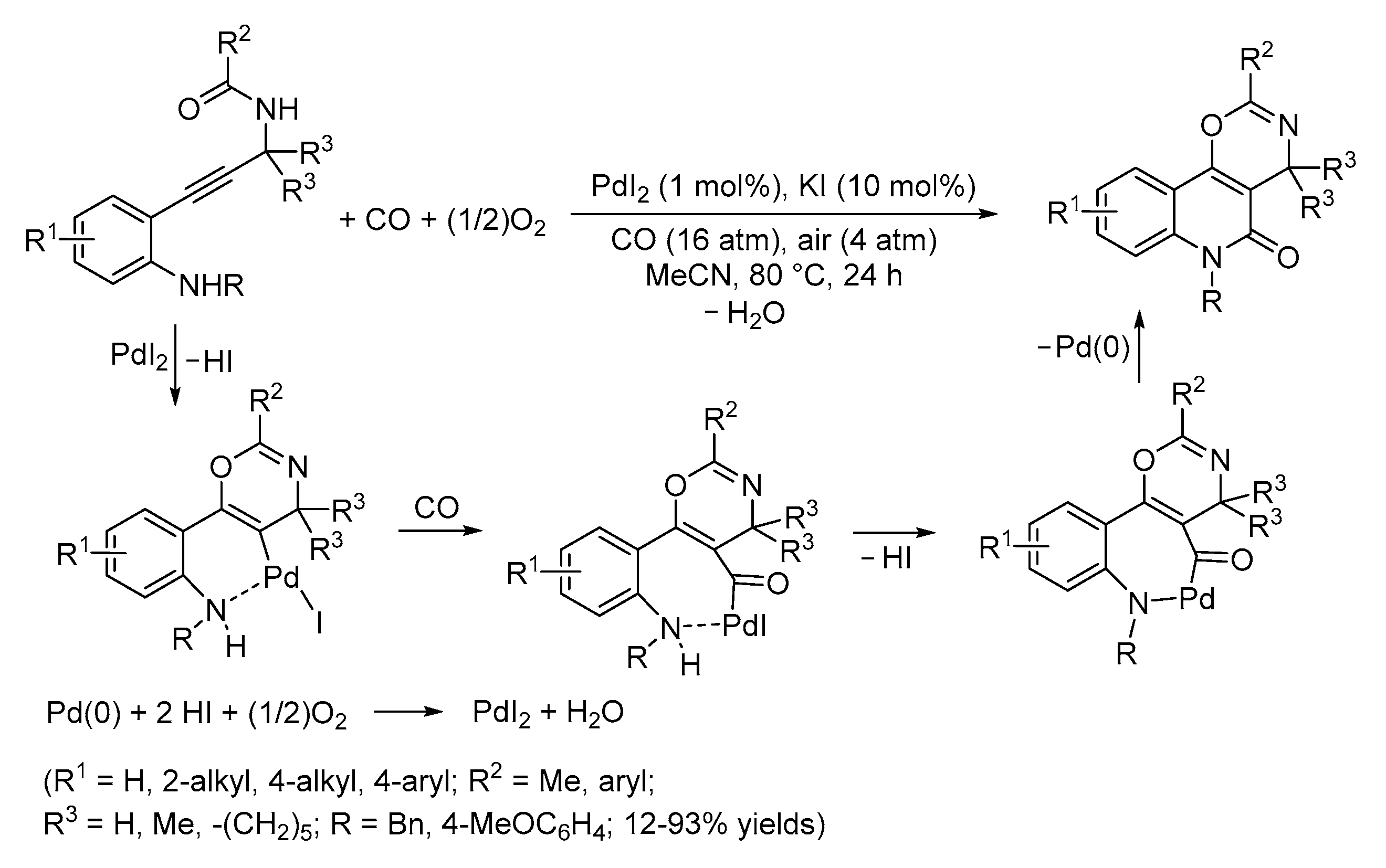
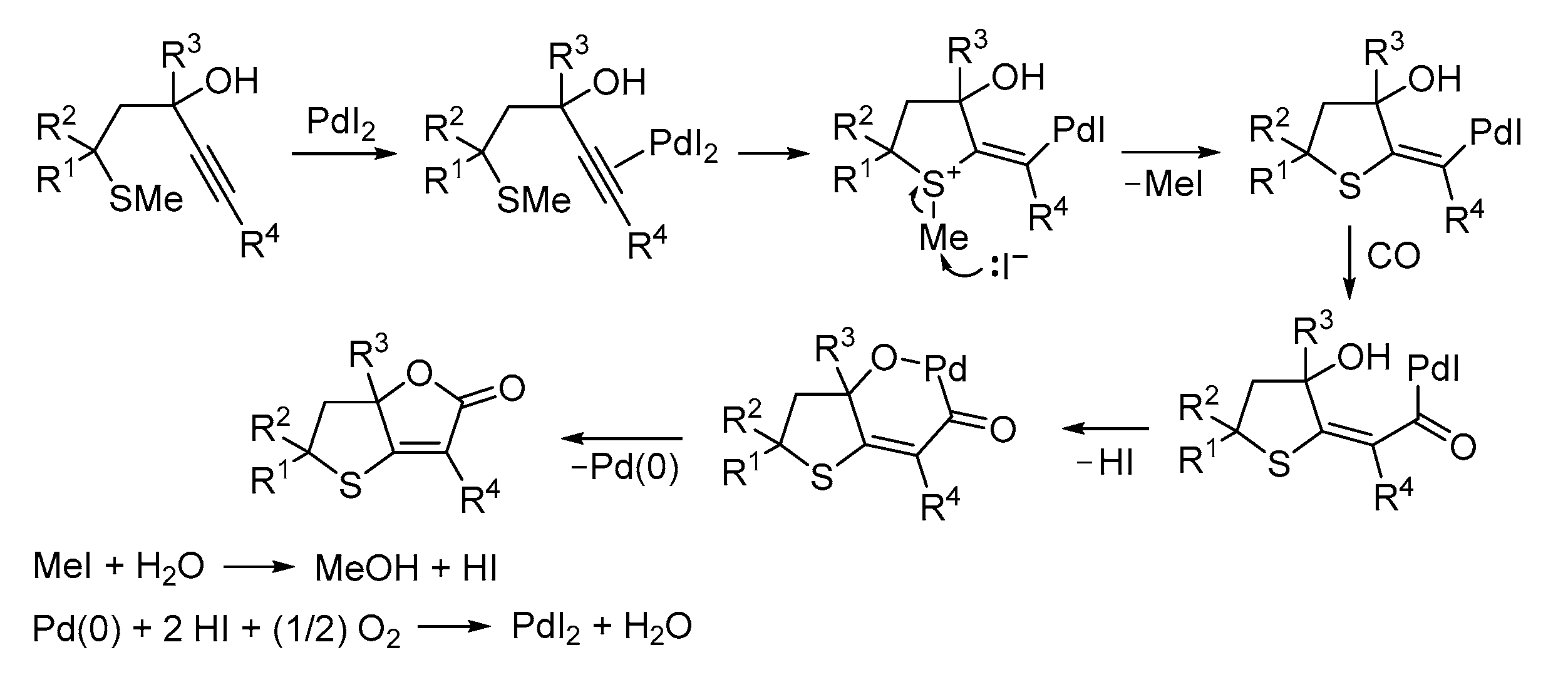
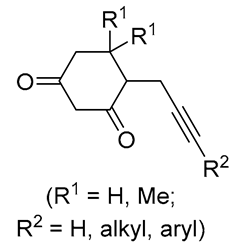
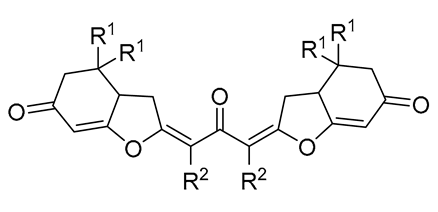
3. Functionalized Acetylenic Substrates
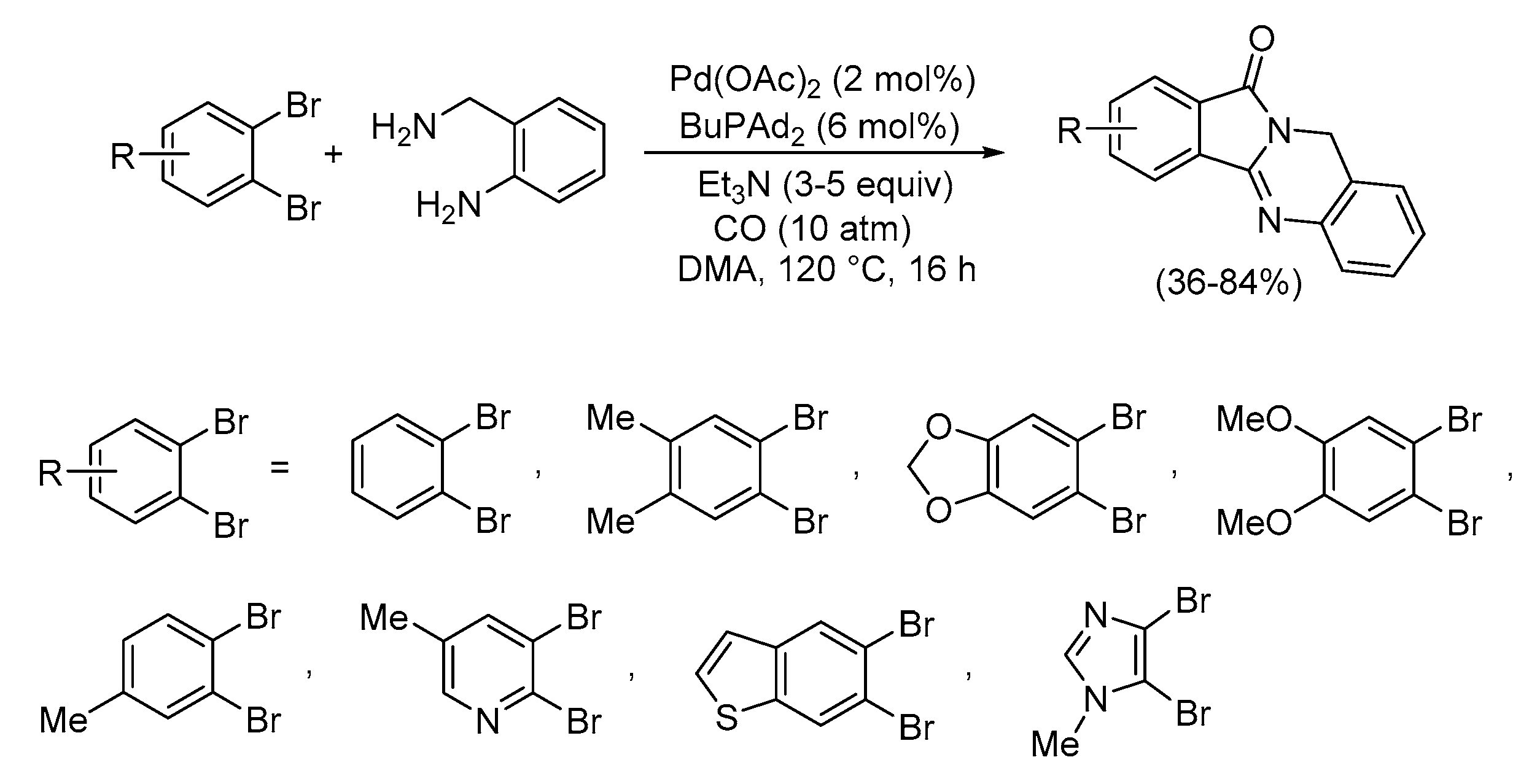
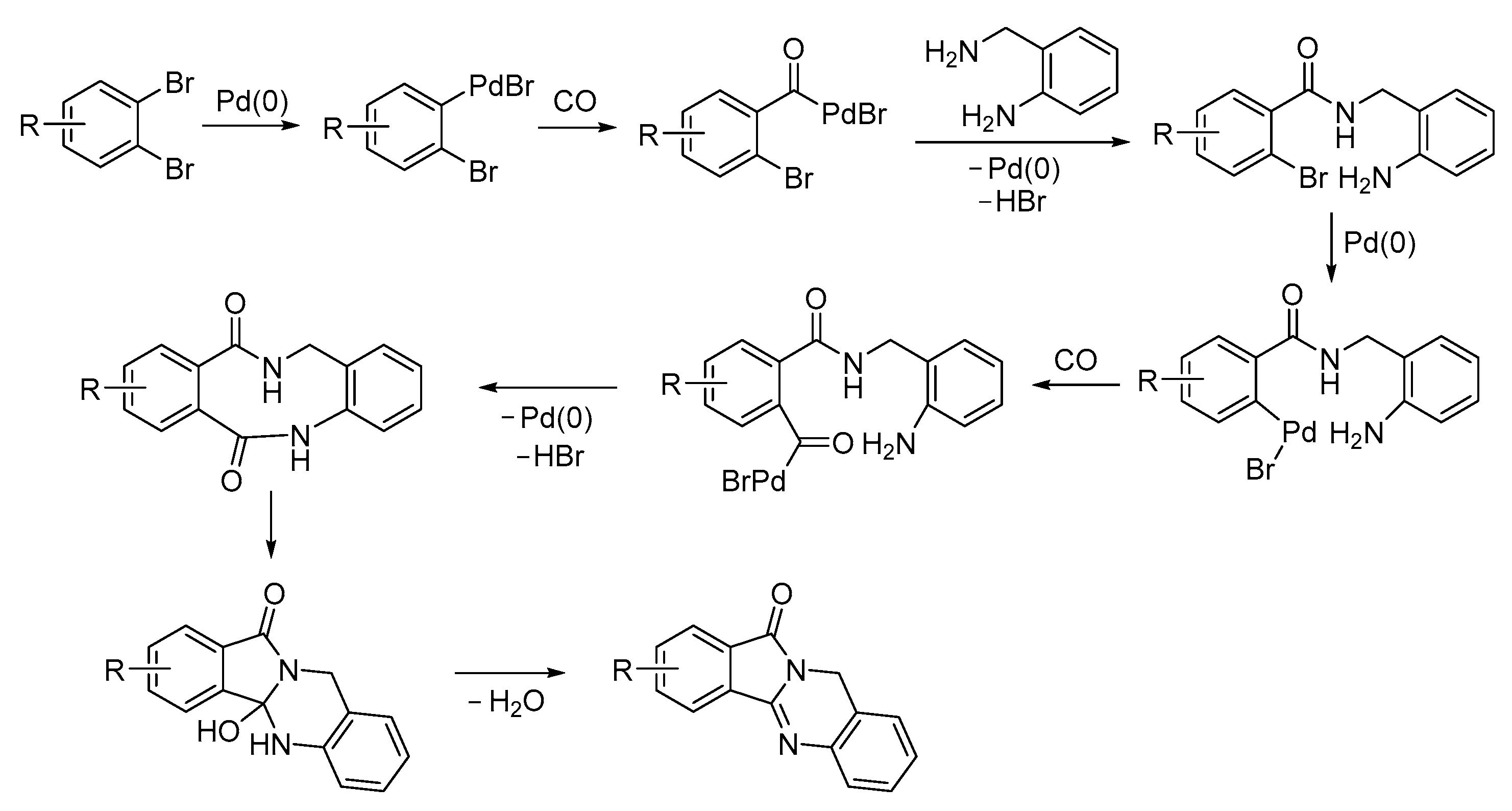
4. Functionalized Halides
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Gabriele, B. (Ed.) Carbon Monoxide in Organic Synthesis – Carbonylation Chemistry; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; and Schaub, G. Gas production, 2. In Ullmann’s Encyclopedia of Industrial Chemistry; Baltes, H., Göpel, W., Hesse, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 423–479. [Google Scholar]
- Karl, J.; Pröll, T. Steam Gasification of Biomass in Dual Fluidized Bed Gasifiers: A Review. Renewable Sustainable Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Figueres, C.; Le Quéré, C.; Mahindra, A.; Bäte, O.; Whiteman, G.; Peters, G.; Guan, D. Emissions Are Still Rising: Ramp up the Cuts. Nature 2018, 564, 27–30. [Google Scholar] [CrossRef]
- Li, J.J.; Gribble, G. (Eds.) Palladium in Heterocyclic Chemistry - A Guide for the Synthetic Chemist, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Gabriele, B.; Della Ca’, N.; Mancuso, R.; Veltri, L.; Ziccarelli, I. Palladium(II)-Catalyzed Carbonylations. In Carbon Monoxide in Organic Synthesis – Carbonylation Chemistry; Gabriele, B., Ed.; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Semmelhack, M.F.; Bodurow, C.; Baum, M. Direct Synthesis of Pyran-Lactones Related to Nafhthoquinone Antibiotics. Tetrahadron Lett. 1984, 25, 3171–3174. [Google Scholar] [CrossRef]
- Tamaru, H.; Higashimura, K.; Hojo, M.; Yoshida, Z. PdII-Catalyzed Stereoselective Bis-Lactonization. Angew. Chem., Int. Ed. 1985, 24, 1045–1046. [Google Scholar] [CrossRef]
- Tamaru, H.; Kobayashi, T.; Kawamura, S.; Hojo, M.; Yoshida, Z. Palladium Catalyzed Oxycarbonylation of 4-Penten-1,3-diols: Efficient Stereoselective Synthesis of cis 3-Hydroxytetrahydrofuran 2-Acetic Acid Lactones. Tetrahedron Lett. 1985, 26, 3207–3210. [Google Scholar] [CrossRef]
- Gracza, T.; Hasenöhrl, T.; Stahl, T.; Jäger, V. Synthesis of 3,5-Anhydro-2-deoxy-1,4-glyconolactones by Palladium(II)-Catalyzed, Regioselective Oxycarbonylation of C5- and C6-enitols. ω-Homologation of Aldoses to Produce Intermediates for C-Glycoside/C-Nucleoside Synthesis. Synthesis 1991, 1991, 1108–1118. [Google Scholar] [CrossRef]
- Kraus, G.A.; Li, J. Regiocontrol by Remote Substituents. An Enantioselective Total Synthesis of Frenolicin B via a Highly Regioselective Diels-Alder Reaction. J. Am. Chem. Soc. 1993, 115, 5859–5860. [Google Scholar] [CrossRef]
- Gracza, T.; Jäger, V. Synthesis of Natural and Unnatural Enantiomers of Goniofufurone and Its 7-Epimers from D-Glucose. Application of Palladium(II) – Catalyzed Oxycarbonylation of Unsaturated Polyols. Synthesis 1994, 1994, 1359–1368. [Google Scholar]
- Boukouvalas, J.; Fortier, G.; Radu, I.-I. Efficient Synthesis of (‒)-trans-Kumausyne via Tandem Intramolecular Alkoxycarbonylation-Lactonization. J. Org. Chem. 1998, 63, 916–917. [Google Scholar] [CrossRef]
- Dixon, D.J.; Ley, S.V.; Gracza, T.; Szolcsanyi, P. Total Synthesis of the Polyenoyltetramic acid Mycotoxin Erythroskyrine. J. Chem. Soc., Perkin Trans. 1 1999, 1999, 831–841. [Google Scholar] [CrossRef]
- Paddon-Jones, G.C.; Hungerford, N.L.; Haynes, P.; Kitching, W. Efficient Palladium(II)-Mediated Construction of Functionalized Plakortone Cores. Org. Lett. 1999, 1, 1905–1908. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Shanmugam, P. Development of an Approach to the Synthesis of the Plakortones. Tetrahedron Lett. 2000, 41, 3567–3571. [Google Scholar] [CrossRef]
- Paddon-Jones, G.C.; McErlean, C.S.P.; Haynes, P.; Moore, C.J.; Konig, W.A.; Kitching, W. Synthesis and Stereochemistry of Some Bicyclic γ-Lactones from Parasitic Wasps (Hymenoptera: Braconidae). Utility of Hydrolytic Kinetic Resolution of Epoxides and Palladium(II)-Catalyzed Hydroxycyclization-Carbonylation-Lactonization of Ene-diols. J. Org. Chem. 2001, 66, 7487–7495. [Google Scholar] [CrossRef]
- Haynes, P.Y.; Kitching, W. Total Synthesis and Absolute Stereochemistry of Plakortone D. J. Am. Chem. Soc. 2002, 124, 9718–9719. [Google Scholar]
- Haynes, P.Y.; Kitching, W. Synthesis of the Plakortone Series: Plakortone E. Heterocycles 2004, 62, 173–177. [Google Scholar]
- Babjak, M.; Kapitán, P.; Gracza, T. Synthesis of (+)-Goniothalesdiol and (+)-7-epi-Goniothalesdiol. Tetrahedron 2005, 61, 2471–2479. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Hooley, R.J.; Kraml, C.M. Synthesis of Plakortone B and Analogs. Org. Lett. 2006, 8, 5203–5206. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Pouliot, M.; Robichaud, J.; MacNeil, S.; Snieckus, V. Asymmetric Total Synthesis of (‒)-Panacene and Correction of Its Relative Configuration. Org. Lett. 2006, 8, 3597–3599. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Stereocontrolled Oxycarbonylation of 4-Benzyloxyhepta-1,6-diene-3,5-diols Promoted by Chiral Palladium(II) Complexes. Tetrahedron: Asymm. 2008, 19, 38–44. [Google Scholar] [CrossRef]
- Nesbitt, C.L.; McErlean, C.S.P. An Expedient Synthesis of 2,5-Disubstituted-3-oxygenated Tetrahydrofurans. Tetrahedron Lett. 2009, 50, 6318–6320. [Google Scholar] [CrossRef]
- Nesbitt, C.L.; McErlean, C.S.P. Total Synthesis of C19 Lipid Diols Containing a 2,5-Disubstituted-3-Oxygenated Tetrahydrofuran. Org. Biomol. Chem. 2011, 9, 2198–2208. [Google Scholar] [CrossRef]
- Haynes, P.Y.; Chow, S.; Rahm, F.; Bernhardt, P.V.; De Voss, J.J.; Kitching, W. Synthesis of the Sponge-Derived Plakortone Series of Bioactive Compounds. J. Org. Chem. 2010, 75, 6489–6501. [Google Scholar]
- Werness, J.B.; Tang, W. Stereoselective Total Synthesis of (‒)-Kumausallene. Org. Lett. 2011, 13, 3664–3666. [Google Scholar] [CrossRef]
- Markovič, M.; Ďuranová, M.; Koóš, P.; Szolcsányi, P.; Gracza, T. Synthesis of bis-Tetrahydrofuran Subunit of (‒)-Neopallavicinin. Tetrahedron 2013, 69, 4185–4189. [Google Scholar] [CrossRef]
- Markovič, M.; Koóš, P.; Čarný, T.; Sokoliová, S.; Bohačiková, N.; Moncol’, J.; Gracza, T. Total Synthesis, Configuration Assignment, and Cytotoxic Activity Evaluation of Protulactone A. J. Nat. Prod. 2017, 80, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Markovič, M.; Koóš, P.; Gracza, T. A Short Asymmetric Synthesis of Sauropunols A–D. Synthesis 2017, 49, 2939–2942. [Google Scholar]
- Lopatka, P.; Gavenda, M.; Markovič, M.; Koóš, P.; Gracza, T. Flow Pd(II)-Catalyzed Cyclisation in the Total Synthesis of Jaspine B. Catalysts 2021, 11, 1513. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Asymmetric Intramolecular Pd(II)-catalyzed Oxycarbonylation of Alkene-1,3-diols. Arkivoc 2008, viii, 8–17. [Google Scholar]
- Doháňošová, J.; Lásikivá, A.; Toffano, M.; Gracza, T.; Vo-Thanh, G. Kinetic Resolution of Pent-4-ene-1,3-diol by Pd(II)-Catalysed Oxycarbonylation in Ionic Liquids. New J. Chem. 2012, 36, 1744–1750. [Google Scholar] [CrossRef]
- Babjak, M.; Markovič, K.; Kandríkova, B.; Gracza, T. Homogeneous Cyclocarbonylation of Alkenols with Iron Pentacarbonyl. Synthesis 2014, 46, 809–816. [Google Scholar] [CrossRef]
- Markovič, K.; Lopatka, P.; Koóš, P.; Gracza, T. Asymmetric Formal Synthesis of (+)-Pyrenolide D. Synthesis 2014, 46, 817–821. [Google Scholar]
- Lopatka, P.; Markovič, K.; Koóš, P.; Ley, S.V.; Gracza, T. Continuous Pd-Catalyzed Carbonylative Cyclization Using Iron Pentacarbonyl as a CO Source. J. Org. Chem. 2019, 84, 14394–14406. [Google Scholar] [CrossRef] [PubMed]
- Babjak, M.; Zálupský, P.; Gracza, T. Regiocontrol in the Palladium(II)-Catalysed Oxycarbonylation of Unsaturated Polyols. Arkivoc 2005, v, 45–57. [Google Scholar] [CrossRef]
- Tamaru, Y.; Kobayashi, T.; Kawamura, S.; Ochiai, H.; Yoshida, Z. Stereoselective Intramolecular Aminocarbonylation of 3-Hydroxypent-4-enylamides Catalyzed by Palladium. Tetrahedron Lett. 1985, 26, 4479–4482. [Google Scholar] [CrossRef]
- Tamaru, Y.; Hojo, M.; Yoshida, Z. Palladium(2+)-Catalyzed Intramolecular Aminocarbonylation of 3-Hydroxy-4-pentenylamines and 4-Hydroxy-5-hexenylamines. J. Org. Chem. 1988, 53, 5731–5741. [Google Scholar] [CrossRef]
- Hümmer, W.; Dubois, E.; Gracza, T.; Jäger, V. Halocyclization and Palladium(II)-Catalyzed Amidocarbonylation of Unsaturated Aminopolyols. Synthesis of 1,4-Iminoglycitols as Potential Glycosidase Inhibitors. Synthesis 1997, 1997, 634–642. [Google Scholar] [CrossRef]
- Caletková, O.; Ďurišová, N.; Gracza, T. Aminohydroxylation of Divinylcarbinol and its Application to the Synthesis of Bicyclic hydroxypyrrolidine and Aminotetrahydrofuran Building Blocks. Chem. Pap. 2013, 67, 66–75. [Google Scholar] [CrossRef]
- Koóš, P.; Špánik, I. , Gracza, T. Asymmetric Intramolecular Pd(II)-Catalysed Amidocarbonylation of Unsaturated Amino Alcohols. Tetrahedron: Asymm. 2009, 20, 2720–2723. [Google Scholar] [CrossRef]
- Lee, H.-W.; Kwong, F.-Y. A Decade of Advancements in Pauson-Khand-Type Reactions. Eur. J. Org. Chem. 2010, 2010, 789–811. [Google Scholar] [CrossRef]
- Shibata, T. Recent Advances in the Catalytic Pauson-Khand-type Reaction. Adv. Synth. Catal. 2006, 348, 2328–2336. [Google Scholar] [CrossRef]
- Blanco-Urgoiti, J.; Añorbe, L.; Pérez-Serrano, L.; Domínguez, G.; Pérez-Castells, J. The Pauson–Khand Reaction, a Powerful Synthetic Tool for the Synthesis of Complex Molecules. Chem. Soc. Rev. 2004, 33, 32–42. [Google Scholar] [PubMed]
- Heravi, M.M.; Mohammadi, L. Application of Pauson-Khand Reaction in the Total Synthesis of Terpenes. RSC Adv. 2021, 11, 38325–38373. [Google Scholar]
- Yang, Z. Navigating the Pauson-Khand Reaction in Total Syntheses of Complex Natural Products. Acc. Chem. Res. 2021, 54, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, C. , Zheng, N., Yang, Z.; Shi, L. Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016-2020). Catalysts 2020, 10, 1199. [Google Scholar]
- Keese, F.; Guidetti-Grept, R.; Herzog, B. Synthesis of [5.5.5.5]Fenestranes by Pd-Catalyzed Carbonylation-Cyclisation. Tetrahedron Lett. 1992, 33, 1207–1210. [Google Scholar]
- Yasuhara, S.; Sasa, M.; Kusakabe, T.; Takayama, H.; Kimura, M.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reactions of Propargyl Acetates and Amides with Palladium(II)–Bisoxazoline Catalysts. Angew. Chem. Int. Ed. 2011, 50, 3912–3915. [Google Scholar]
- Shen, R.; Kusakabe, T.; Yatsu, T.; Kanno, Y.; Takahashi, K.; Nemoto, K.; Kato, K. Palladium(II) Catalyzed Cyclization-Carbonylation-Cyclization Coupling Reaction of (ortho-Alkynyl Phenyl) (Methoxymethyl) Sulfides Using Molecular Oxygen as the Terminal Oxidant. Molecules 2016, 21, 1177. [Google Scholar] [PubMed]
- Bartish, C.M.; Drissel, G.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Grayson, M., Eckroth, D., Bushey, G.J., Campbell, L., Klingsberg, A., van Nes, L., Eds.; John Wiley & Sons: New York, 1978; Vol. 4, p. 774. [Google Scholar]
- Kusakabe, T.; Kawaguchi, K.; Kawamura, M.; Niimura, N.; Shen, R.; Takayama, H.; Kato, K. Cyclization-Carbonylation-Cyclization Coupling Reaction of Propargyl Ureas with Palladium(II)-bisoxazoline Catalyst. Molecules 2012, 17, 9220–9230. [Google Scholar]
- Kusakabe, T.; Kawai, Y.; Shen, R.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reaction of γ-Propynyl-1,3-diketones with Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2012, 10, 3192–3194. [Google Scholar] [CrossRef]
- Kusakabe, T.; Sekiyama, E.; Ishino, Y.; Motodate, S.; Kato, S.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reactions of N-Propargylanilines and o-Alkynylphenols with Palladium(II)–bisoxazoline Catalysts. Synthesis 2012, 44, 1825–1832. [Google Scholar] [CrossRef]
- Kusakabe, T.; Sagae, H.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling reaction of α,β-Alkynic Hydrazones with Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2013, 11, 4943–4948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kusakabe, T.; Takahashi, K.; Kato, K. A Cyclization–Carbonylation–Cyclization Coupling Reaction of (ortho-Alkynyl phenyl) (Methoxymethyl) Sulfides with the Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2014, 12, 3380–3385. [Google Scholar] [CrossRef]
- Shen, R.; Kusakabe, T.; Takahashi, K.; Kato, K. Pd(II)-Catalyzed Ligand Controlled Synthesis of Methyl 1-benzyl-1H-indole-3-carboxylates and Bis(1-benzyl-1H-indol-3-yl)methanones. Org. Biomol. Chem. 2014, 12, 4602–4609. [Google Scholar] [CrossRef]
- Ariyama, T.; Kusakabe, T.; Sato, K.; Funatogawa, M.; Lee, D.; Takahashi, K.; Kato, K. Pd(II)-Catalyzed Ligand-Controlled Synthesis of 2,3-Dihydroisoxazole-4-carboxylates and Bis(2,3-dihydroisoxazol-4-yl)methanones. Heterocycles 2016, 93, 512–518. [Google Scholar]
- Kubasabe, T.; Mochida, T.; Ariyama, T.; Lee, D.; Ohkubo, S.; Takahashi, K.; Kato, K. PdII Catalyzed Ligand Controlled Synthesis of Bis(3-furanyl)methanones and Methyl 3-furancarboxylates. Org. Biomol. Chem. 2019, 17, 6860–6865. [Google Scholar]
- Gabriele, B.; Chimento, A.; Mancuso, R.; Pezzi, V.; Ziccarelli, I.; Sirianni, R. Derivati 6,6a-diidrofuro[3,2-b]furan-2-(5H)onici, loro Preparazione e Uso nel Trattamento dei Tumori. Italian Pat. 102017000078586, filed on 13 July 2017, granted on 8 October 2019.
- Gabriele, B.; Chimento, A.; Mancuso, R.; Pezzi, V.; Ziccarelli, I.; Sirianni, R. 6,6a-Dihydrofuro[3,2-b]furan-2-(5H)one Derivatives, their Preparation and Use for Treating Tumors. Eur. Pat. EP3428169, filed on 12 July 2018, granted on 20 January 2021.
- Mancuso, R.; Ziccarelli, I.; Chimento, A.; Marino, N.; Della Ca’, N.; Sirianni, R.; Pezzi, V.; Gabriele, B. Catalytic Double Cyclization Process for Antitumor Agents against Breast Cancer Cell Lines. iScience 2018, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
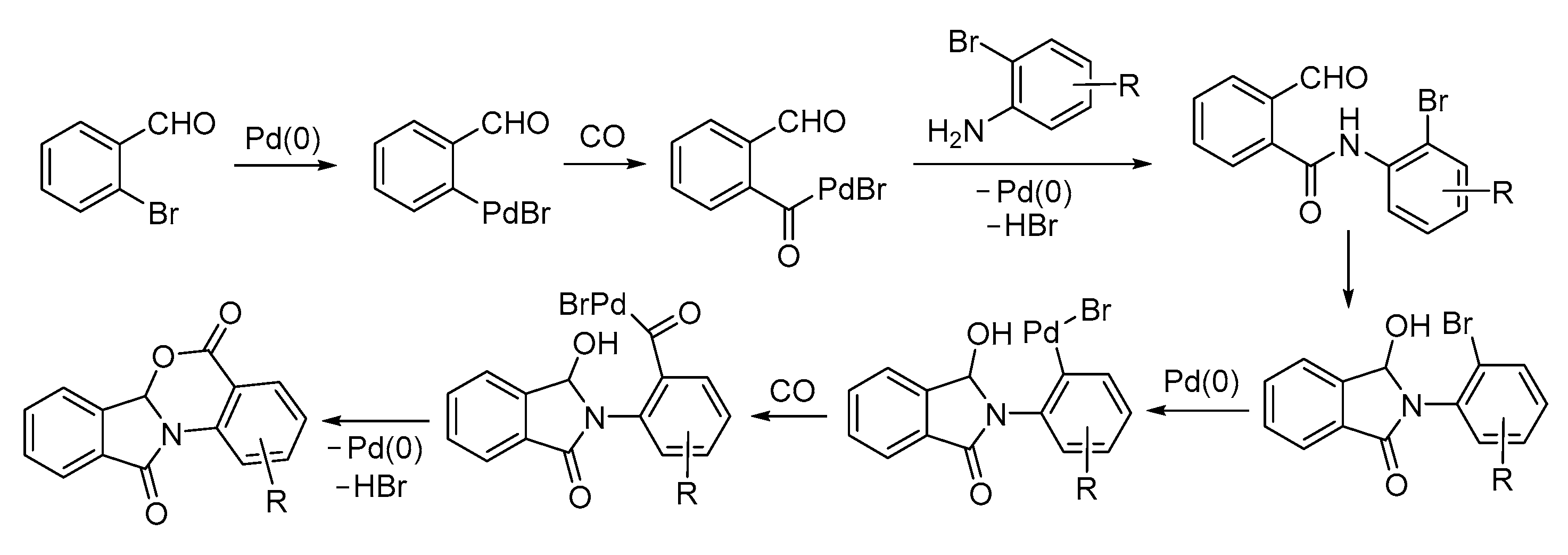
- Acerbi, A.; Carfagna, C.; Costa, M.; Mancuso, R.; Gabriele, B.; Della Ca’, N. An Unprecedented Pd-Catyalyzed Carbonylative Route to Fused Furo[3,4-b]indol-1-ones. Chem. Eur. J. 2018, 24, 4835–4840. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2‒Based Catalysis for Carbonylation Reactions: A Personal Account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef]
- Gabriele, B. Recent Advances in the PdI2-Catalyzed Carbonylative Synthesis of Heterocycles from Acetylenic Substrates: A Personal Account. Targets Heterocycl Syst. 2018, 22, 41–55. [Google Scholar]
- Gabriele, B.; Salerno, G. PdI2. In e-EROS (Electronic Encyclopedia of Reagents for Organic Synthesis); Crich, D., Ed.; Wiley–Interscience: New York, NY, USA, 2006. [Google Scholar]
- Gabriele, B.; Salerno, G.; Costa, M. PdI2-Catalyzed Synthesis of Heterocycles. Synlett 2004, 2004, 2468–2483. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G.P. Recent Developments in the Synthesis of Heterocyclic Derivatives by PdI2-Catalyzed Oxidative Carbonylation Reactions. J. Organomet. Chem. 2003, 687, 219–228. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. An Efficient and Selective Palladium-Catalysed Oxidative Sicarbonylation of Alkynes to Alkyl- or Aryl-Maleic Esters. J. Chem. Soc., Perkin Trans. 1 1994, 1994, 83–87. [Google Scholar] [CrossRef]
- Mancuso, R.; Miliè, R.; Palumbo Piccionello, A. , Olivieri, D.; Della Ca’. N.; Carfagna, C.; Gabriele, B. Catalytic Carbonylative Double Cyclization of 2-(3-Hydroxy-1-yn-1-yl)phenols in Ionic Liquids Leading to Furobenzofuranone Derivatives. J. Org. Chem. 2019, 84, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Pancrazzi, F.; Sarti, N.; Mazzeo, P.P.; Bacchi, A.; Carfagna, C.; Mancuso, R.; Gabriele, B.; Stirling, A.; Della Ca’, N. Site-Selective Double and Tetracyclization Routes to Fused Polyheterocyclic Structures by Pd-Catalyzed Carbonylation Reactions. Org. Lett. 2020, 22, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Della Ca’, N.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-Catalyzed Synthesis of Symmetrical Urea Derivatives by Oxidative Carbonylation of Primary Amines in Carbon Dioxide Medium. J. Catal. 2011, 282, 120–127. [Google Scholar]
- Mancuso, R.; Russo, P.; Lettieri, M.; Santandrea, M.; Cuocci, C.; Gabriele, B. Disclosing Polycyclic Heterocycles: Synthesis of Furothienopyran and Pyranothienopyran Derivatives by Palladium Iodide Catalyzed Carbonylative Double Cyclization. Adv. Synth. Catal. 2022, 364, 3917–3926. [Google Scholar] [CrossRef]
- Mancuso, R.; Strangis, R.; Ziccarelli, I.; Della Ca’, N.; Gabriele, B. Palladium Catalysis with Sulfurated Substrates under Aerobic Conditions: A Direct Oxidative Carbonylation Approach to Thiophene-3-carboxylic Esters. J. Catal. 2021, 393, 335–343. [Google Scholar] [CrossRef]
- Mancuso, R.; Cuglietta, S.; Strangis, R.; Gabriele, B. Synthesis of Benzothiophene-3-carboxylic Esters by Palladium Iodide-Catalyzed Oxidative Cyclization−Deprotection−Alkoxycarbonylation Sequence under Aerobic Conditions. J. Org. Chem. 2023, 88, 5180–5186. [Google Scholar] [CrossRef]
- Mancuso, R.; Russo, P.; Miliè. R.; Dell’Aera, M.; Grande, F.; Della Ca’, N.; Gabriele, B. Palladium Iodide Catalyzed Carbonylative Double Cyclization to a New Class of S,O-Bicyclic Heterocycles. Catal. Today 2022, 397-399, 631–638. [Google Scholar] [CrossRef]
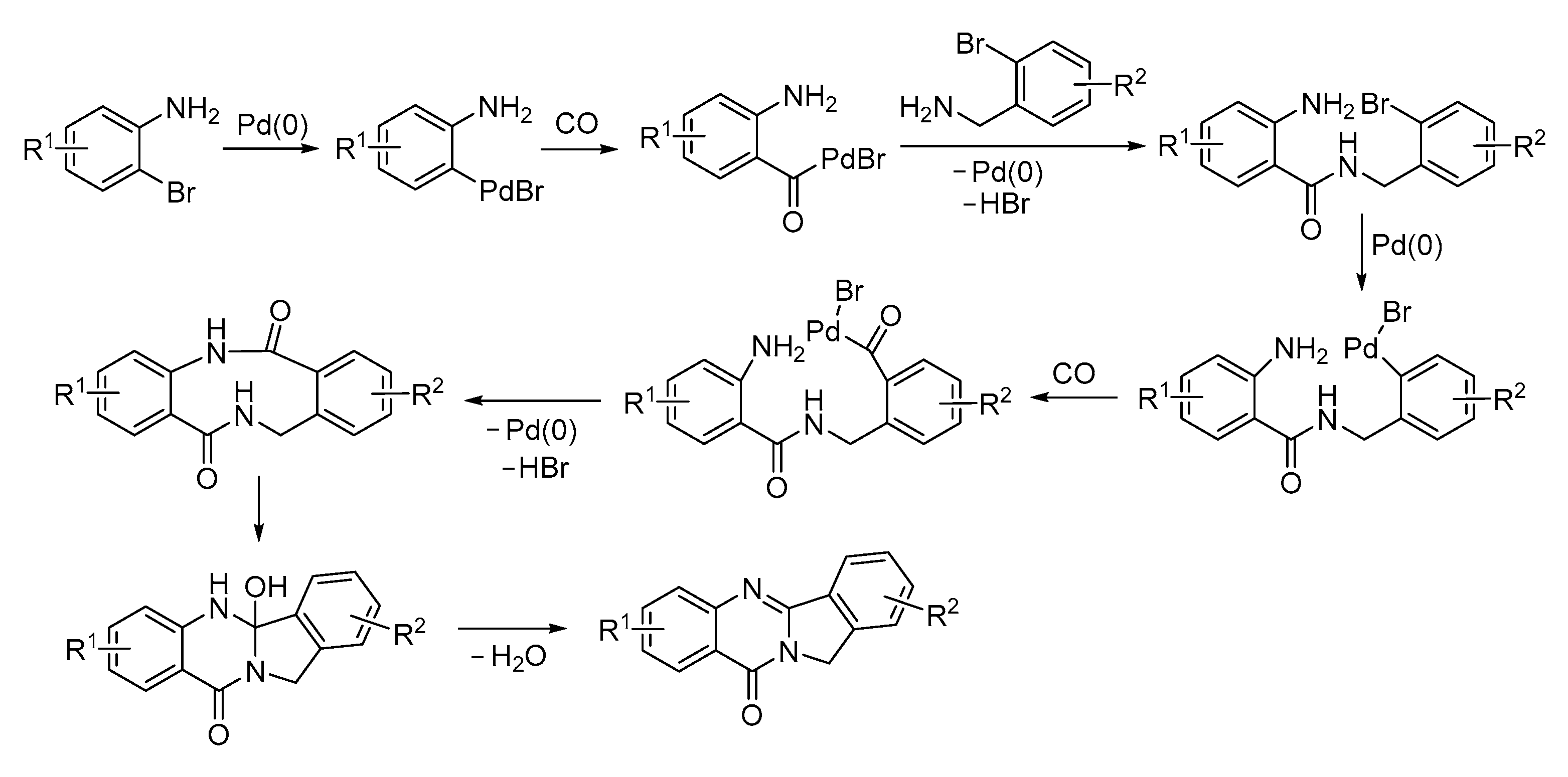
- Chen, J.; Neumann, H.; Beller, M.; Wu, X.-F. Palladium-Catalyzed Synthesis of Isoindoloquinazolinones via Dicarbonylation of 1,2-Dibromoarenes. Org. Biomol. Chem. 2014, 12, 5835–5838. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Man, N.Y.T.; Stewart, S.; Wu, X.-F. Palladium-Catalyzed Dicarbonylative Synthesis of Tetracycle Quinazolinones. Org. Biomol. Chem. 2015, 13, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Natte, K.; Chen, J.; Li, H.; Neumann, H.; Beller, M.; Wu, X.-F. Palladium-Catalyzed Carbonylation of 2-Bromoanilines with 2-Formylbenzoic Acid and 2-Halobenzaldehydes: Efficient Synthesis of Functionalized Isoindolinones. Chem. Eur. J. 2014, 20, 14184–14188. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Spannenberg, A.; Baumann, W.; Neumann, H.; Beller, M.; Wu, X.-F. A Novel Domino Synthesis of Quinazolinediones by Palladium-Catalyzed Double Carbonylation. Chem. Eur. J. 2014, 20, 8541–8544. [Google Scholar] [CrossRef]
- Frutos-Pedreño, R.; García-López, J.-A. 2-Arylacetamides as Versatile Precursors for 3-Aminoisocoumarin and Homophthalimide Derivatives: Palladium-Catalyzed Cascade Double Carbonylation Reactions. Adv. Synth. Catal. 2016, 358, 2692–2700. [Google Scholar] [CrossRef]

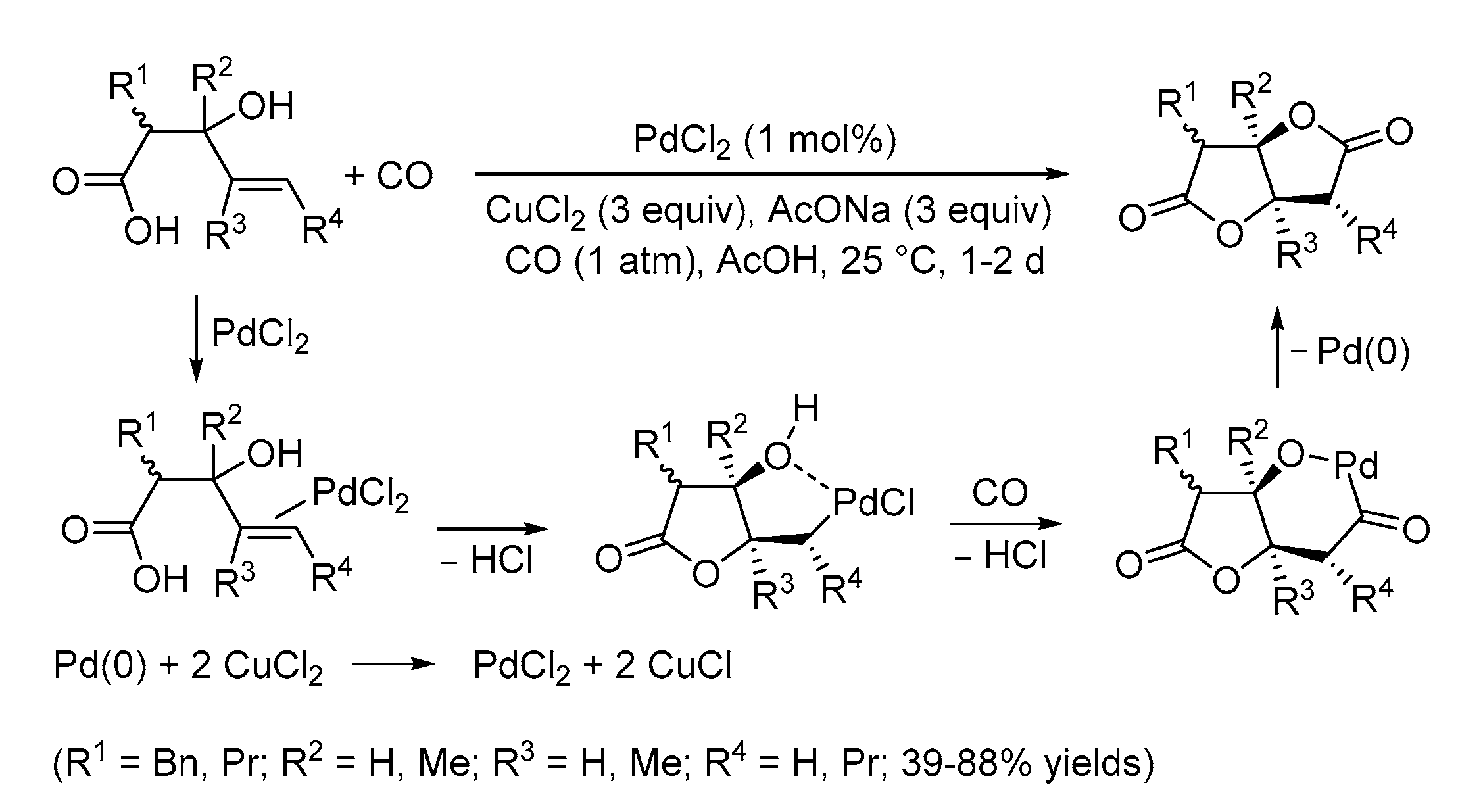
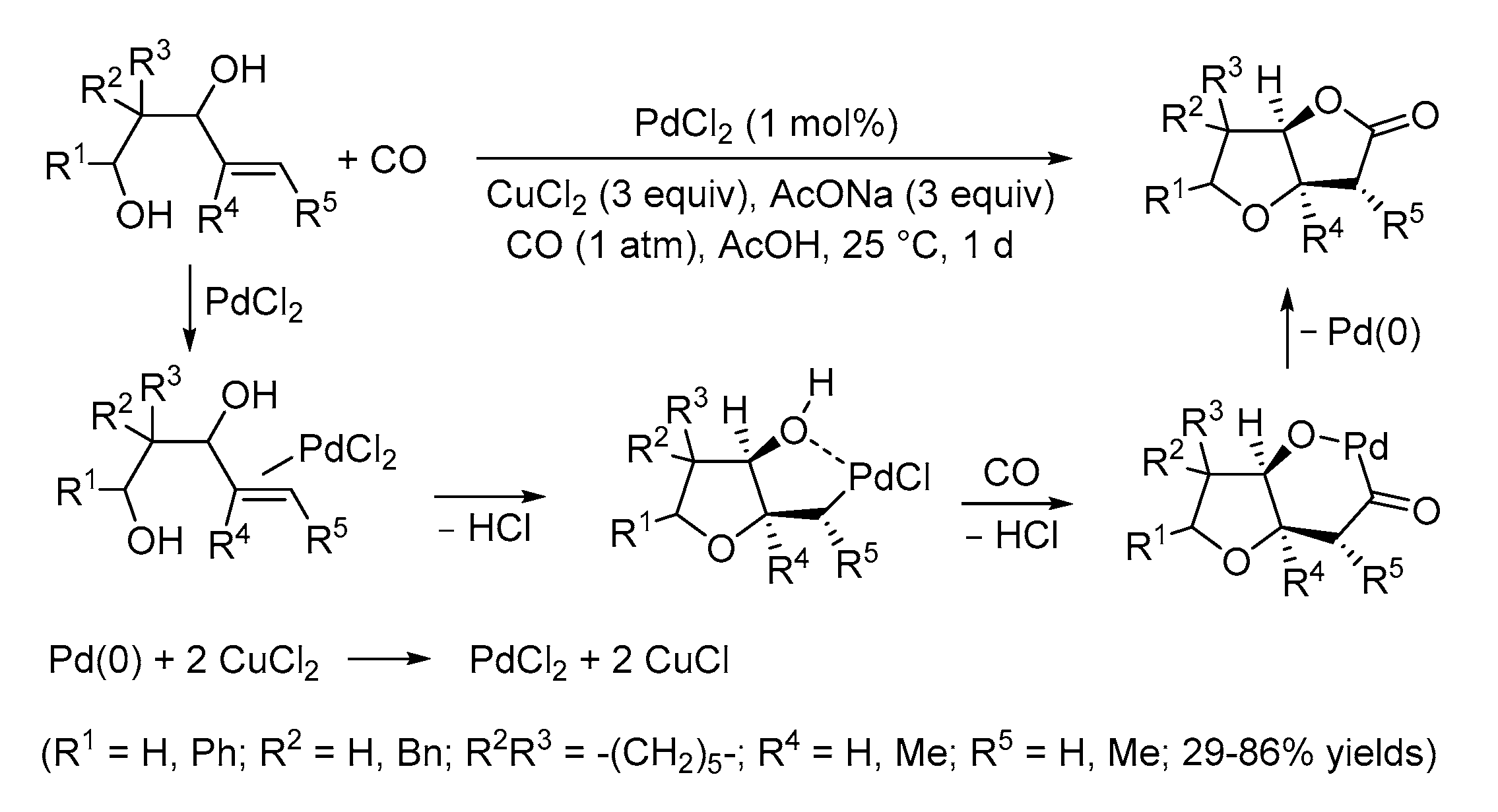
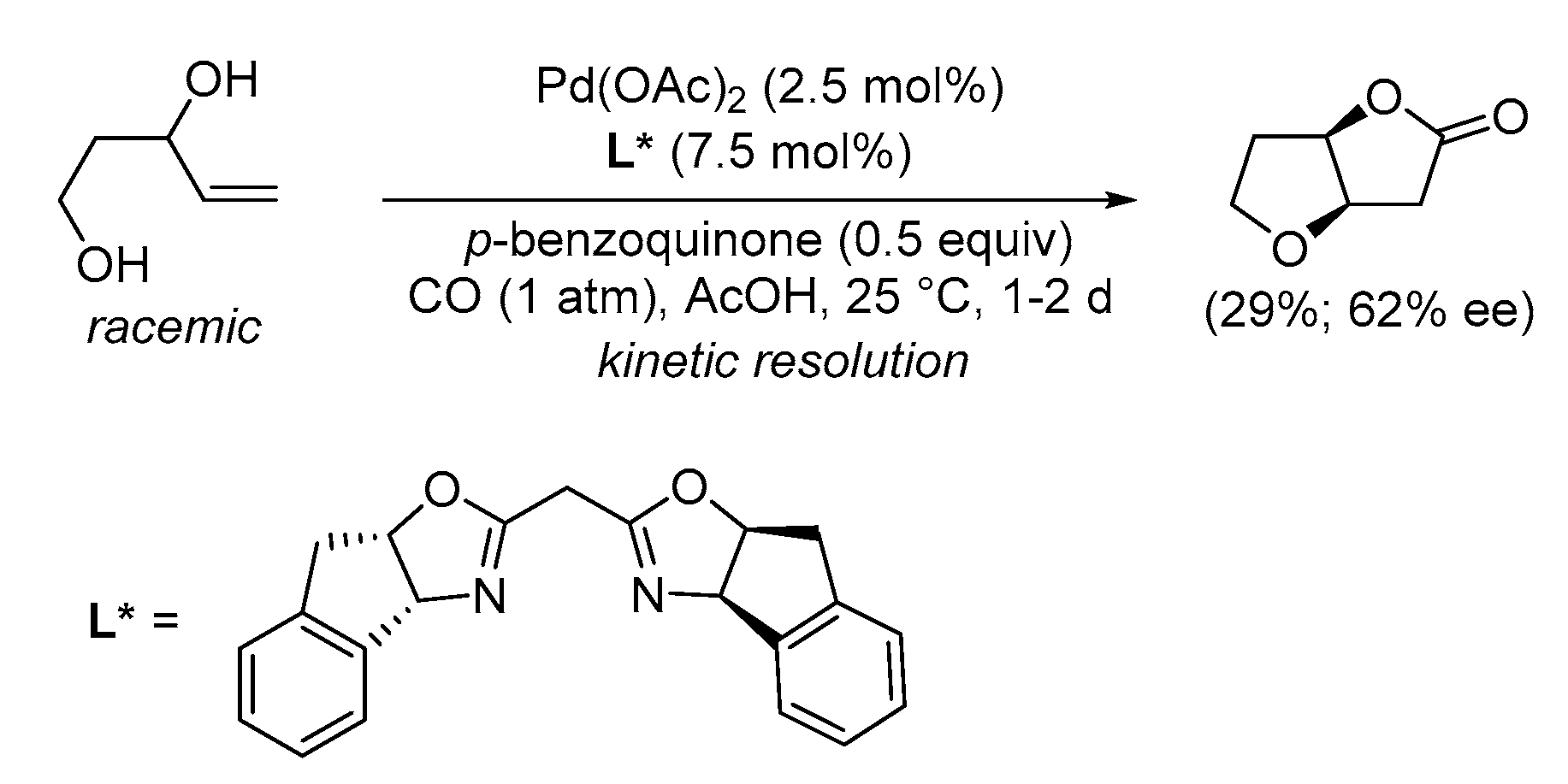
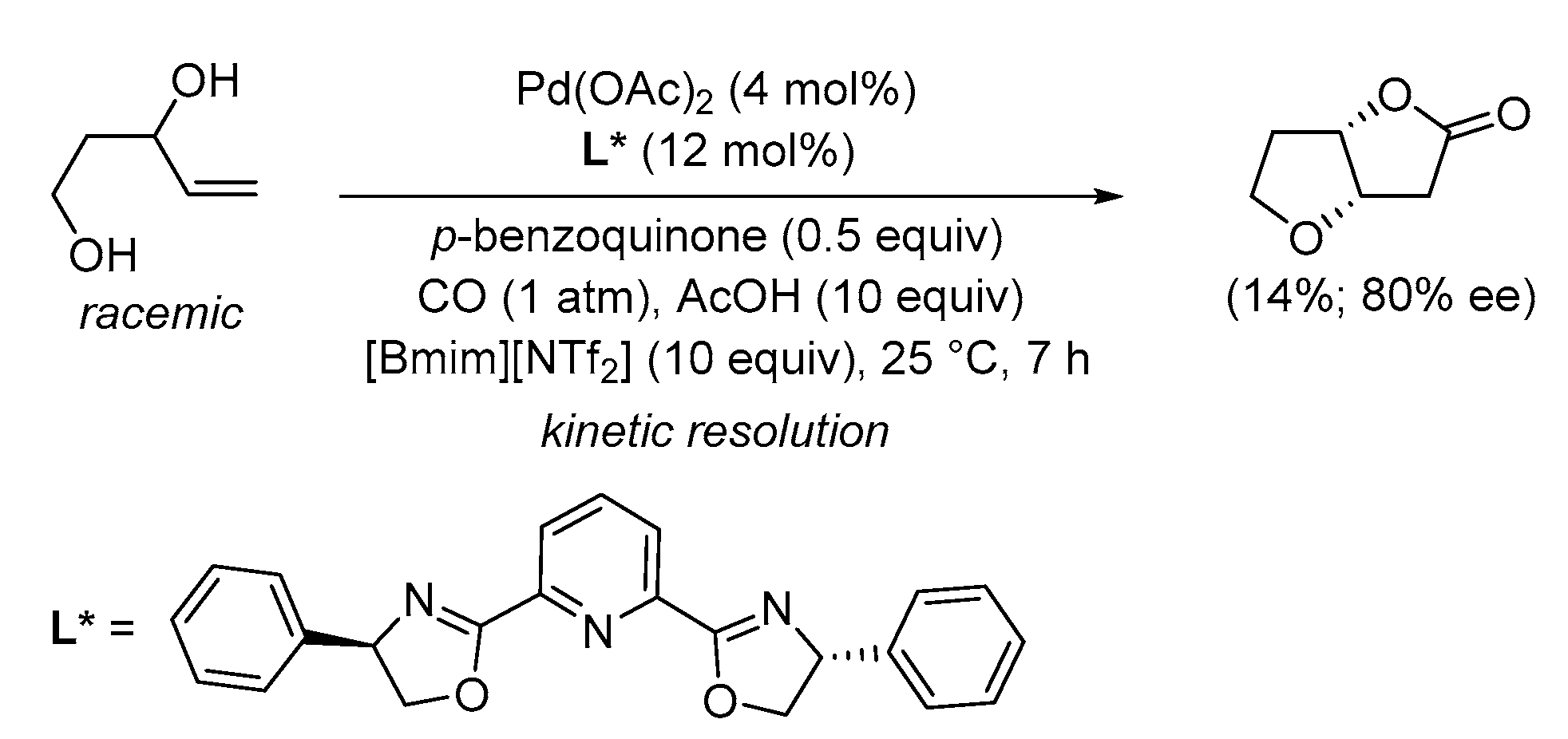

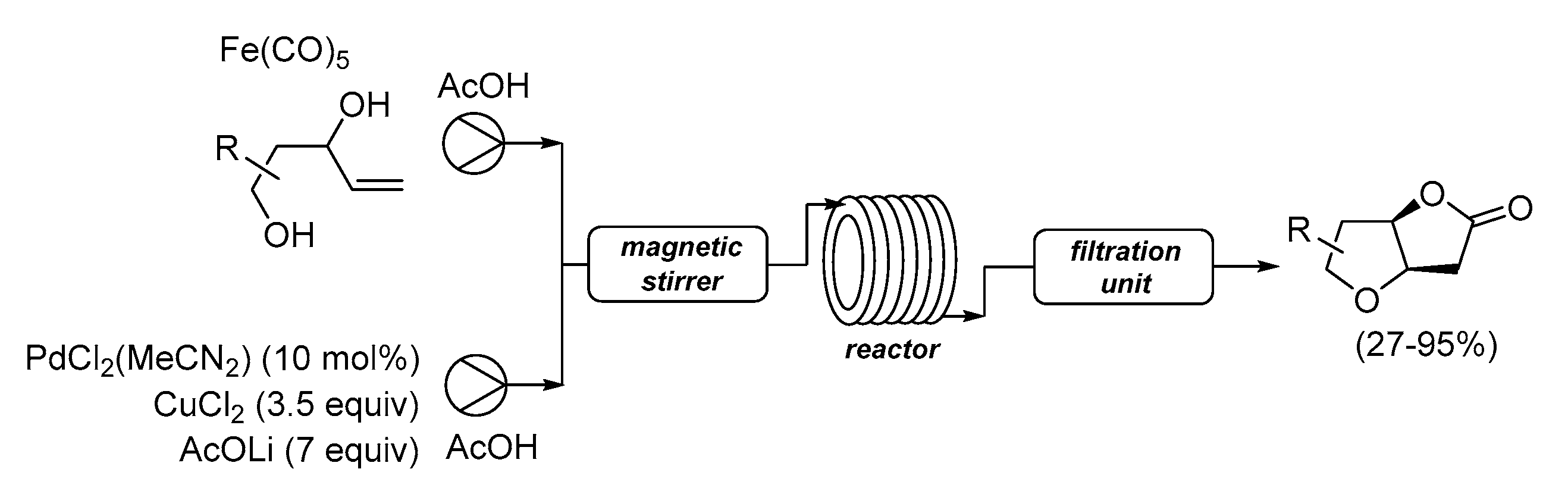
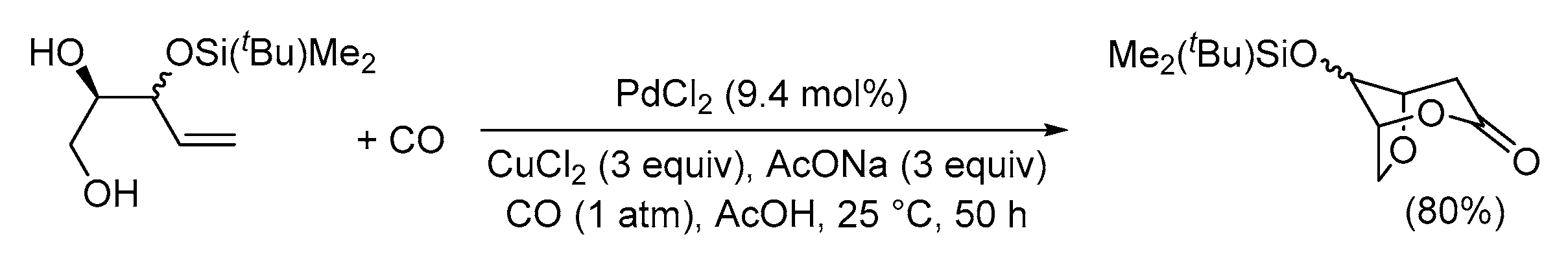
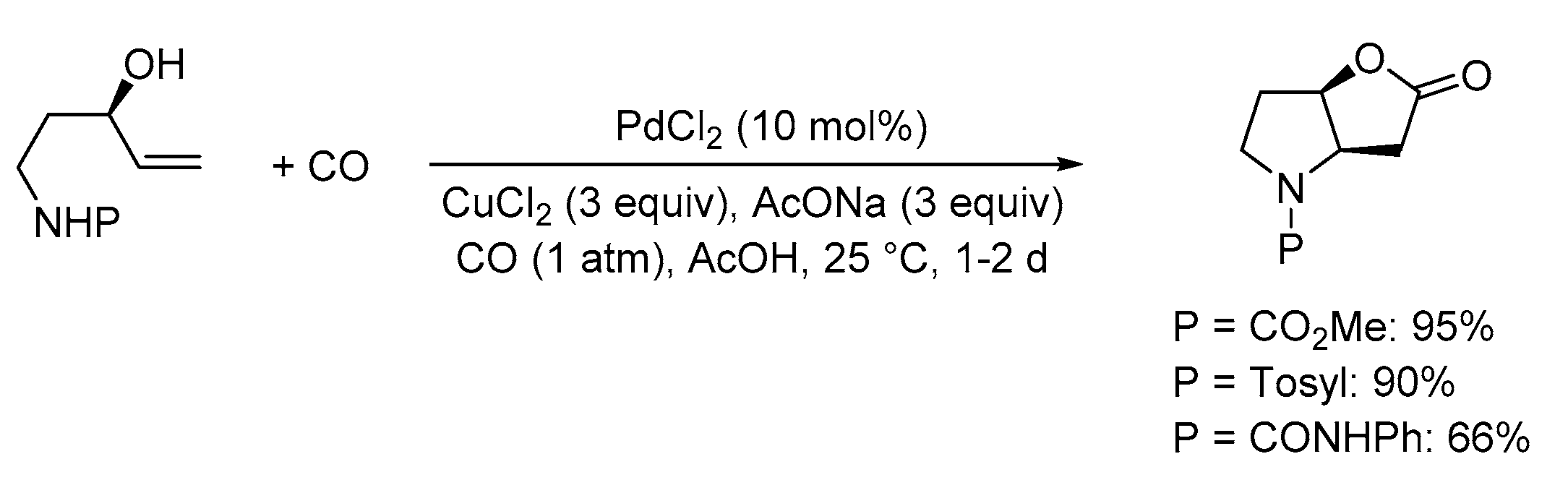
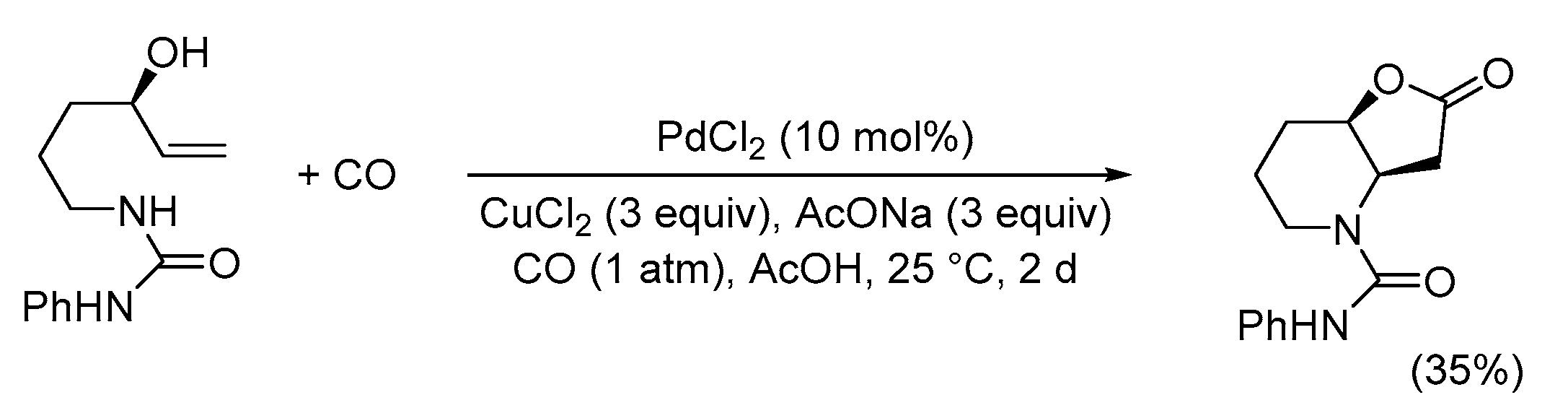
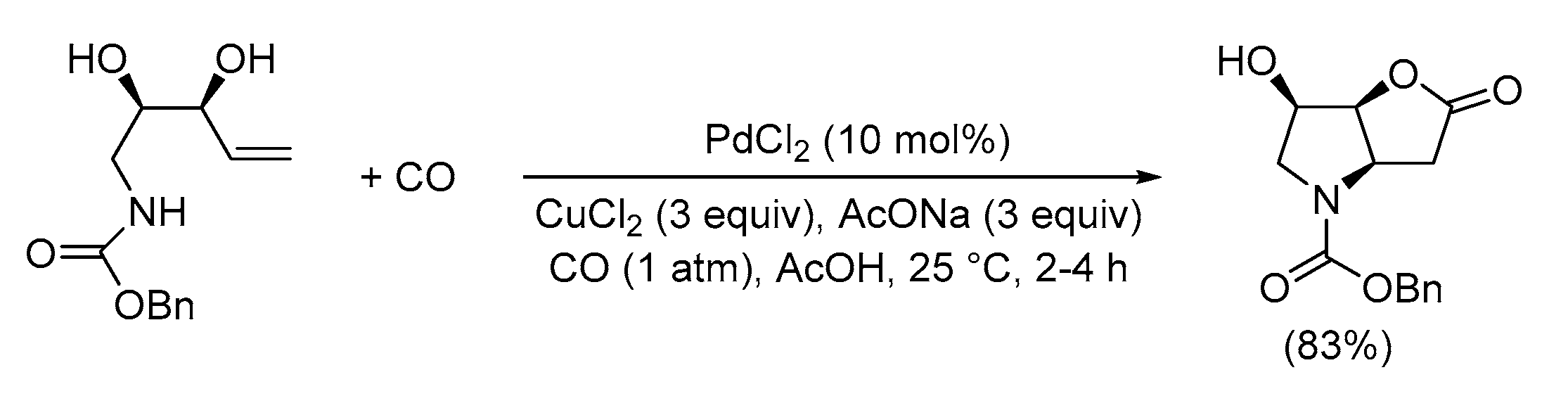
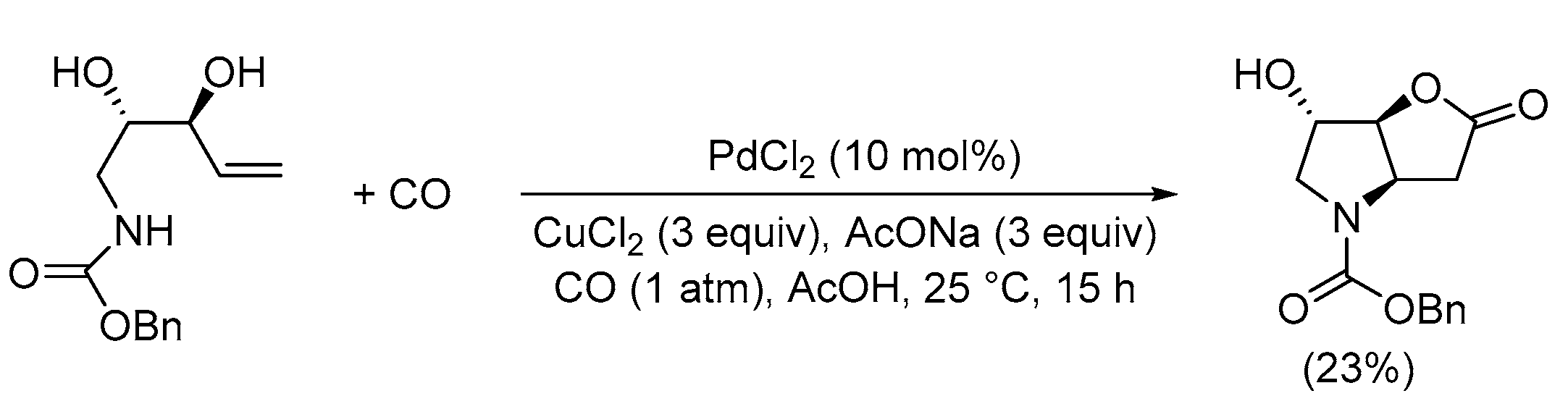
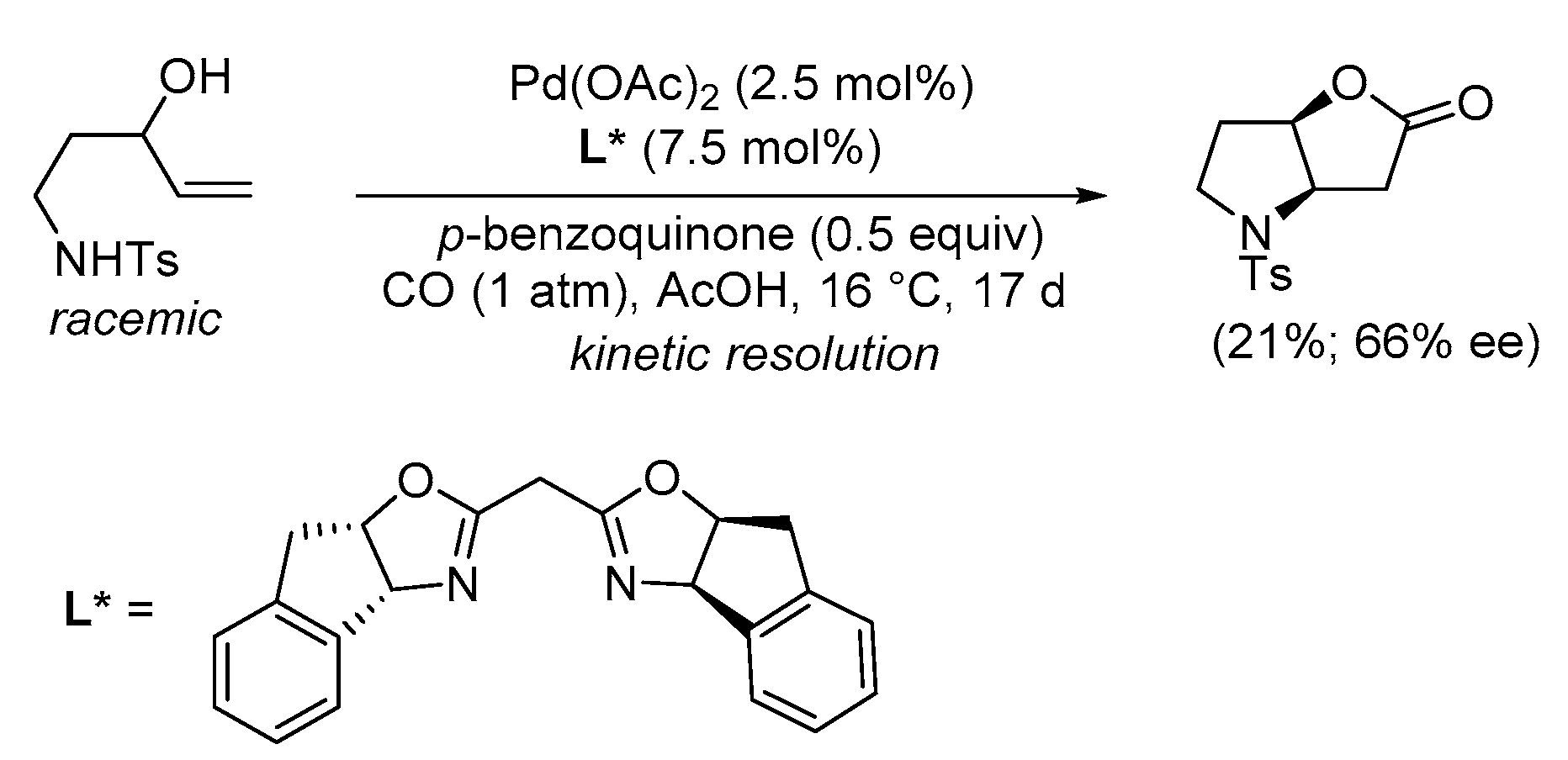
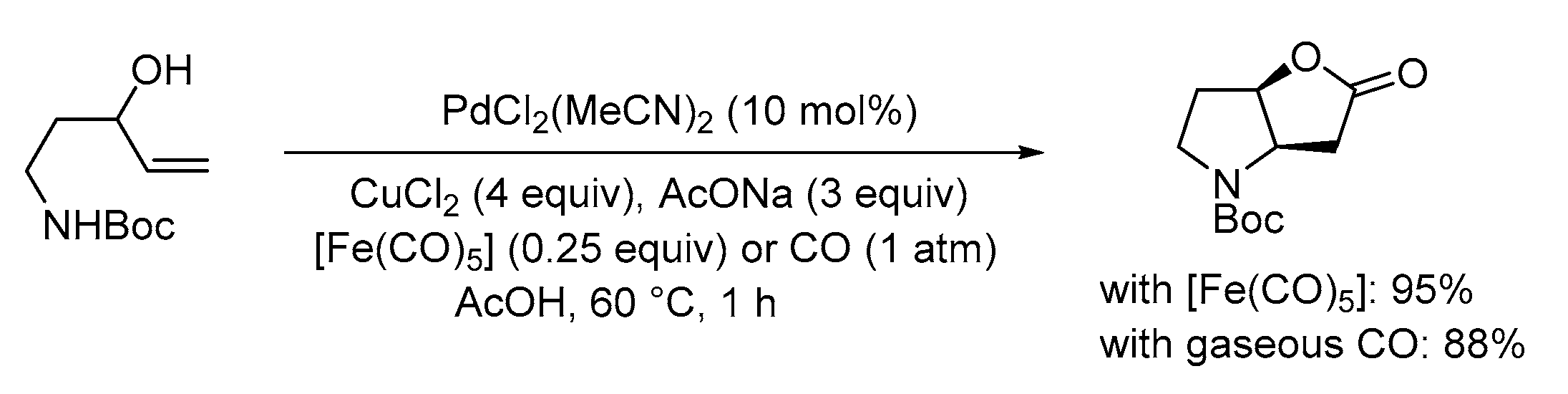
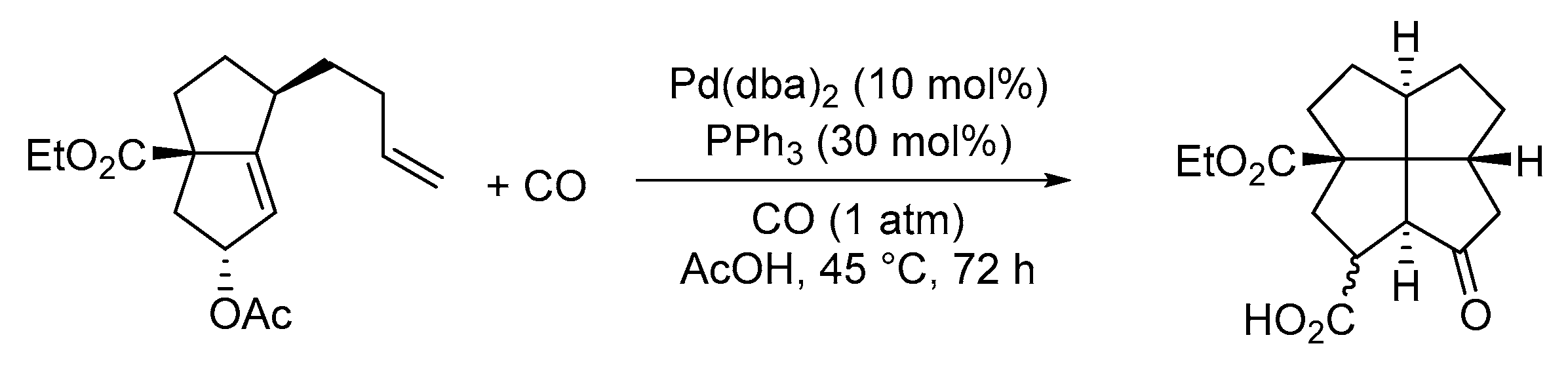
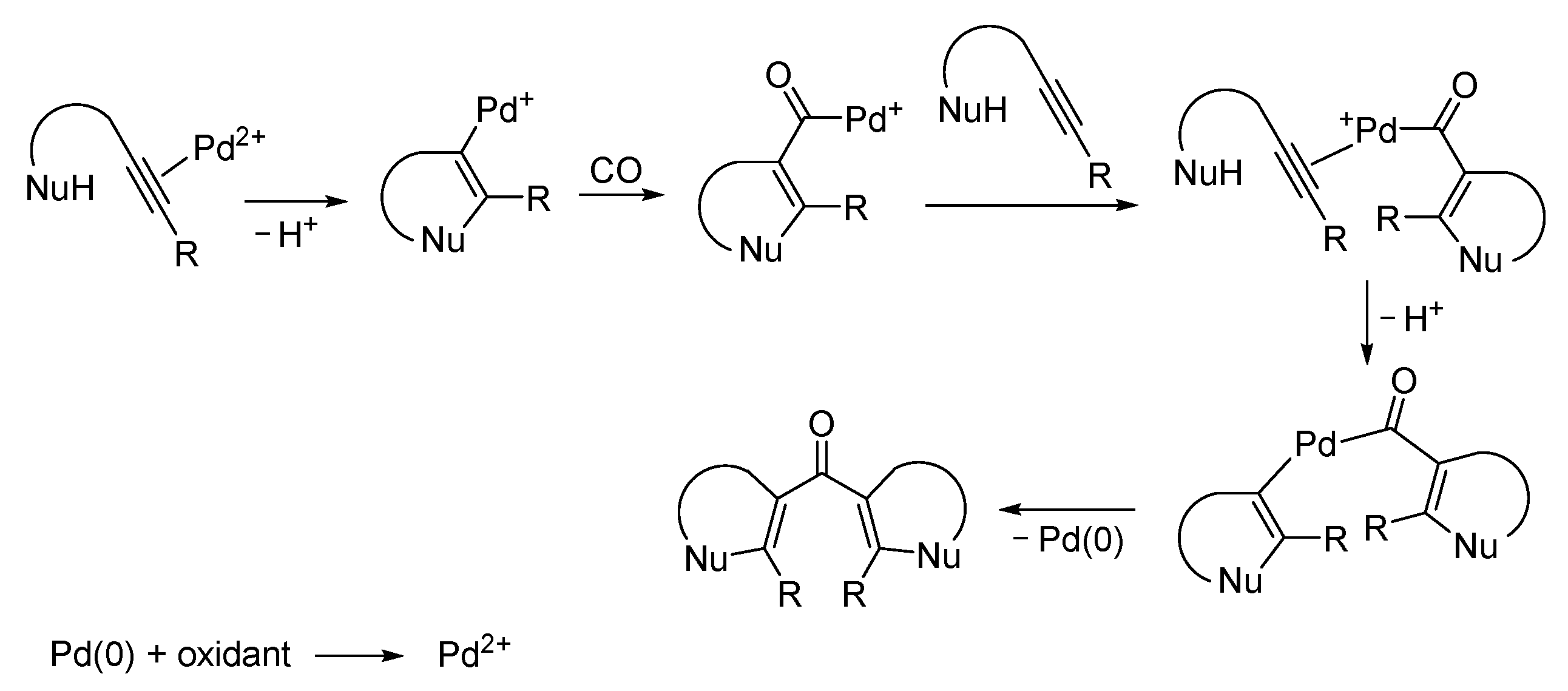
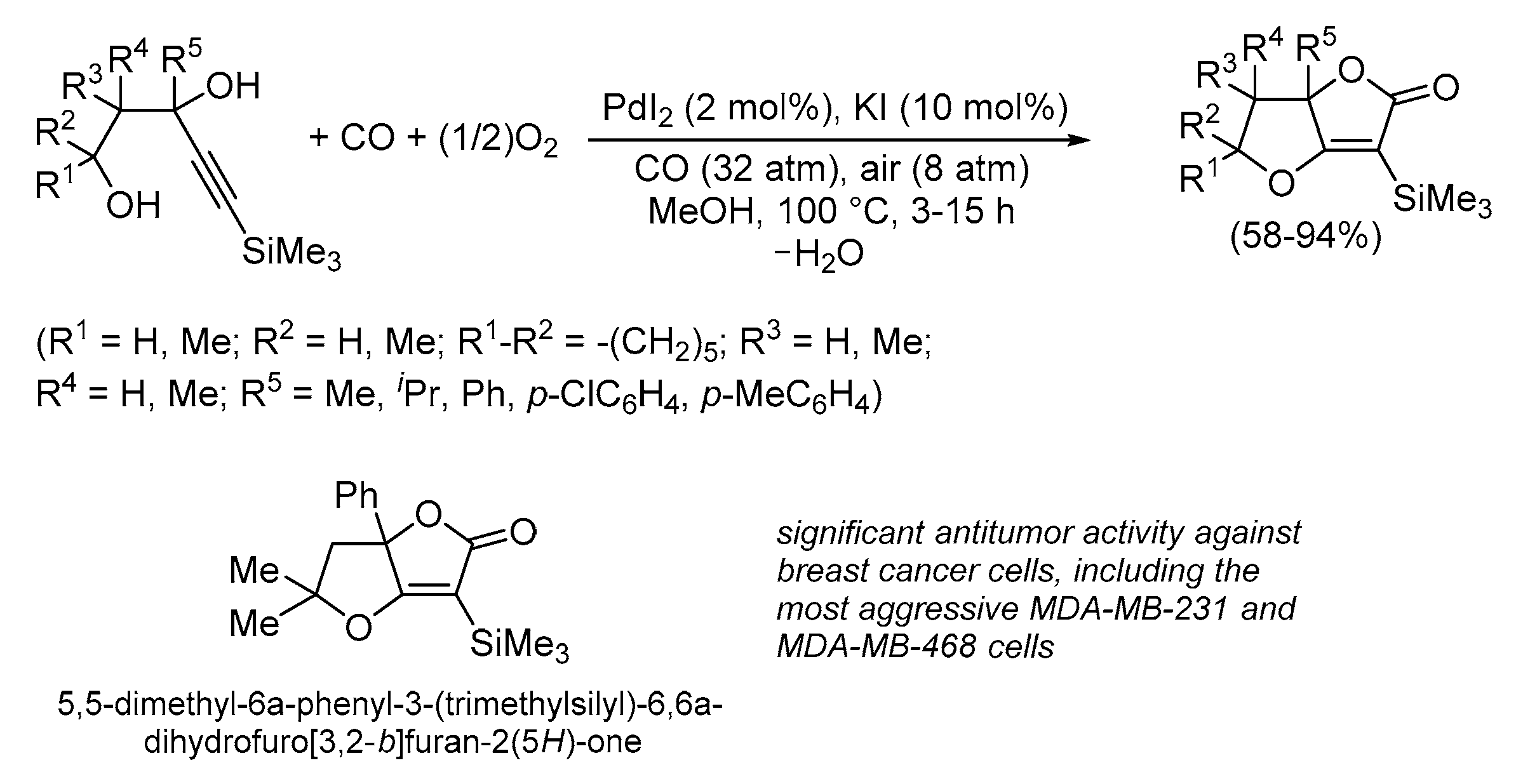
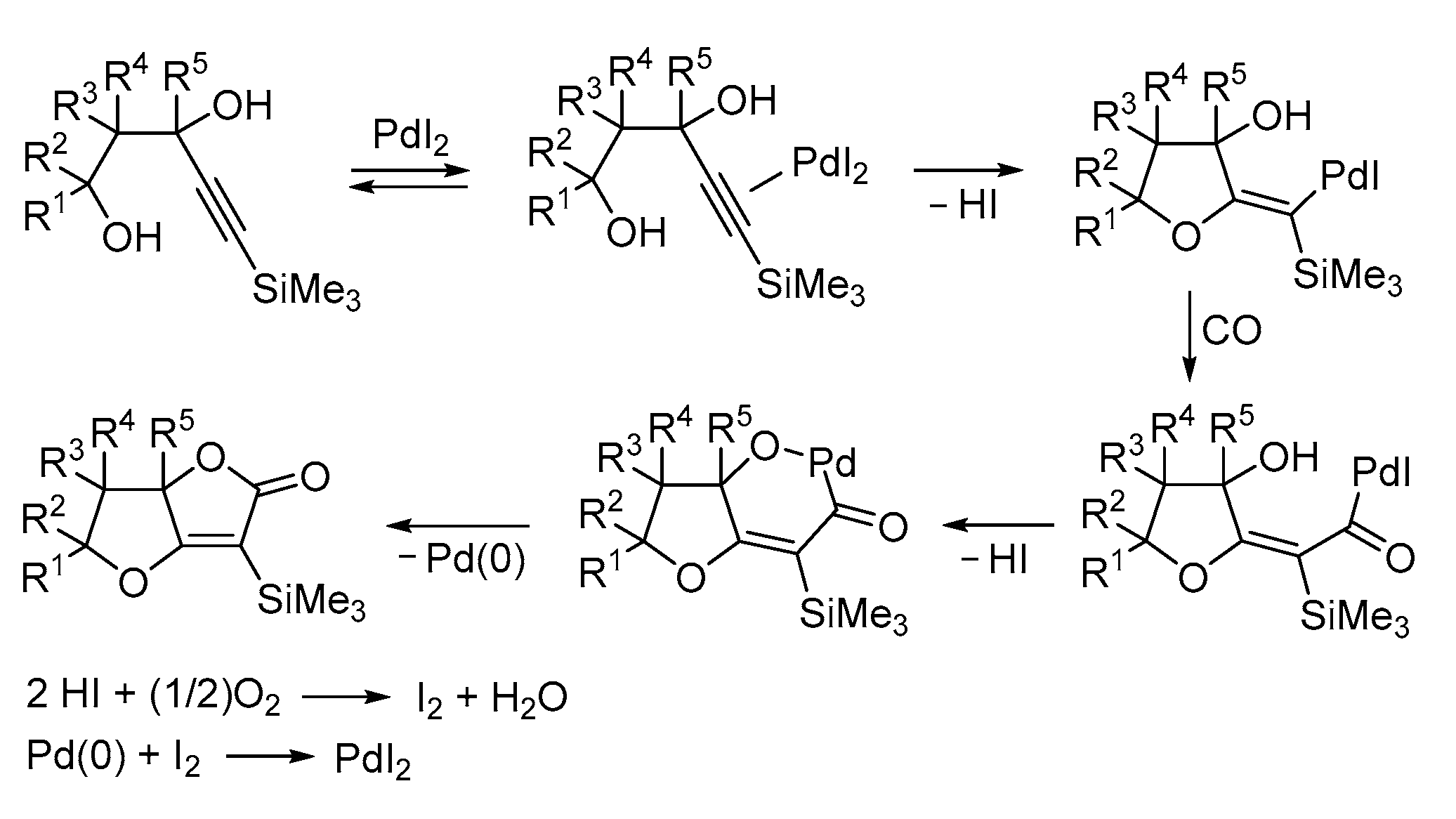
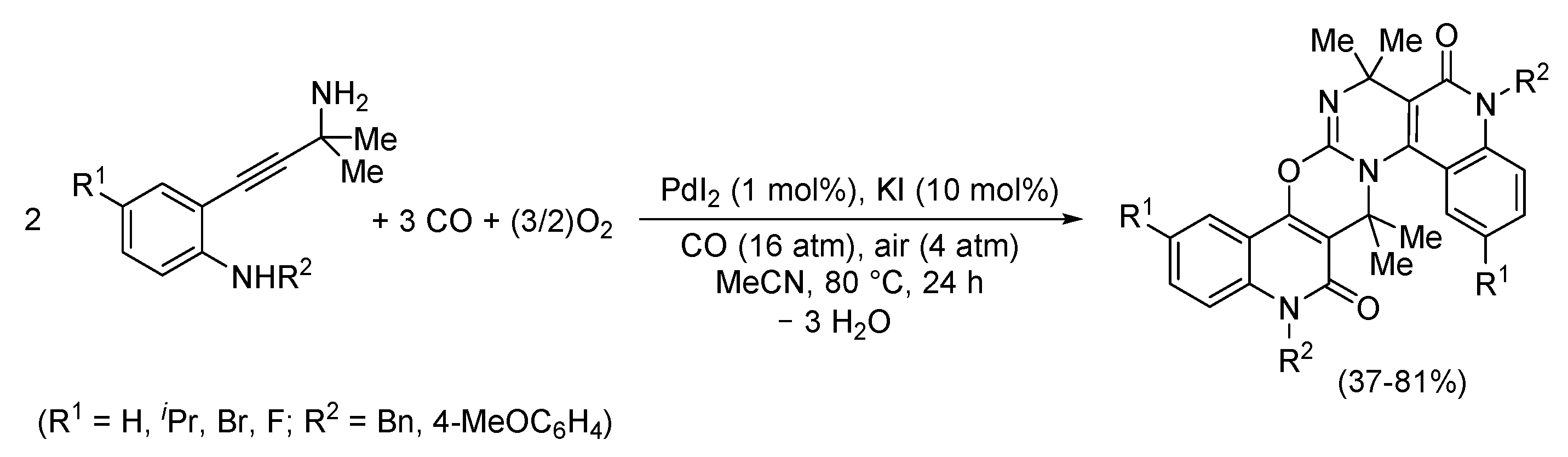
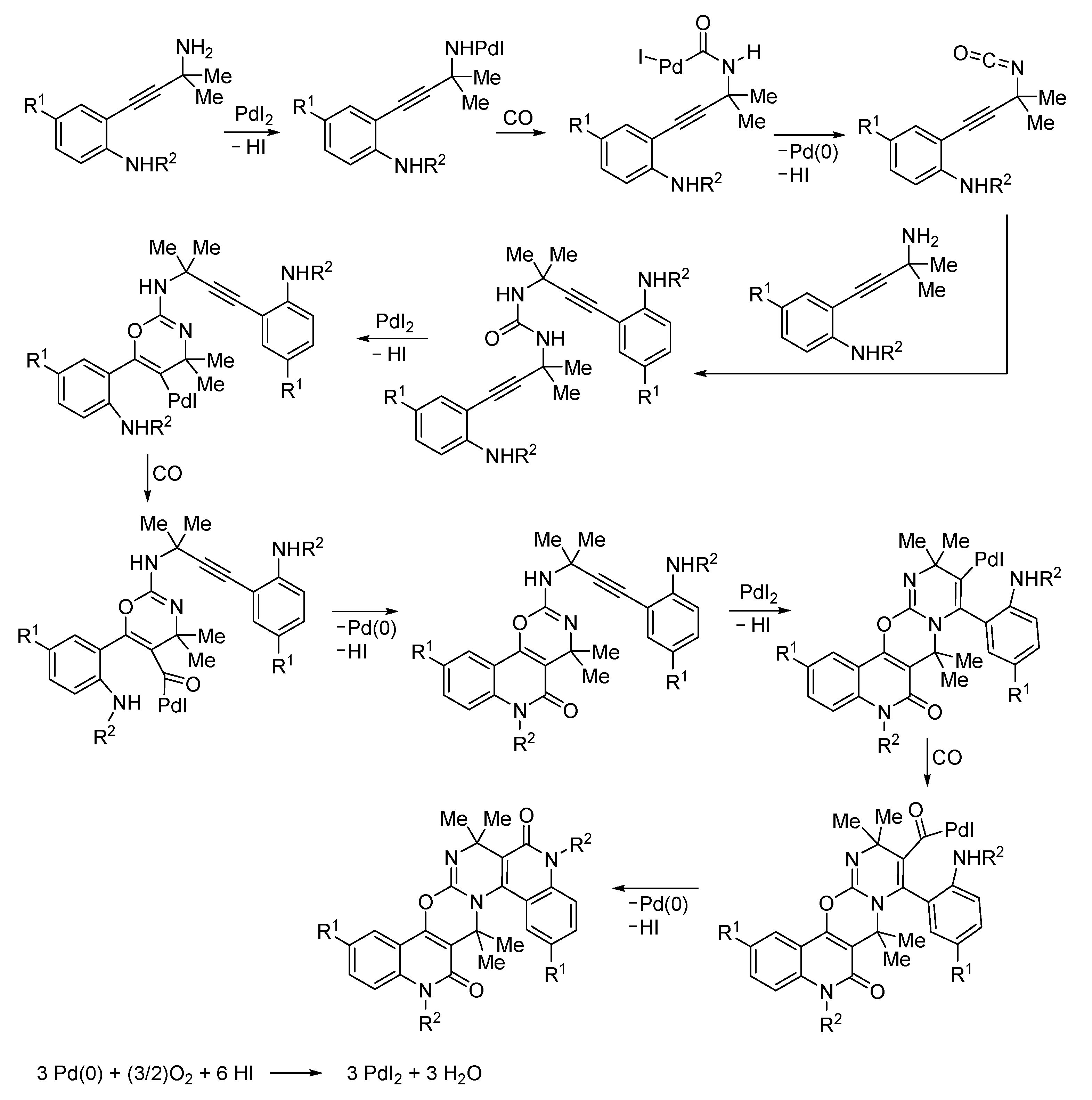
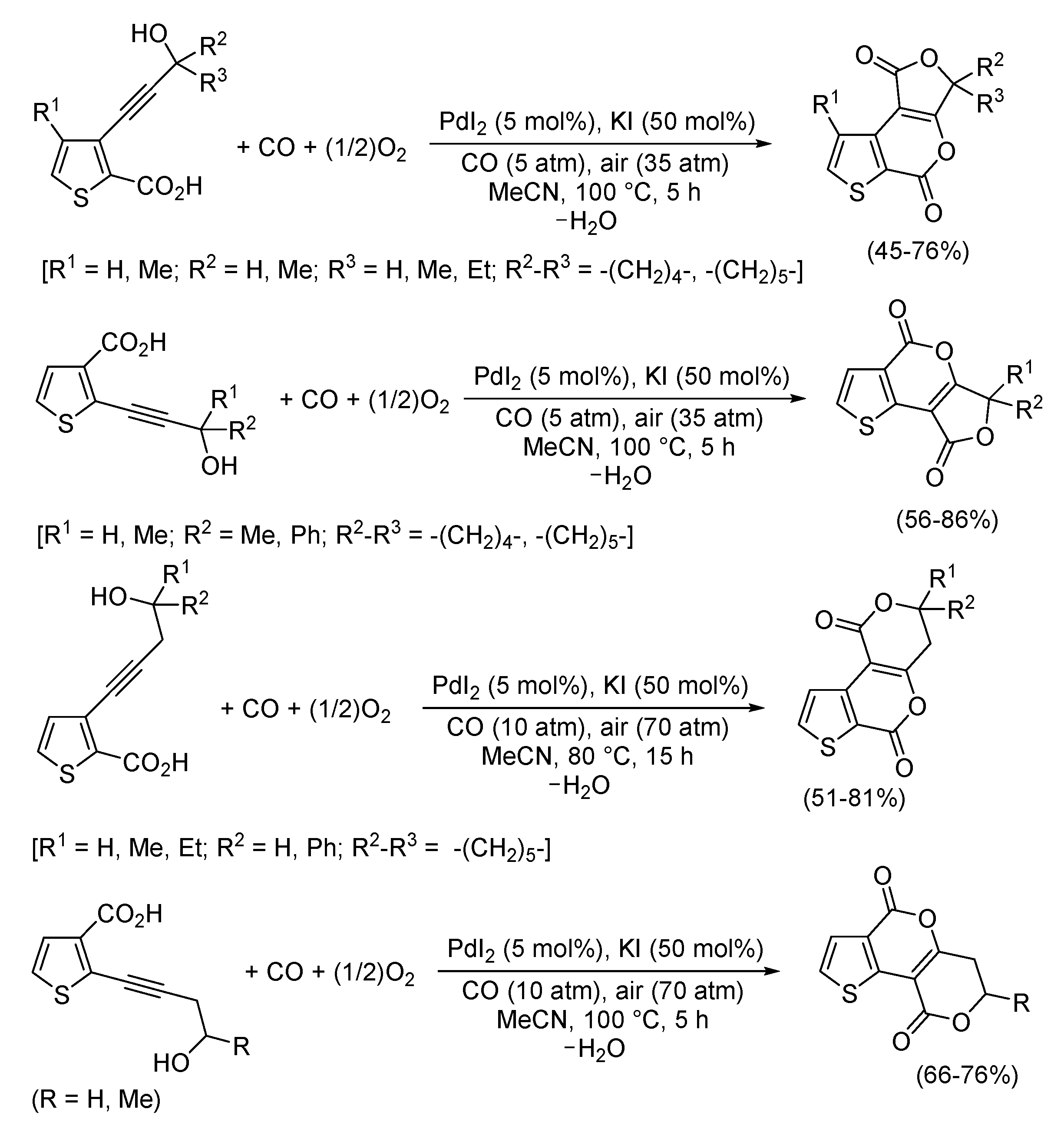
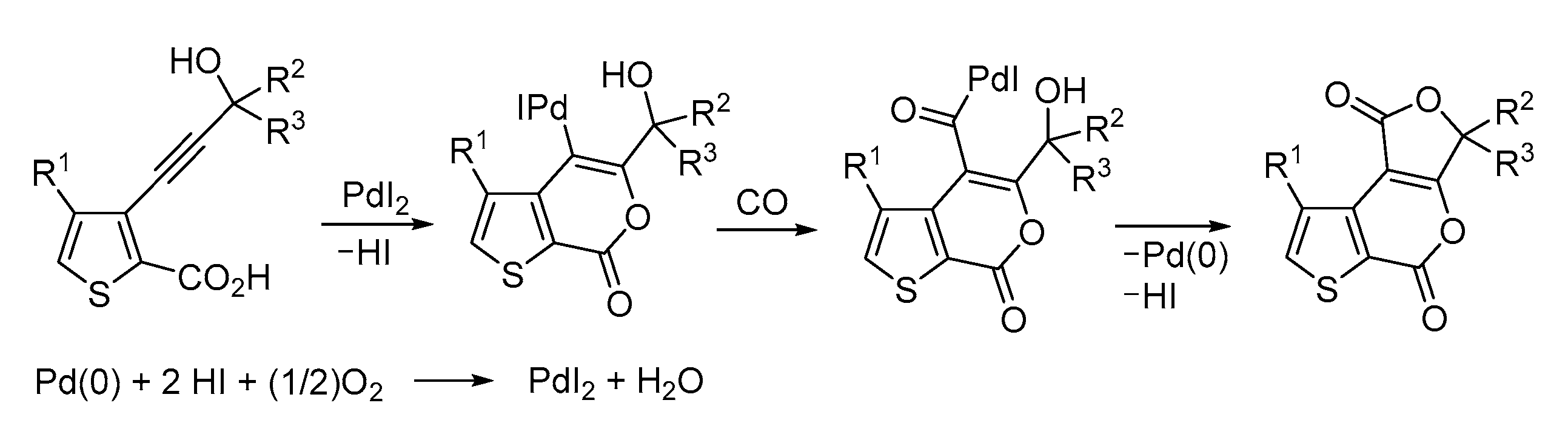
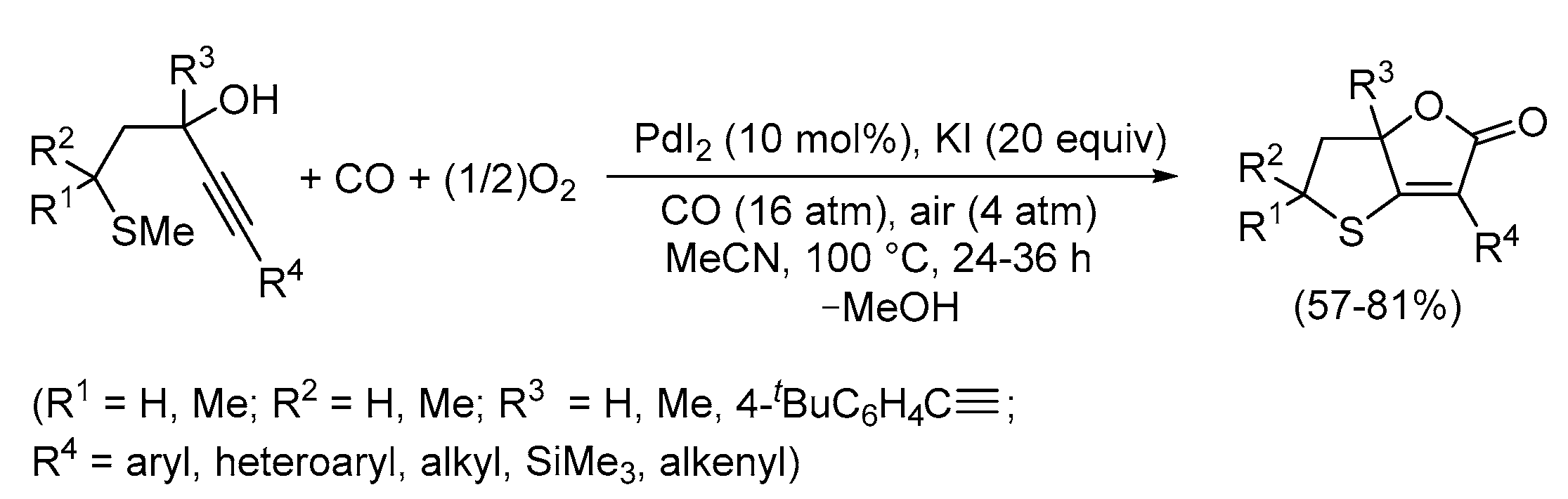
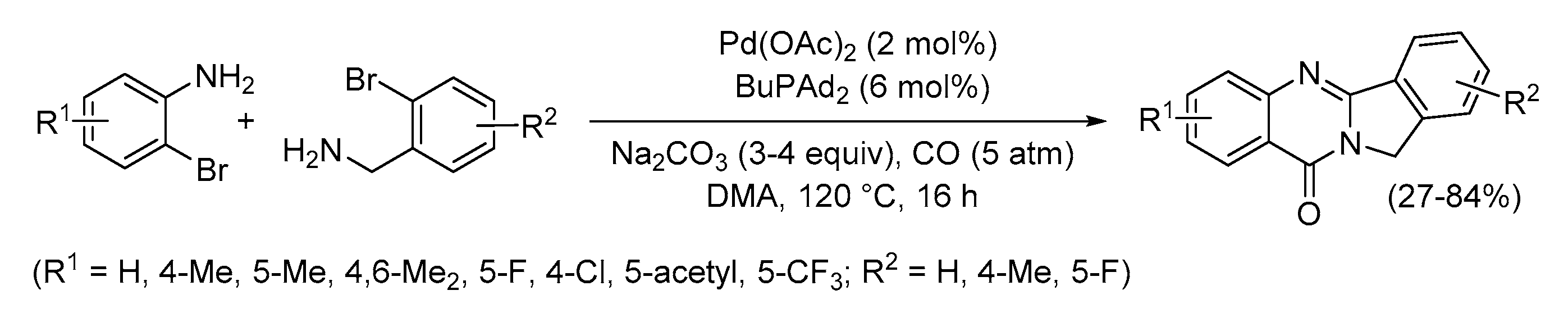
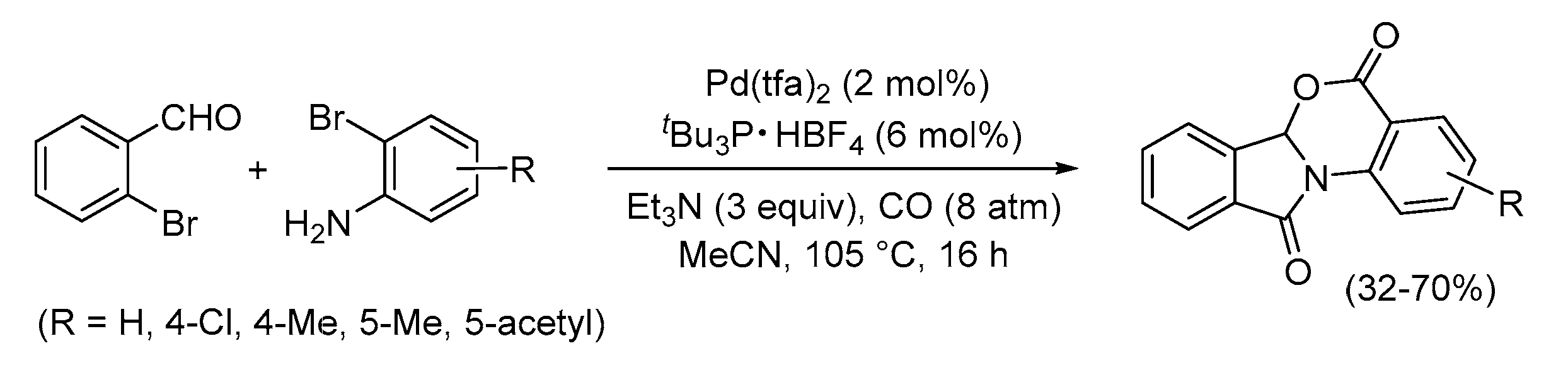
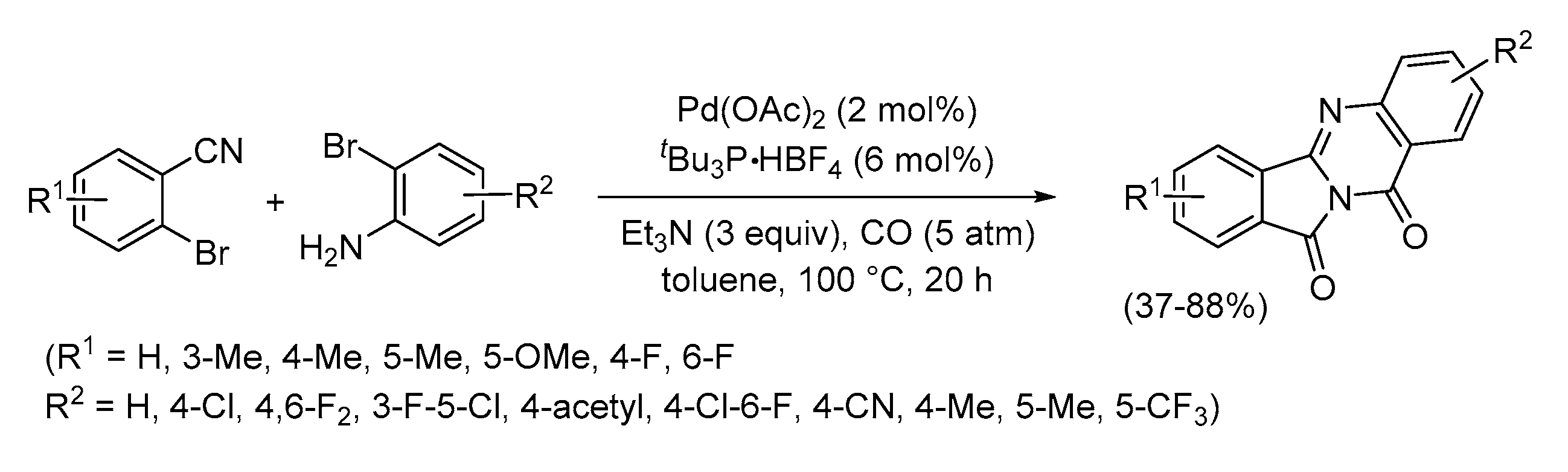

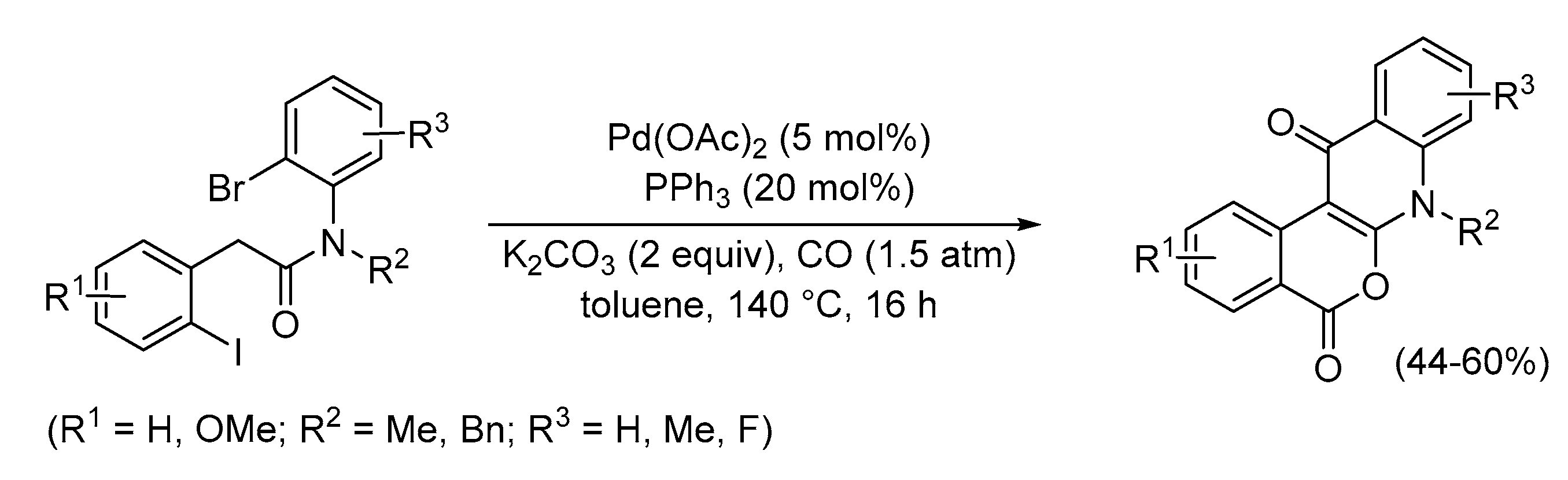
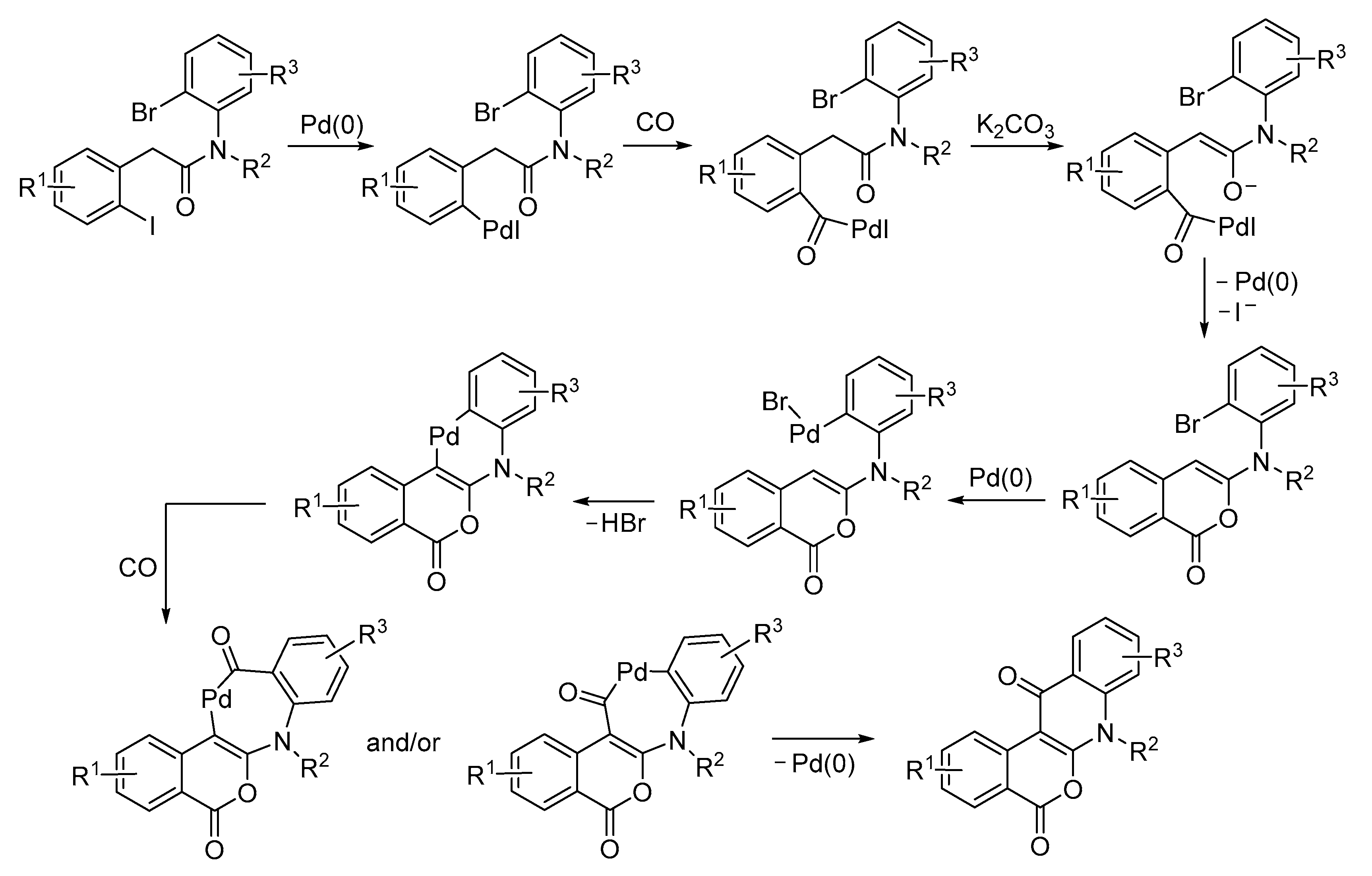
| Entry | Conditions | Substrate | Product | Yield (%) | Ref. |
|---|---|---|---|---|---|
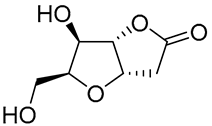
| 1 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 41 h | 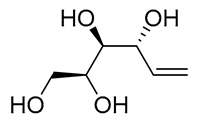 |
 |
63 | [10] |
| 2 | PdCl2(MeCN)2, (10 mol%), CuCl2 (2.4 equiv), CO (1 atm), THF, 25 °C, 24 h | 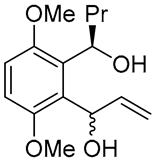 |
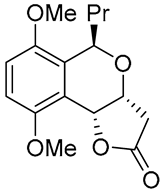 |
65 | [11] |
| 3 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 8 h | 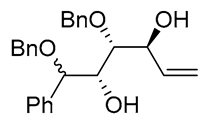 |
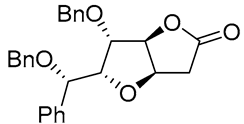 |
85 | [12] |
| 4 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (4 equiv), CO (1 atm), AcOH, 25 °C, 24 h | 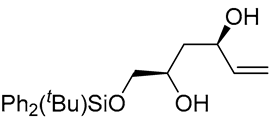 |
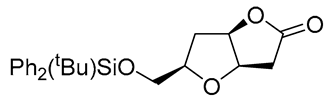 |
93 | [13] |
| 5 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 33 h | 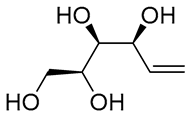 |
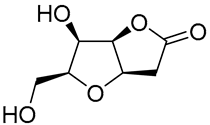 |
38 | [14] |
| 6 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 15 h | 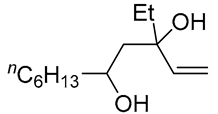 |
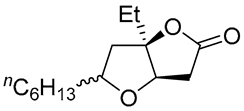 |
>80 | [15] |
| 7 | Pd(OAc)2 (1.5 equiv), CO (1.1 atm), THF, 23 °C, 4 h | 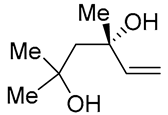 |
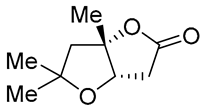 |
87 | [16] |
| 8 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 15 h |  |
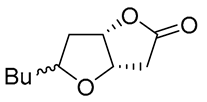 |
81 | [17] |
| 9 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C | 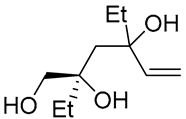 |
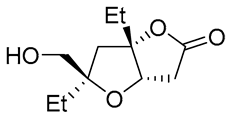 |
63 | [18] |
| 10 | PdCl2, CuCl, AcONa, CO, AcOH | 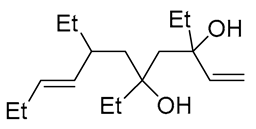 |
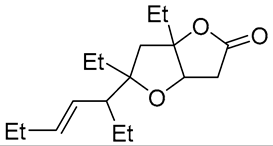 |
33 | [19] |
| 11 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 10 h | 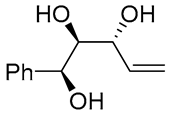 |
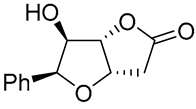 |
85 | [20] |
| 12 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 23 °C, 24 h | 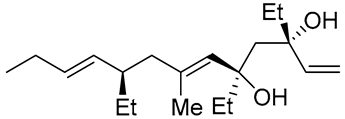 |
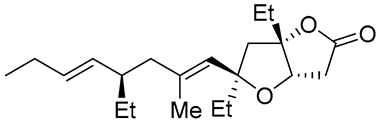 |
75 | [21] |
| 13 | Pd(OAc)2 (1.5 equiv), N-methylmorpholine (3 equiv), CO, THF | 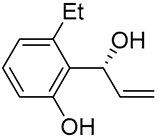 |
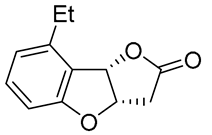 |
58 | [22] |
| 14 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 20 h | 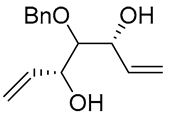 |
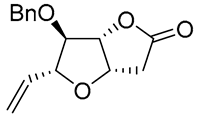 |
65 | [23] |
| 15 | Pd(OAc)2 (10 mol%), CuCl2 (3 equiv), AcONa (39 equiv), CO (1 atm), AcOH, 25 °C, 15 h | 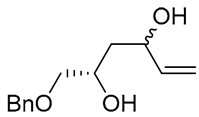 |
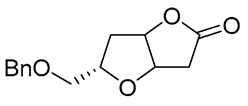 |
63, 70 | [24,25] |
| 16 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 24 h |  |
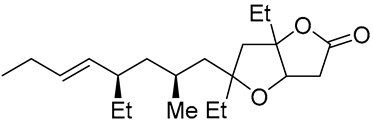 |
33 | [26] |
| 17 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 24 h | 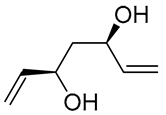 |
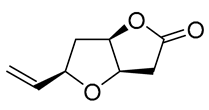 |
87 | [27] |
| 18 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 12 h | 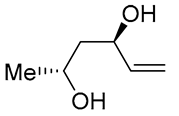 |
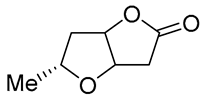 |
61 | [28] |
| 19 | PdCl2(MeCN)2 (10 mol%), CuCl2 (5 equiv), AcOLi (5 equiv), [Fe(CO)5] (0.5 equiv), AcOH, 60 °C, 1 h | 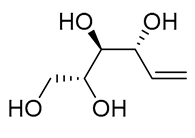 |
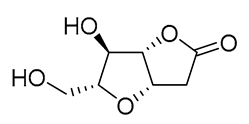 |
47 | [29] |
| 20 | PdCl2(MeCN)2 (10 mol%), Cu(OAc)2 (4 equiv), LiCl (4 equiv), [Fe(CO)5] (0.25 equiv), AcOH, 60 °C, 15 min | 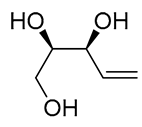 |
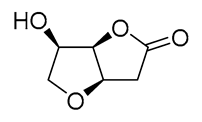 |
67 | [30] |
| 21 | PdCl2(MeCN)2 (10 mol%), CuCl2 (4 equiv), AcOLi (4 equiv), [Fe(CO)5] (0.3 equiv), AcOH, 60 °C, 30 min | 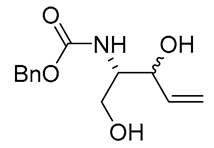 |
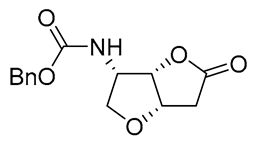 |
75 | [31] |
| Entry | Conditions | Substrate | Product | Yields (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Pd(tfa)2 (5 mol%), 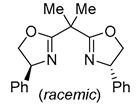 (10 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 0 °C, 5-12 h (10 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 0 °C, 5-12 h |
 |
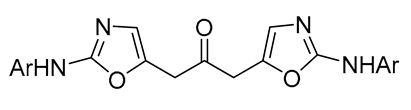 |
90-92 | [50] |
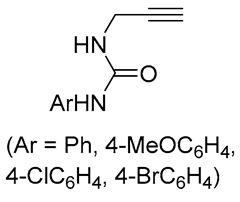
| 2 | Pd(L)(tfa)2 (5 mol%), L =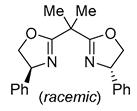 p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, 7 °C to 25 °C, 18-48 h p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, 7 °C to 25 °C, 18-48 h |
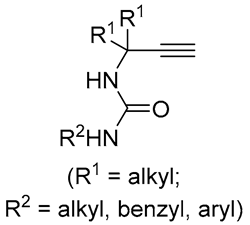 |
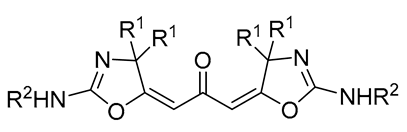 |
24-89 | [53] |
| 3 | Pd(tfa)2 (5-10 mol%), 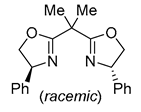 (7.5-12 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, ‒30 to 0 °C, 2-53 h (7.5-12 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, ‒30 to 0 °C, 2-53 h |
 |
 |
71-99 | [54] |
| 4 | Pd(tfa)2 (5 mol%), 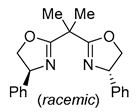 (7.5 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 25 °C, 1-63 h (7.5 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 25 °C, 1-63 h |
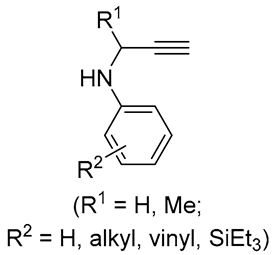 |
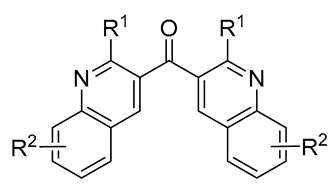 |
10-89 | [55] |
| 5 | Pd(L)(tfa)2 (5 mol%), L =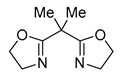 p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒5 °C to 25 °C, 1-46 h p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒5 °C to 25 °C, 1-46 h |
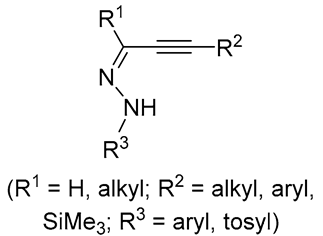 |
 |
70-94 | [56] |
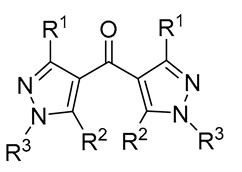
| 6 | Pd(tfa)2 (5 mol%), 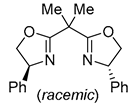 (7.5 mol%), p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒30 °C to 25 °C, 24-144 h (7.5 mol%), p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒30 °C to 25 °C, 24-144 h |
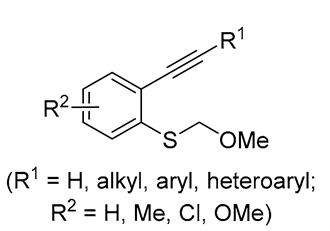 |
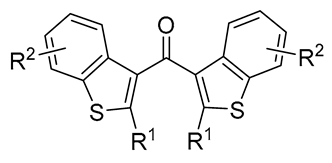 |
75-100 | [51,57] |
| 7 | Pd(L)(tfa)2 (5 mol%), L =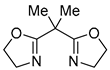 p-benzoquinone (1.5 equiv), CO (1 atm), iPrOH, ‒5 °C to 15 °C, 47-72 h p-benzoquinone (1.5 equiv), CO (1 atm), iPrOH, ‒5 °C to 15 °C, 47-72 h |
 |
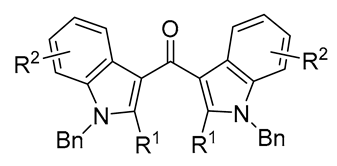 |
73-92 | [58] |
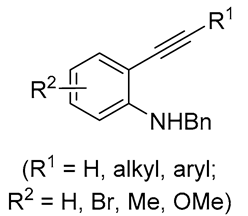
| 8 | Pd(L)(tfa)2 (5 mol%), L = p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒20 °C to 0 °C, 24-76 h p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, ‒20 °C to 0 °C, 24-76 h |
 |
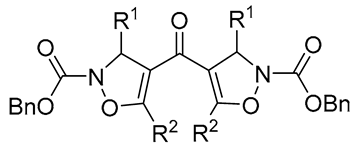 |
70-94 | [59] |
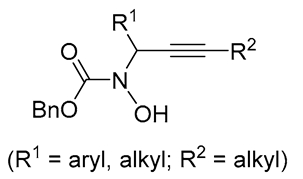
| 9 | Pd(L)(tfa)2 (5 mol%), L =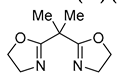 p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, 0 °C to 25 °C, 24-55 h p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, 0 °C to 25 °C, 24-55 h |
 |
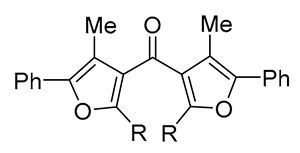 |
12-86 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





