Submitted:
25 May 2023
Posted:
29 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cardiac voltage-gated calcium channels (VGCCs)
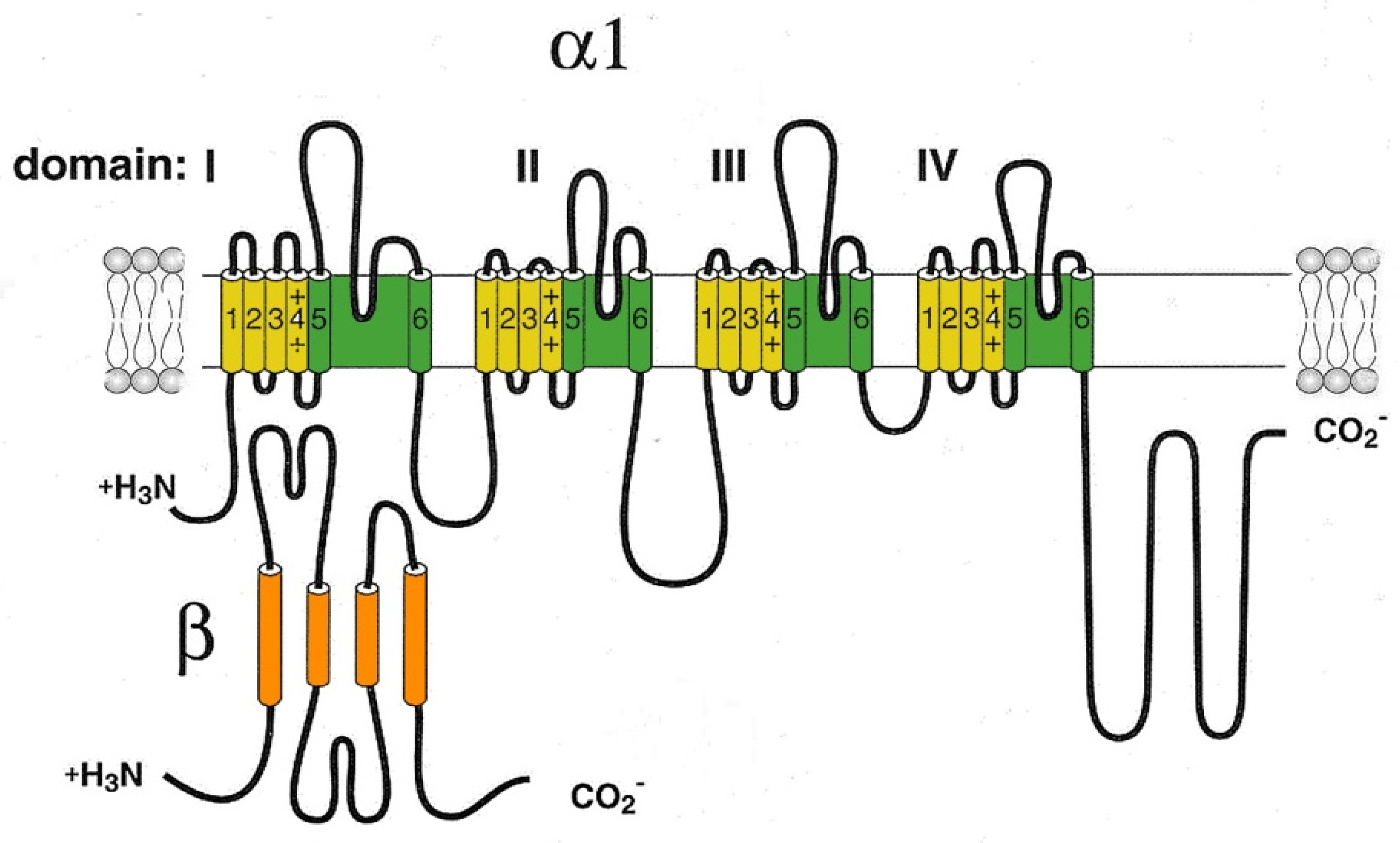
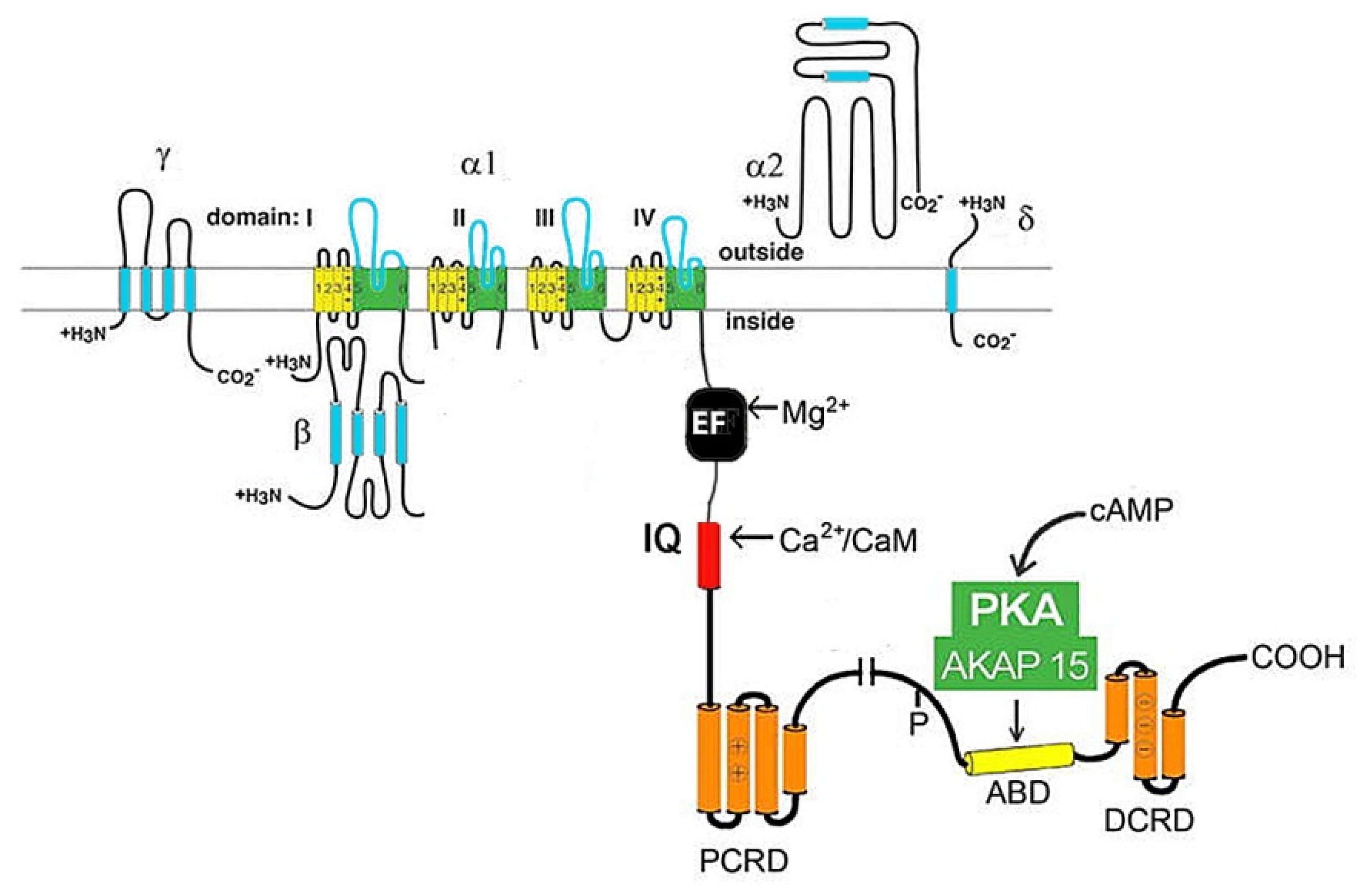
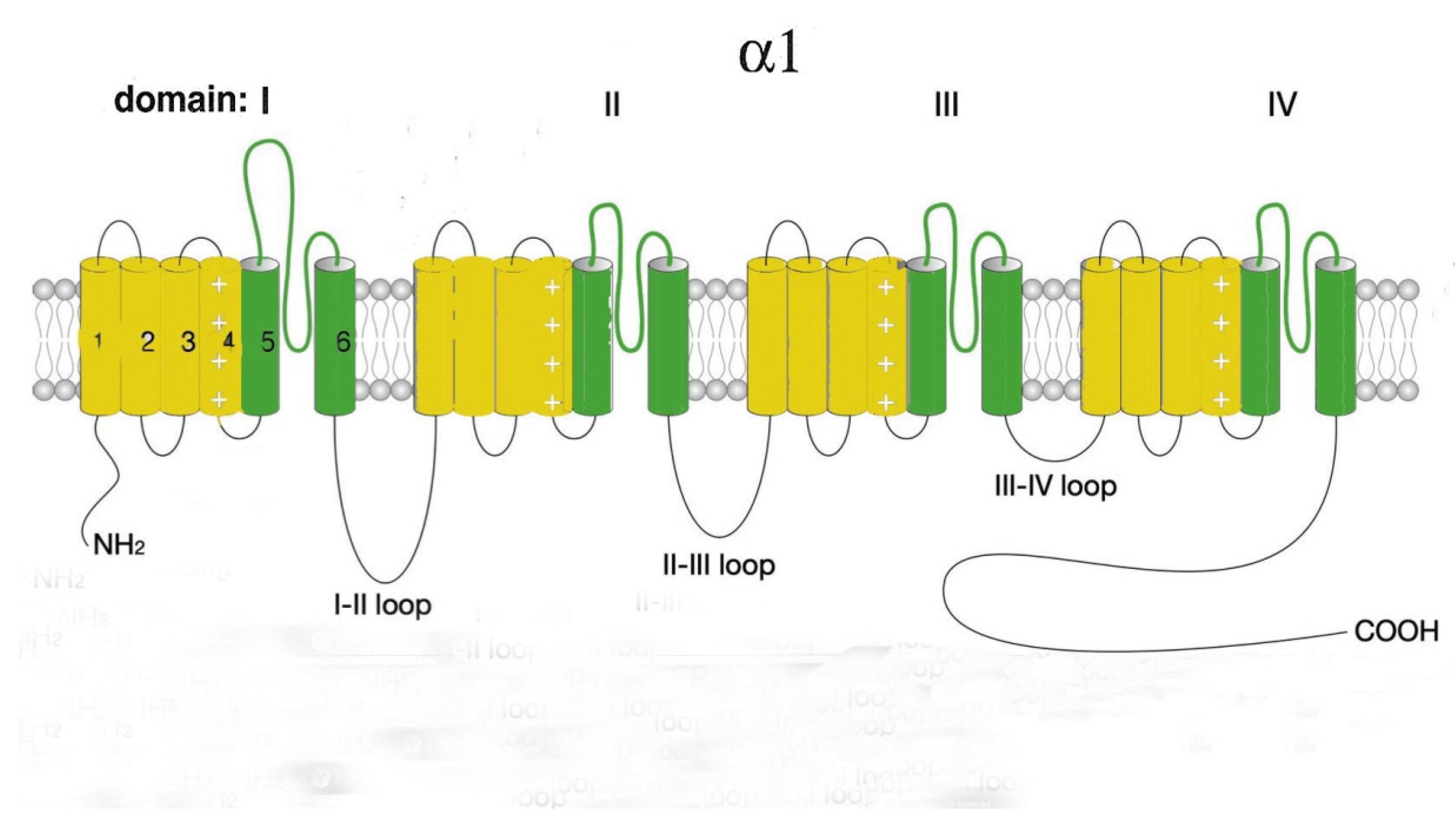
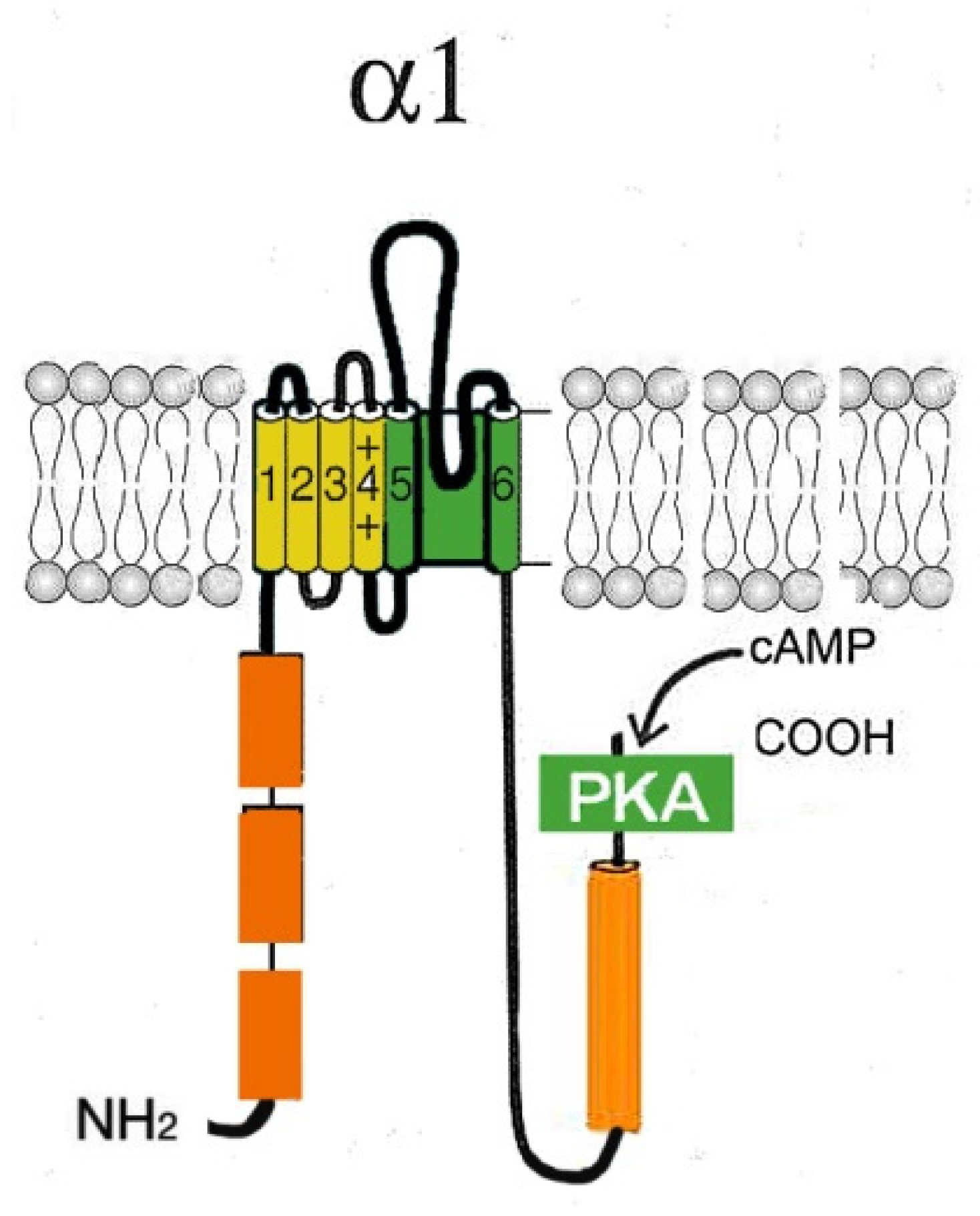
2.1. L-type Cav channels (Cav1)
2.2. R-type Ca2+ channels (Cav2.3)
2.3. T-type Ca2+ channels
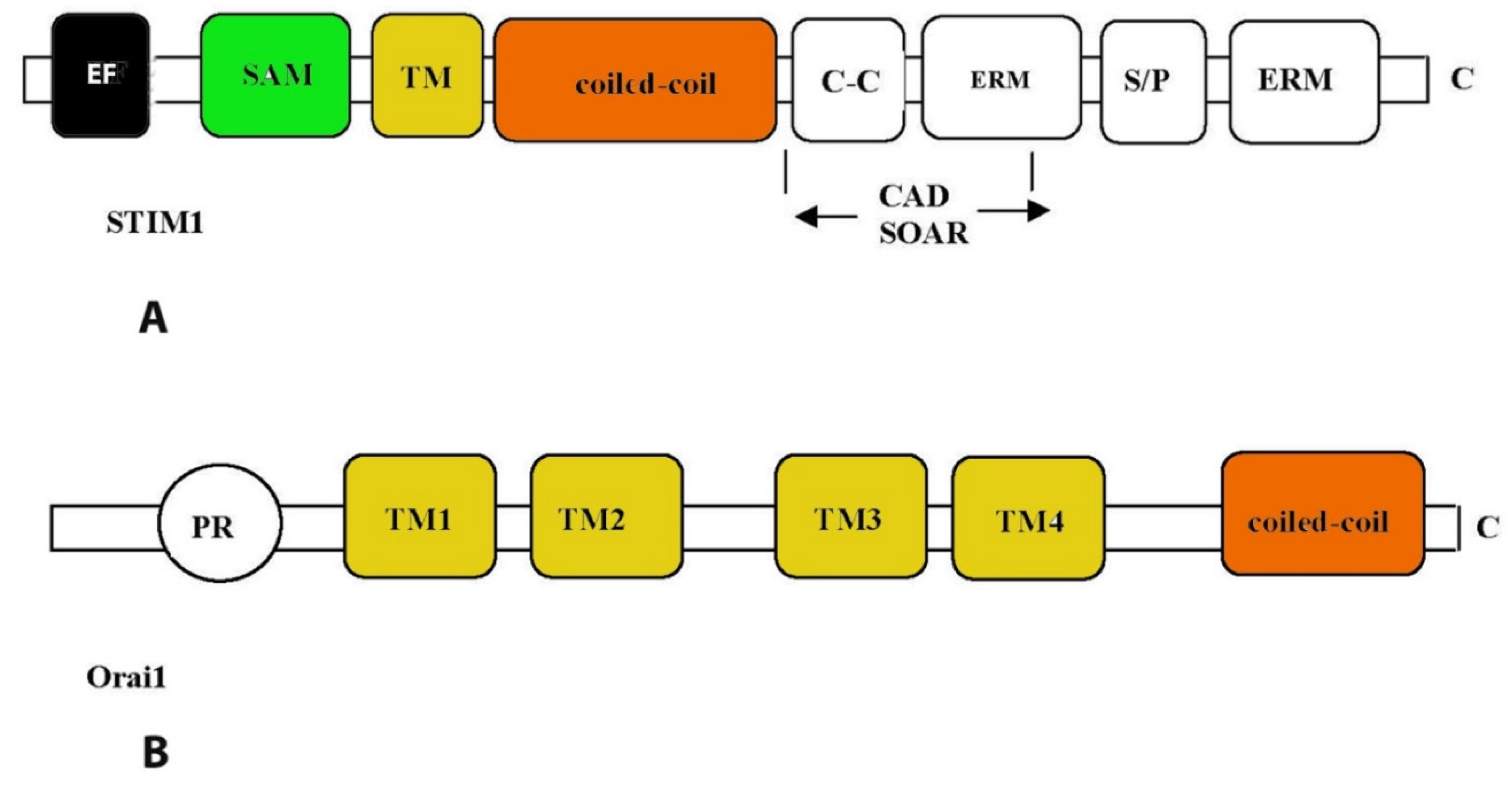
3. Store-operated calcium Ca2+ channels
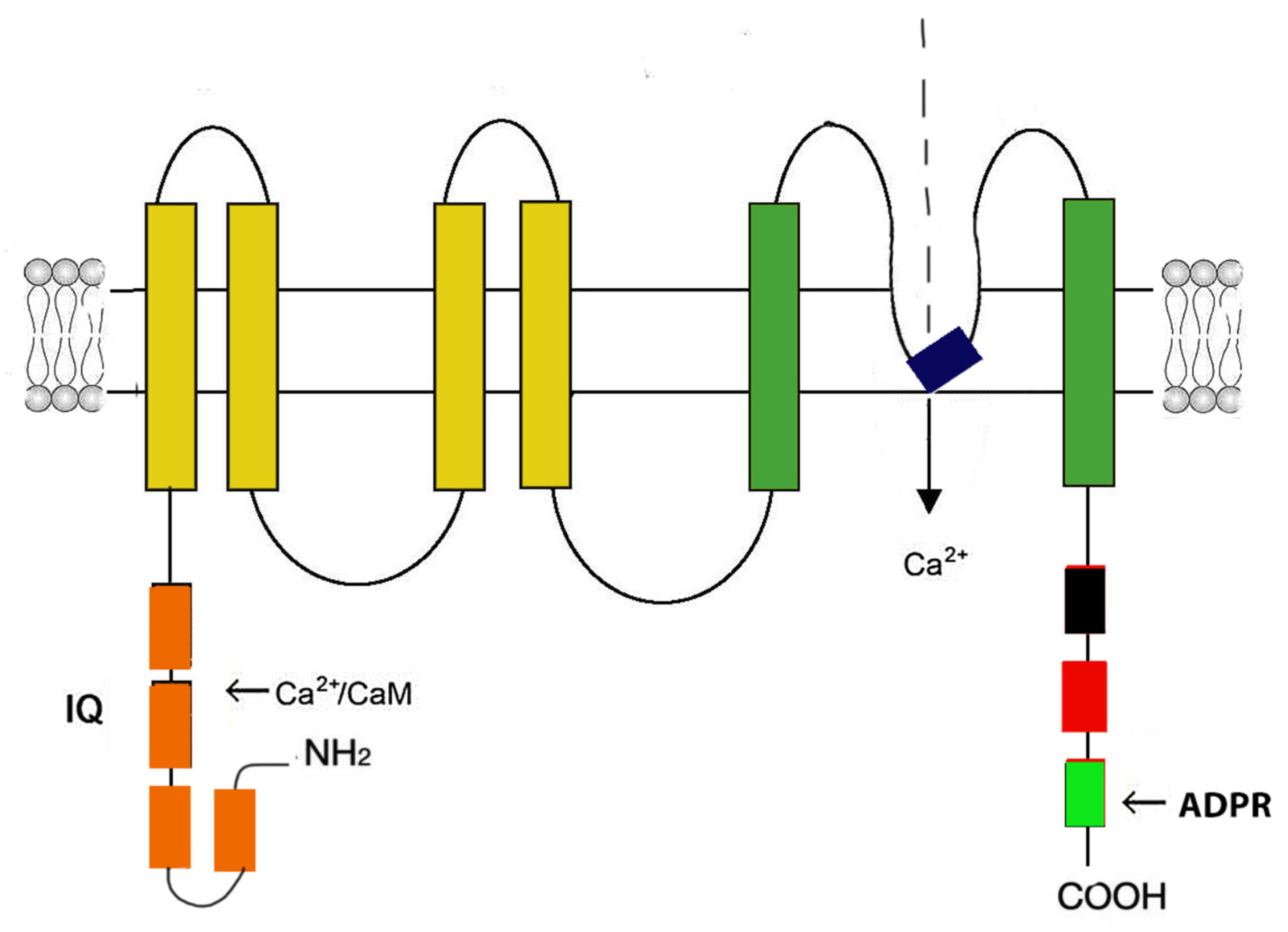
3.1. Cardiac ryanodine receptor (RyR2)
3.2. Ion channels with transient receptor potential (TRPC, TRPM7, TRPA1)
4. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels
5. Prospects of using the achievements of molecular biology in the treatment of chronic heart diseases
Funding
Conflicts of Interest
Abbreviations
| ADPR | ADP-ribose |
| AP | action potential |
| ARVC/D | arrhythmogenic right ventricular cardiomyopathy/dysplasia type 2 |
| AVN | atrioventricular node |
| BS | Brugada syndrome |
| Ca2+ | calcium |
| [Ca(2+)]i | intracellular Ca2+ concentration |
| CAD | сoronary artery disease |
| СаМ | calmodulin |
| Cav channels | voltage-gated calcium channels |
| Cav1 | L-type Cav channels |
| CCB | calcium channel blockers |
| CCs | calcium channelopathies |
| CHD | coronary heart disease |
| CIRC | Ca2+-induced Ca2+ release |
| CM | cardiomyocyte |
| CNBD | cyclic nucleotide-binding domain |
| CPVT | catecholaminergic polymorphic ventricular tachycardia |
| CRAC | calcium-release activated calcium channel |
| CVD | сardiovascular diseases |
| DADs | afterdepolarizations |
| DM1 | myotonic dystrophy type I |
| ECC | excitation-contraction coupling |
| ECG | electrocardiogram |
| EDHF | endothelial derived hyperpolarizing factor |
| GPCR | G-protein-coupled receptors |
| HF | heart failure |
| HR | heart rate |
| HVA | high voltage-activated voltage-gated calcium channels |
| IHD | ischemic heart disease |
| I/R | ischemia/reperfusion |
| LCRs | local diastolic intracellular Ca2+ release |
| LTCC | L-type Cav channels (Cav1) |
| MI | myocardial infarction |
| MP | membrane potential |
| NCX1 | Na+/Ca2+ exchange |
| PDE | phosphodiesterase |
| PFHBI | progressive familial heart block type I |
| PKA | cAMP-dependent protein kinase A |
| PLC | phospholipase C |
| PM | plasma membrane |
| PP | resting potential |
| ROS | reactive oxygen species |
| RP | resting potential |
| RyR2 | ryanodine receptor type 2 |
| SAN | sinus node |
| SOCE | store-operated calcium entry |
| SQTS | Short QT syndrome |
| SR | sarcoplasmic reticulum |
| STIM1 | stromal interacting molecule 1 |
| ТМ | transmembrane domain |
| TS | Timothy syndrome |
| TRP | transient receptor potential (canonical, vallinoid-related, melastatin-related) |
| SCD | Sudden Cardiac Death |
| SMC | smooth muscle cells |
| VGCC | voltage-gate calcium channel |
| VSMCs | vascular smooth muscle cells |
References
- Atlas, D. Voltage-gated calcium channels function as Ca2+-activated signaling receptors. Trends Biochem. Sci. 2014, 39, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E.; Zhang, D; Chatham, J. C. STIM and Orai Mediated Regulation of Calcium Signaling in Age-Related Diseases. Front. Aging. 2022, 3, 876785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Tong, X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, A.S.; Peigneur, S.; Tytga,t. J. Review: HCN Channels in the Heart. Curr. Cardiol. Rev. 2022, 18, e040222200836. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D'Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; Maestrini, V.; Mancone, M.; Chilian, W.M.; Fedele, F. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, D. Small and Intermediate Calcium Activated Potassium Channels in the Heart: Role and Strategies in the Treatment of Cardiovascular Diseases. Front. Physiol. 2020, 11, 590534. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; Kang, H.; Brown, A.L.; Park, S.J.; Xu, X.; Zandee van Rilland, E.; Kim, M.K.; Cohen, J.I.; Kaplan, M.J.; Shoshan-Barmatz, V.; Chung, J.H. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Rosenberg, P. VDAC2 as a novel target for heart failure: Ca2+ at the sarcomere, mitochondria and SR. Cell Calcium. 2022, 104, 102586. [Google Scholar] [CrossRef]
- Martin, C.A.; Huang, C.L.; Matthews, G.D. The role of ion channelopathies in sudden cardiac death: implications for clinical practice. Ann Med. 2013, 45, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, C.T.; Owen, T.; Lawal, S.; Cao, S.; Pandey, S.S.; Yang, H.Y.; Song, W.; Wilkinson, R.; Alvarez-Laviada, A.; Gehmlich, K.; Marston, S.B.; MacLeod, K.T. Age- and strain-related aberrant Ca2+ release is associated with sudden cardiac death in the ACTC E99K mouse model of hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1213–H1226. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J. Voltage-Gated Ca2+/ Channel α1-Subunit de novo Missense Mutations: Gain or Loss of Function - Implications for Potential Therapies. Front. Synaptic Neurosci. 2021, 13, 634760. [Google Scholar] [CrossRef] [PubMed]
- Galetin, T.; Tevoufouet, E.E.; Sandmeyer, J.; Matthes, J.; Nguemo, F.; Hescheler, J.; Weiergräber, M.; Schneider,T. Pharmacoresistant Cav2·3 (E-type/R-type) voltage-gated calcium channels influence heart rate dynamics and may contribute to cardiac impulse conduction. Cell Biochemistry Funct. 2013, 31, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Priest, B.T.; McDermott, J.S. Cardiac ion channels. Channels (Austin). 2015, 9, 52–359.
- Fernández-Quintero, M.L.; El Ghaleb, Y.; Tuluc, P.; Campiglio, M.; Liedl, K.R.; Flucher, B.E. Structural determinants of voltage-gating properties in calcium channels. Elife. PMCID: PMC8099428. 2021, 10, e64087. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Signaling complexes of voltage-gated sodium and calcium channels, 486,107–16. Neurosci Lett.
- Mangoni, M.E.; Couette, B.; Marger, L.; Bourinet, E.; Striessnig, J.; Nargeot, J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog. Biophys. Mol. Biol. 2006, 90, 38–63. [Google Scholar] [CrossRef] [PubMed]
- Harraz, O.F.; Jensen, L.J. Vascular calcium signalling and ageing. J. Physiol. 2021, 599, 5361–5377. [Google Scholar] [CrossRef]
- Betzenhauser, M.J.; Pitt, G.S.; Antzelevitch, C. Calcium Channel Mutations in Cardiac Arrhythmia Syndromes. Curr. Mol. Pharmacol. 2015, 8, 133–142. [Google Scholar] [CrossRef]
- Torrente, A.G.; Mesirca, P.; Bidaud, I.; Mangoni, M.E. Channelopathies of voltage-gated L-type Cav1.3/α1D and T-type Cav3.1/α1G Ca2+channels in dysfunction of heart automaticity. Pflugers Arch. 2020, 472, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Baudot, M.; Louradour, J.; Torrente, A.G.; Fossier, L.; Talssi, L.; Nargeot, J.; Barrère-Lemaire, S.; Mesirca, P.; Mangoni, M.E. Concomitant genetic ablation of L-type Cav1.3 (α1D) and T-type Cav3.1 (α1G) Ca2+ channels disrupts heart automaticity. Sci Rep. 2020, 10, 18906. [Google Scholar] [CrossRef]
- Harvey, R.D.; Hell, J.W. Cav1.2 signaling complexes in the heart. J. Mol. Cell Cardiol. 2013, 58, 143–152. [Google Scholar] [CrossRef]
- Varró, A.; Tomek, J.; Nagy, N.; Virág, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef] [PubMed]
- Tester, D.J.; Ackerman, M.J. Genetics of long QT syndrome. Methodist Debakey Cardiovasc. J. 2014, 10, 29–33. [Google Scholar] [CrossRef]
- Gakenheimer-Smith, L.; Meyers, L.; Lundahl, D.; Menon, S.C.; Bunch, T.J.; Sawyer, B.L.; Tristani-Firouzi, M.; Etheridge, S.P. Expanding the phenotype of CACNA1C mutation disorders. Mol. Genet. Genomic. Med. 2021, 9, e1673. [Google Scholar] [CrossRef]
- Harrell, D.T.; Ashihara, T.; Ishikawa, T.; Tominaga, I.; Mazzanti, A.; Takahashi, K.; Oginosawa, Y.; Abe, H.; Maemura, K.; Sumitomo, N.; Uno, K.; Takano, M.; Priori, S.G.; Makita, N. Genotype-dependent differences in age of manifestation and arrhythmia complications in short QT syndrome. Int. J. Cardiol. 2015, 190, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Zhang, F.; Yang. Z.; Li, Y.; Xu, Q.; Lan, H.; Cyganek, L.; El-Battrawy, I.; Zhou X.; Akin, I.; Borggrefe, M. Epigenetic mechanism of L-type calcium channel β-subunit downregulation in short QT human induced pluripotent stem cell-derived cardiomyocytes with CACNB2 mutation. Europace. 2022, 24, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Van Petegem, F.; Clark, K.A.; Chatelain, F.C.; Minor, D.L. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004, 429, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Alings; M. ; Wilde, A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999, 99, 666–673. [Google Scholar] [CrossRef]
- Ednie, A.R.; Bennett, E.S. Intracellular O-linked glycosylation directly regulates cardiomyocyte L-type Ca(2+) channel activity and excitation-contraction coupling. Basic Res. Cardiol. 2020, 115, 59. [Google Scholar] [CrossRef]
- Angelini, M.; Pezhouman, A.; Savalli, N.; Chang, M.G.; Steccanella, F.; Scranton, K.; Calmettes, G.; Ottolia, M.; Pantazis, A.; Karagueuzian, H.S.; Weiss, J.N; Olcese, R. Suppression of ventricular arrhythmias by targeting late L-type Ca2+ current. J. Gen. Physiol. 2021, 153, e202012584. [Google Scholar] [CrossRef]
- Ito, D.W.; Hannigan, K.I.; Ghosh, D.; Xu, B.; Del Villar, S.G.; Xiang, Y.K; Dickson, E.J.; Navedo, M.F.; Dixon, R.E. β-adrenergic-mediated dynamic augmentation of sarcolemmal CaV1.2 clustering and co-operativity in ventricular myocytes. J. Physiol, 2019; 597, 2139–2162, PMCID: PMC6462464. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, F.; Schneider, T.; Albanna, W. Cav2.3 channel function and Zn2+induced modulation: potential mechanisms and (patho)physiological relevance. Channels (Austin). 2020, 14, 362–379. [Google Scholar] [CrossRef]
- Tevoufouet, E.E.; Nembo, E.N.; Distler, F.; Neumaier, F.; Hescheler, J.; Nguemo, F.; Schneider, T. Multiple nickel-sensitive targets elicit cardiac arrhythmia inisolated mouse hearts after pituitary adenylate cyclase-activatingpolypeptide-mediated chronotropy. Pharmacol Res. 2017, 117, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Alpdogan, S.; Hescheler, J.; Neumaier, F. In vitro and in vivo phosphorylation of the Cav2.3 voltage-gated R-type calcium channel. Channels (Austin). 2018, 12, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, Y.; Wang, Z.; Fan, X.; Wu, D.; Huang, J.; Mueller, A.; Gao, S.; Hu, M.; Robinson, C.V.; Yu, Y.; Gao, S.; Yan, N. Structures of the R-type human Cav2.3 channel reveal conformational crosstalk of the intracellular segments. Nat. Commun. 2022, 13, 7358. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Neumaier, F.; Hescheler, J.; Alpdogan, S. Cav2.3 R-type calcium channels: from its discovery to pathogenic de novo CACNA1E variants: a historical perspective. Pflugers Arch. 2020, 472, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Vassort, G.; Talavera, K.; Alvarez, J.L. Role of T-type Ca2+ channels in the heart. Cell Calcium. 2006, 40, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Zamponi, G.W. Genetic T-type calcium channelopathies. J. Med. Genet. 2020, 57, 1–10. [Google Scholar] [CrossRef]
- Cribbs, L.L.; Lee, J.H.; Yang, J.; Satin, J.; Zhang, Y.; Daud, A.; Barclay, J.; Williamson, M.P.; Fox, M.; Rees, M.; Perez-Reyes, E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 1998, 83, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.; Majumdar, D.; Tipanee, J.; Singh, K.; Klein, A.F.; Furling, D.; Chuah, M.K.; VandenDriessche, T. Comprehensive transcriptome-wide analysis of spliceopathy correction of myotonic dystrophy using CRISPR-Cas9 in iPSCs-derived cardiomyocytes. Mol. Ther., 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Petri, H.; Vissing, J.; Witting, N.; Bundgaard, H.; Køber, L. Cardiac manifestations of myotonic dystrophy type 1. Int. J. Cardiol. 2012, 160, 82–88. [Google Scholar] [CrossRef]
- Yasui, K.; Niwa, N.; Takemura, H.; Opthof, T.; Muto, T.; Horiba, M.; Shimizu, A.; Lee, J.K.; Honjo, H.; Kamiya, K.; Kodama, I. Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased heart. J. Pharmacol. Sci. 2005, 99, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Bodi, I.; Correll, R.N.; Chen, X.; Lorenz, J.; Houser, S.R.; Robbins, J. α1G-dependent T-type Ca2+ current antagonizes cardiac hypertrophy through a NOS3-dependent mechanism in mice. J. Clin. Invest. 2009, 119, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, A.; Wajsberg, B.; Marks, A.R. Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kurebayashi, N.; Yamazawa, T.; Murayama, T. Regulatory mechanisms of ryanodine receptor/Ca2+release channel revealed by recent advancements in structural studies. J. Muscle Res. Cell Motil. 2021, 42, 291–304. [Google Scholar] [CrossRef]
- Hamilton, S.L.; Serysheva, II. Ryanodine receptor structure: progress and challenges. J. Biol. Chem. 2009, 284, 4047–4051. [Google Scholar] [CrossRef] [PubMed]
- Correll, R.N.; Goonasekera, S.A.; van Berlo, J.H.; Burr, A.R.; Accornero, F.; Zhang, H.; Makarewich, C.A.; York, A.J.; Sargent, M.A.; Chen, X.; Houser, S.R.; Molkentin, J.D. STIM1 elevation in the heart results in aberrant Ca2+ handling and cardiomyopathy. J. Mol. Cell Cardiol. 2015, 87, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, M.Y.; Kuang SJ, Qin, X. Y.; Cai, Y.J.; Chen, S.Z.; Li, S.M.; Rao, F.; Yang, H.; Deng, C.Y. Differential role of STIM1 in calcium handling in coronary and intrarenal arterial smooth muscles. Eur. J. Pharmacol. 2022, 937, 175386. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Katz, D.; Bryson, V. SOCE and STIM1 signaling in the heart: Timing and location matter. Cell Calcium. 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Vinogradova, T.M.; Maltsev, V.A. The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann. N. Y. Acad. Sci. 2008, 1123, 41–57. [Google Scholar] [CrossRef]
- Xue, J.B.; Val-Blasco, A.; Davoodi, M.; Gómez, S.; Yaniv, Y.; Benitah, J.P.; Gómez, A.M. Heart failure in mice induces a dysfunction of the sinus node associated with reduced CaMKII signaling. J. Gen. Physiol. 2022, 154, e202112895. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Mesirca, P.; Marqués-Sulé, E.; Zahradnikova, A. Jr.; Villejoubert, O.; D'Ocon, P.; Ruiz, C.; Domingo, D.; Zorio, E.; Mangoni, M.E.; Benitah, J.P.; Gómez, A.M. RyR2R420Q catecholaminergic polymorphic ventricular tachycardia mutation induces bradycardia by disturbing the coupled clock pacemaker mechanism. JCI Insight. 2017, 2, e91872. [Google Scholar] [CrossRef]
- Wei, J.; Yao, J.; Belke, D.; Guo, W.; Zhong, X.; Sun, B.; Wang, R.; Paul Estillore, J.; Vallmitjana, A.; Benitez, R.; Hove-Madsen, L.; Alvarez-Lacalle, E.; Echebarria, B.; Chen, S.R.W. Ca2+-CaM Dependent Inactivation of RyR2 Underlies Ca2+ Alternans in Intact Heart. Circ. Res. 2021, 128, e63–e83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, T.; Oda, T.; Chakraborty, A.; Chen, L.; Uchinoumi, H.; Knowlton, A.A.; Fruen, B.R.; Cornea, R.L.; Meissner, G.; Bers, D.M. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ. Res. 2014, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Takahashi, N.; Xu, L.; Smithies, O.; Meissner, G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca2+ release channel. J. Clin. Invest. 2007, 117, 1344–1353. [Google Scholar] [CrossRef]
- Liu, B.; Walton, S.D.; Ho, H.T.; Belevych, A.E.; Tikunova, S.B.; Bonilla, I.; Shettigar, V.; Knollmann, B.C.; Priori, S.G.; Volpe, P.; Radwański, P.B.; Davis, J.P.; Györke, S. Gene Transfer of Engineered Calmodulin Alleviates Ventricular Arrhythmias in a Calsequestrin-Associated Mouse Model of Catecholaminergic Polymorphic Ventricular Tachycardia. J. Am. Heart Assoc. 2018, 7, e008155. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Hazan, A.; Lotteau, S.; Zhang, R.; Torrente, A.G.; Philipson, K.D.; Ottolia, M.; Goldhaber, J.I. Na/Ca exchange in the atrium: Role in sinoatrial node pacemaking and excitation-contraction coupling. Cell Calcium. 2020, 87, 102167. [Google Scholar] [CrossRef]
- Li, M.C.H.; O'Brien, T.J.; Todaro, M.; Powell, K.L. Acquired cardiac channelopathies in epilepsy: Evidence, mechanisms, and clinical significance. Epilepsia. 2019, 60, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Humphries, E.S.A.; Kamishima, T.; Quayle, J.M.; Dart, C. Calcium/calmodulin-dependent kinase 2 mediates Epac-induced spontaneous transient outward currents in rat vascular smooth muscle. J. Physiol. 2017, 595, 6147–6164. [Google Scholar] [CrossRef]
- Ezeani, M.; Prabhu, S. PI3K(p110α) as a determinant and gene therapy for atrial enlargement in atrial fibrillation. Mol. Cell Biochem. 2022, 478, 471–490. [Google Scholar] [CrossRef]
- Potenza, D.M.; Janicek, R.; Fernandez-Tenorio, M.; Camors, E.; Ramos-Mondragón, R.; Valdivia, H.H.; Niggli, E. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete β-adrenergic response. J. Gen. Physiol. 2019, 151, 131–145. [Google Scholar] [CrossRef]
- Sirenko, S.T., Zahanich, I.; Li, Y.; Lukyanenko, Y.O.; Lyashkov, A.E.; Ziman, B.D.; Tarasov, K.V.; Younes, A.; Riordon, D.R.; Tarasova, Y.S.; Yang, D.; Vinogradova, T.M.; Maltsev, V.A.; Lakatta EG. Phosphoprotein Phosphatase 1 but Not 2A Activity Modulates Coupled-Clock Mechanisms to Impact on Intrinsic Automaticity of Sinoatrial Nodal Pacemaker Cells. Cells PMCID: PMC8623309. 2021, 10, 3106. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Kushner, J.; Marx, S.O. Adrenergic Regulation of Calcium Channels in the Heart. Annu Rev Physiol. 2022, 84, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Hulsurkar, M.M.; Lahiri, S.K.; Karch, J.; Wang, M.C.; Wehrens, X.H.T. Targeting calcium-mediated inter-organellar crosstalk in cardiac diseases. Expert Opin, Ther, Targets. 2022, 26, 303–317. [Google Scholar] [CrossRef]
- Chopra, N.; Knollmann, B.C. Triadin regulates cardiac muscle couplon structure and microdomain Ca(2+) signalling: a path towards ventricular arrhythmias. Cardiovasc. Res. 2013, 98, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mirza, S.; Richardson, S.J.; Gallant, E.M.; Thekkedam, C.; Pace, S.M.; Zorzato, F.; Liu, D.; Beard, N.A.; Dulhunty, A.F. A new cytoplasmic interaction between junctin and ryanodine receptor Ca2+ release channels. J. Cell Sci. 2015, 128, 951–963, PMCID: PMC4342579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Han, L.; Wang, Y.; Zhou, Y.; Li, Q.; Wu, Y.; Talabieke, S.; Hou, Y.; Wu, L.; Liu, R.; Fu, Z.; You, H.; Li, B.Y.; Zheng, Y.; Luo, D. Functional Calsequestrin-1 Is Expressed in the Heart and Its Deficiency Is Causally Related to Malignant Hyperthermia-Like Arrhythmia. Circulation. 2021, 144, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, S.; Pawlowitz, J.; Steckmeister, V.; Subramanian, H.; Uhlenkamp, D.; Scardigli, M.; Mushtaq, M.; Amlaz, S.I.; Kohl, T.; Wegener, J.W.; Arvanitis, D.A.; Sanoudou, D.; Sacconi, L.; Hasenfub, G.; Voigt, N.; Nikolaev, V.O.; Lehnart, S.E. A junctional cAMP compartment regulates rapid Ca2+signaling in atrial myocytes. J. Mol. Cell Cardiol. 2022, 165, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, X.; Gong, Y.; Zhang, P.; Qiang, S.; Zhao, Q.; Guo, R.; Qian, Y.; Wang, L.; Zhu, L.; Wang, R.; Hao, Z.; Wen, H.; Zhang, J.; Tang, K.; Zang, W.F.; Yuchi, Z.; Chen, H.; Chen, S.R.W.; Zheng, W.; Wang, S.Q.; Xu, Y.W.; Liu, Z. Pathogenic mechanism of a catecholaminergic polymorphic ventricular tachycardia causing-mutation in cardiac calcium release channel RyR2. J. Mol. Cell Cardiol. 2018, 117, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Ghezelbash, S.; Wehrens, X.H.; Dobrev, D. Serine/Threonine Phosphatases in Atrial Fibrillation. J. Mol. Cell Cardiol. 2017, 103, 110–120. [Google Scholar] [CrossRef]
- Klapproth, E.; Kämmerer, S.; El-Armouche, A. Function and regulation of phosphatase 1 in healthy and diseased heart. Cell Signal. 2022, 90, 110203. [Google Scholar] [CrossRef] [PubMed]
- Sovari, A.A.; Iravanian, S.; Dolmatova, E.; Jiao, Z.; Liu, H.; Zandieh, S.; Kumar, V.; Wang, K.; Bernstein, K.E.; Bonini, M.G.; Duffy, H.S.; Dudley, S.C. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J. Am. Cell Cardiol. 2011, 58, 2332–2339. [Google Scholar] [CrossRef]
- Potenza, D.M.; Janicek, R.; Fernandez-Tenorio, M.; Niggli, E. Activation of endogenous protein phosphatase 1 enhances the calcium sensitivity of the ryanodine receptor type 2 in murine ventricular cardiomyocytes. J. Physiol. 2020, 598, 1131–1150. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; Becerra, R.; Valverde, C.A.; Kaetzel, M.A.; Dedman, J.R.; Mundiña-Weilenmann, C.; Wehrens, X.H.; Vittone, L.; Mattiazzi, A. Calcium-calmodulin dependent protein kinase II (CaMKII): a main signal responsible for early reperfusion arrhythmias. J. Mol. Cell Cardiol. 2011, 51, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Grandi, E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009, 54, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Chuang, H.L.; Chen, Y.C.; Kao, Y.H.; Lin, Y.K.; Yeh, Y.H.; Chen, S.A.; Chen, Y.J. Heart failure modulates electropharmacological characteristics of sinoatrial nodes. Exp. Ther. Med. 2017, 13, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Liu, Y.; Zhang, J.; Zheng, X.; Sun, X.; Lei, S.; Kang, Z.; Chen, X.; Lei, M.; Hu, H.; Zeng, X.; Hao, L. Distinct roles of calmodulin and Ca2+calmodulin-dependent protein kinase II in isopreterenol-induced cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2020, 526, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Q.; Wang, L.P.; Gong, Y.Y.; Fan, X.X.; Zhu, S.Y.; Wang, X.T.; Wang, Y.P.; Li, L.L.; Xing, X.; Liu, X.X.; Ji, G.S.; Hou, T.; Zhang, Y.; Xiao, R.P.; Wang, S.Q. β2-Adrenergic Stimulation Compartmentalizes β1- Signaling Into Nanoscale Local Domains by Targeting the C-Terminus of β1-Adrenoceptors. Circ. Res. 2019, 124, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Demydenko, K.; Sipido, K.R.; Roderick, H.L. Ca2+ release via InsP3Rs enhances RyR recruitment during Ca2+ transients by increasing dyadic [Ca2+] in cardiomyocytes. J. Cell Sci. 2021, 134, jcs258671. [Google Scholar] [CrossRef]
- Jin, X.; Amoni, M.; Gilbert, G.; Dries, E.; Doñate Puertas, R.; Tomar, A.; Nagaraju, C.K.; Pradhan, A.; Yule, D.I.; Martens, T.; Menten, R.; Vanden Berghe, P.; Rega, F.; Sipido, K.; Roderick, H.L. InsP3R-RyR Ca2+ channel crosstalk facilitates arrhythmias in the failing human ventricle. Basic. Res. Cardiol. 2022, 117. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, X.H.; Lehnart, S.E.; Huang, F.; Vest, J.A.; Reiken, S.R.; Mohler, P.J.; Sun, J.; Guatimosim, S.; Song, L.S.; Rosemblit, N.; D'Armiento, J.M.; Napolitano, C.; Memmi, M.; Priori, S.G.; Lederer, W.J.; Marks, A.R. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003, 113, 829-840. [CrossRef] [PubMed]
- Taur, Y.; Frishman, W.H. The cardiac ryanodine receptor (RyR2) and its role in heart disease. Cardiol. Rev. 2005, 13, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Dridi, H.; Kushnir, A.; Zalk, R.; Yuan, Q.; Melville, Z.; Marks, A.R. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target. Nat. Rev. Cardiol. 2020, 17, 732–747. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Wang, W.; Tang, C.; Ran, J.; Huang, W.; Li, S.; Liu, J. Chai-Hu-San-Shen Capsule Ameliorates Ventricular Arrhythmia Through Inhibition of the CaMKII/FKBP12.6/RyR2/Ca2+ Signaling Pathway in Rats with Myocardial Ischemia. Evid. Based Complement. Alternat. Med. 2022, 3, 2670473. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, C.; Mazzanti, A.; Priori, S.G. Genetic risk stratification in cardiac arrhythmias. Curr. Opin. Cardiol. 2018, 33, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Wleklinski, M.J.; Kannankeril, P.J.; Knollmann, B.C. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J. Physiol. 2020, 598, 2817–2834. [Google Scholar] [CrossRef] [PubMed]
- Tiso, N.; Stephan, D.A.; Nava, A.; Bagattin, A.; Devaney, J.M.; Stanchi, F.; Larderet, G.; Brahmbhatt, B.; Brown, K.; Bauce, B.; Muriago, M.; Basso, C.; Thiene, G.; Danieli, G.A; Rampazzo, A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum. Mol. Genet. 2001, 10, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Falcón, D.; Galeano-Otero, I.; Martín-Bórnez, M.; Fernández-Velasco, M.; Gallardo- Castillo, I.; Rosado, J.A.; Ordóñez, A.; Smani, T. TRPC Channels: Dysregulation and Ca2+/Mishandling in Ischemic Heart Disease. Cells. 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Molkentin, J.D. TRPC channels as effectors of cardiac hypertrophy. Circ. Res. 2011, 108, 265–272. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhang, J.; Luo, D.; Yu, Y.; Chen, T.; Yang, Y.; Yu, F.; Li, M. Upregulation of Transient Receptor Potential Canonical Type 3 Channel via AT1R/TGF-iβ/i1/Smad2/3 Induces Atrial Fibrosis in Aging and Spontaneously Hypertensive Rats. Oxid. Med. Cell Longev. 2019, 23, 4025496, PMCID: PMC6906806. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.; Beresneva, O.; Galkina, O.; Zubina, I.; Ivanova, G.; Parastaeva, M.; Semenova, N.; Dobronravov, V. Myocardial Hypertrophy and Fibrosis Are Associated with Cardiomyocyte Beta-Catenin and TRPC6/Calcineurin/NFAT Signaling in Spontaneously Hypertensive Rats with 5/6 Nephrectomy. Int. J. Mol. Sci. 2021, 22, 4645. [Google Scholar] [CrossRef]
- Yan, J.; Honglei, Y.; Yun, W.; Sheng, D.; Yun, H.; Anhua, Z.; Na, F.; Min, L.; Dandan, S.; Jing, W.; Junming, T.; Wenjun, Z.; Xiju, H. Puerarin ameliorates myocardial remodeling of spontaneously hypertensive rats through inhibiting TRPC6-CaN-NFATc3 pathway. Eur. J. Pharmacol. 2022, 933, 175254. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kurahara, L.H.; Hiraishi, K. TRP channels in cardiac and intestinal fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalamo, K.; Montell, C. TRP channels. Annu Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Numaga-Tomita, T.; Nishida, M. TRPC Channels in Cardiac Plasticity. Cells. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Wilkin, B.J.; Bodi, I.; Molkentin, J.D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006, 20, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Tanaka, H.; Ueda, Y.; Oyama, J.; Sugano, M.; Sumimoto, H.; Mori, Y.; Makino, N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+channel mediating angiotensin II-induced myocardial apoptosis. Mol. Cell Biochem. 2007, 294, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Plummer, N.W.; George, M.D.; Abramowitz, J.; Zhu, M.X.; Birnbaumer, L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC: Orai complex may mediate store and receptor operated Ca2+ entry. Proc. Natl. Acad. Sci U S A. 2009, 106, 3202–3206. [Google Scholar] [CrossRef]
- Ambudkar, I.S.; de Souza, L.B.; Ong, H.L. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium. 2017, 63, 33–39. [Google Scholar] [CrossRef]
- Dyrda, A.; Koenig, S.; Frieden, M. STIM1 long and STIM1 gate differently TRPC1 during store-operated calcium entry. Cell Calcium. 2020, 86, 102134. [Google Scholar] [CrossRef] [PubMed]
- Kühn, F.J.; Heiner, I.; Lückhoff, A. TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflugers Arch. 2005, 451, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ru, X.; Yao, X. TRPM2: a multifunctional ion channel for oxidative stress sensing. Sheng Li Xue Bao. 2014, 66, 7–15. [Google Scholar] [PubMed]
- Cheung, J.Y.; Miller, B.A. Transient Receptor Potential-Melastatin Channel Family Member 2: Friend or Foe. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 308–329. [Google Scholar]
- Kruse, M.; Schulze-Bahr, E.; Corfield, V.; Beckmann, A.; Stallmeyer, B.; Kurtbay, G.; Ohmert, I.; Schulze-Bahr, E.; Brink, P.; Pongs, O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J. Clin. Invest. 2009, 119, 2737–44. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zong, P.; Yan, J.; Yue, Z.; Li, X.; Smith, C.; Ai, X.; Yue, L. Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients. Pflugers Arch. 2021, 473, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Du, R.; Fan, L.L.; Jin, J.Y.; Huang, H.; Chen, Y.Q.; Bi, D.D.; Xiang, R. Whole-Exome Sequencing Identifies a Novel TRPM4 Mutation in a Chinese Family with Atrioventricular Block. Biomed. Res. Int. 2021, 17, 9247541. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y,; Li, Q. ; Kurahara, L.H.; Shioi, N.; Hiraishi, K.; Fujita, T.; Zhu, X.; Inoue, R. An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction. Cells. 2021, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Mesirca, P.; Mason, X.; Gibson, W.; Bates-Withers, C.; Van den Boogert, M.; Chaudhuri, D.; Pu, W.T.; Mangoni, M.E.; Clapham, D.E. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation. 2013, 128, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.H.; Aziz, Q.; Harmer, S.C.; Thayyil, S.; Tinker, A.; Munroe, P.B. Genetic variants in TRPM7 associated with unexplained stillbirth modify ion channel function. Hum. Mol. Genet. 2020, 29, 1797–1807. [Google Scholar] [CrossRef]
- Conklin, D.J.; Guo, Y.; Nystoriak, M.A.; Jagatheesan, G.; Obal, D.; Kilfoil, P.J.; Hoetker, J.D.; Guo, L.; Bolli, R.; Bhatnagar, A. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H889–H899. [Google Scholar] [CrossRef]
- Martín-Bórnez, M.; Galeano-Otero, I.; Del Toro, R.; Smani, T. ; TRPC and TRPV Channels' Role in Vascular Remodeling and Disease. Int. J. Mol. Sci. 2020, 21, 6125. [Google Scholar] [CrossRef]
- Üstünel, L.; Özgüler, I.M. The effects of iloprost and beta3 receptor agonist on TRPA1 and TRPC1 immunreactivity in an experimental lower extremty ischemia-reperfusion injury model. Turk. J. Med. Sci. 2021, 51, 2763–2770. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; Osawa, T. Protein-bound acrolein: potential markers for oxidative stress. Proc. Natl. Acad. Sci U S A. 1998, 95, 4882–4887. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. , Ahmad, A.A.; Seidel, T.; Hunter, C.; Streiff, M.; Nikolova, L.; Spitzer, K.W.; Sachse, F.B. Location and function of transient receptor potential canonical channel 1 in ventricular myocytes. J. Mol. Cell Cardiol. 2020, 139, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.L.; Remillard, C.V.; Yuan, J.X. TRP channels in hypertension. Biochim. Biophys. Acta. 2007, 1772, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. ; Role of TRP Channels in the Cardiovascular System. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef] [PubMed]
- Andriulė, I.; Pangonytė, D.; Gwanyanya, A.; Karčiauskas, D.; Mubagwa, K.; Mačianskienė, R. Detection of TRPM6 and TRPM7 Proteins in Normal and Diseased Cardiac Atrial Tissue and Isolated Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 14860. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, M.G. Endothelial Ca2+ signaling-dependent vasodilation through transient receptor potential channels. Korean J. Physiol. Pharmacol. 2020, 24, 287–298. [Google Scholar] [CrossRef]
- Kochukov, M.Y.; Balasubramanian, A.; Noel, R.C.; Marrelli, S.P. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. J. Vasc. Res. 2013, 50, 11–20. [Google Scholar] [CrossRef]
- Willette, R.N.; Bao, W.; Nerurkar, S.; Yue, T.L.; Doe, C.P.; Stankus, G.; Turner, G.H. , Ju, H.; Thomas, H.; Fishman, C.E.; Sulpizio, A.; Behm, D.J.; Hoffman, S.; Lin, Z.; Lozinskaya, I.; Casillas, L.N.; Lin, M.; Trout, R.E.; Votta, B.J.; Thorneloe, K.; Lashinger, E.S.; Figueroa, D.J.; Marquis, R.; Xu, X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Ther. 2008, 326, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Miguel, I.; Cidad, P.; Pérez-García, M.T.; López-López, J.R. Differences in TRPC3 and TRPC6 channels assembly in mesenteric vascular smooth muscle cells in essential hypertension. J. Physiol. 2017, 595, 1497–1513. [Google Scholar] [CrossRef]
- Mathar, I.; Vennekens, R.; Meissner, M.; Kees, F.; Van der Mieren, G.; Camacho Londoño, J.E.; Uhl, S.; Voets, T.; Hummel, B.; van den Bergh, A.; Herijgers, P.; Nilius, B.; Flockerzi, V.; Schweda, F.; Freichel, M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J. Clin. Invest. 2010, 120, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ching, L.C.; Zhao, J.F.; Shyue, S.K.; Lee, H.F.; Kou, Y.R.; Lee, T.S. Essential Role of Transient Receptor Potential Vanilloid Type 1 in Evodiamine-Mediated Protection Against Atherosclerosis. Acta Physiol. 2013, 207, 299–307. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Mangmool, S.; Nishiyama, K.; Nishimura, A. Canonical Transient Receptor Potential Channels and Vascular Smooth Muscle Cell Plasticity. J. Lipid Atheroscler. 2020, 9, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Rahaman, S.G.; Goswami, R.; Dutta, B.; Mahanty, M.; Rahaman, S.O. Role of mechanosensitive channels/receptors in atherosclerosis. Am. J. Physiol. Cell Physiol. 2022, 322, C927–C938. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, Z.; Tepel, M. The role of transient receptor potential channels in metabolic syndrome. Hypertens Res. 2008, 31, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Tanaka, T.; Nishimura, A.; Nishida, M. TRPC3-Based Protein Signaling Complex as a Therapeutic Target of Myocardial Atrophy. Curr. Mol. Pharmacol. 2021, 14, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, K.; Kurahara, L.H.; Ishikawa, K.; Go, T.; Yokota, N.; Hu, Y.; Fujita, T.; Inoue, R.; Hirano, K. Potential of the TRPM7 channel as a novel therapeutic target for pulmonary arterial hypertension. J. Smooth Muscle Res. 2022, 58, 50–62. [Google Scholar] [CrossRef]
- Lüthi, A.; McCormick, D.A. H-current: properties of a neuronal and network pacemaker. Neuron. 1998, 21, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, A.S.; Peigneur, S.; Tytgat, J. Review: HCN Channels in the Heart. Curr. Cardiol. Rev. 2022, 18, e040222200836. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, A.; Bauer, D.; Giese, M.H.; Swuec, P.; Porro, A.; Gasparri, F.; Sharifzadeh, A.S.; Chaves-Sanjuan, A.; Alberio, L.; Parisi, G.; Cerutti, G.; Clarke, O.B.; Hamacher, K.; Colecraft, H.M.; Mancia, F.; Hendrickson. W.A.; Siegelbaum, S.A.; DiFrancesco, D.; Bolognesi, M.; Thiel, G.; Santoro, B.; Moroni, A. Gating movements and ion permeation in HCN4 pacemaker channels. Mol. Cell. 2021, 81, 2929–2943. [Google Scholar] [CrossRef] [PubMed]
- Santoro, B.; Tibbs, G.R. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann. N. Y. Acad. Sci. 1999, 868, 741–764. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.; Schneider, A.; Wahl, C. Cardiac HCN channels: Structure, function, and modulation. Trends Cardiovasc. Med. 2002, 12, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.S.; Robinson, R.B. Pacemaker current and automatic rhythms: toward a molecular understanding. Handb. Exp. Pharmacol. 2006, 171, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Farraha, M; Kumar, S. ; Chong, J.; Cho, H.C.; Kizana, E. Gene Therapy Approaches to Biological Pacemakers. J. Cardiovasc. Dev. Dis. 2018, 5, 50, PMCID: PMC6306875. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, D.R.; Lindsay, B.D. Phosphodiesterase-4 activity: a critical modulator of atrial contractility and arrhythmogenesis. J. Am. Coll. Cardiol. 2012, 59, 2191–2192. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.W.; Zhou, P.; Yao, L.; Liu, T.; Zhang, B.; Li, Y.; Zheng, H.; Zheng, L.H.; Zhang, C.X.; Bruce, I.; Ge, J.B.; Wang, S.Q.; Hu, Z.A.; Yu, H.G.; Zhou, Z. Calcium influx through If channels in rat ventricular myocytes. Am. J. Physiol Cell Physiol. 2007, 292, C1147–C1155. [Google Scholar] [CrossRef]
- Michels, G.; Brandt, M.C.; Zagidullin, N.; Khan, I.F.; Larbig, R.; van Aaken, S.; Wippermann, J.; Hoppe, U.C. Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc. Res. 2008, 78, 466–475. [Google Scholar] [CrossRef]
- Ide, T.; Ohtani, K.; Higo, T.; Tanaka, M.; Kawasaki, Y.; Tsutsui, H. Ivabradine for the treatment of cardiovascular diseases. Circ. J. 2019, 83, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. 2020, 472, 931–951. [Google Scholar] [CrossRef] [PubMed]
- Proenza, C.; Tran, N.; Angoli, D.; Zahynacz, K.; Balcar, P.; Accili, E.A. Different roles for the cyclic nucleotide binding domain and amino terminus in assembly and expression of hyperpolarization-activated, cyclic nucleotide-gated channels. J. Biol. Chem. 2002, 277, 29634–29642. [Google Scholar] [CrossRef]
- Pan, Y.; Laird, J.G.; Yamaguchi, D.M.; Baker, S.A. An N-terminal ER export signal facilitates the plasma membrane targeting of HCN1 channels in photoreceptors. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Blanco, I.; Hayoz, S.; Brelidze, T.I. The HCN domain is required for HCN channel cell-surface expression and couples voltage- and cAMP-dependent gating mechanisms. J. Biol. Chem. 2020, 295, 8164–8173. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Bucchi, A.; Difrancesco, D. Physiology and pharmacology of the cardiac pacemaker ("funny") current. Pharmacol. Ther. 2005, 107, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Hennis, K.; Rötzer, R.D.; Piantoni, C.; Biel, M.; Wahl-Schott, C.; Fenske, S. Speeding Up the Heart? Traditional and New Perspectives on HCN4 Function. Front Physiol. 2021, 12, 669029. [Google Scholar] [CrossRef] [PubMed]
- Wahl-Schott, C.; Biel, M. HCN channels: structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2009, 66, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Carbonara, S.; Ricci, G.; Mandurino, C.; Locorotondo, M.; Bulzis, G.; Gesualdo. M.; Zito, A.; Carbonara, R.; Dentamaro, I.; Riccioni, G.; Ciccone, M.M. HCN channels and heart rate. Molecules. 2012, 17, 4225–4235. [Google Scholar] [CrossRef]
- Hummert, S.; Thon, S.; Eick, T.; Schmauder, R.; Schulz, E.; Benndorf, K. Activation gating in HCN2 channels. PLoS Comput. Biol. 2018, 14, e1006045. [Google Scholar] [CrossRef]
- VanSchouwen, B.; Melacini, G. Regulation of HCN Ion Channels by Non-canonical Cyclic Nucleotides. Handb. Exp. Pharmacol. 2017, 238, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.H.; Singh, R.K.; Bankston, J.R.; Proenza, C. Regulation of HCN Channels by Protein Interactions. Front. Physiol. 2022, 13, 928507. [Google Scholar] [CrossRef]
- Erlenhardt, N.; Kletke, O.; Wohlfarth, F.; Komadowski, M.A.; Clasen, L.; Makimoto, H; Rinné, S. ; Kelm, M.; Jungen, C.; Decher, N.; Meyer, C.; Klöcker, N. Disease-associated HCN4 V759I variant is not sufficient to impair cardiac pacemaking. Pflugers Arch. 2020, 472, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Nakamura, K.; Hisatomi, Y.; Matsumoto, S.; Suzuki, M.; Harvey, R.P.; Kurihara, H.; Hattori, S.; Yamamoto, T.; Michalak, M.; Endo, F. Arrhythmia induced by spatiotemporal overexpression of calreticulin in the heart. Mol. Genet. Metab. 2007, 91, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Y.; Temple, I.P.; Borbas, Z.; Atkinson, A.; Yanni, J.; Maczewski, M.; Mackiewicz, U.; Aly, M.; Logantha, S.J.R.J.; Garratt, C.J.; Dobrzynski, H. Structural and functional remodeling of the atrioventricular node with aging in rats: The role of hyperpolarization-activated cyclic nucleotide-gated and ryanodine 2 channels. Heart Rhythm. 2018, 15, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Farraha, M.; Kumar, S.; Chong, J.; Cho, H.C.; Kizana, E. Gene Therapy Approaches to Biological Pacemakers. J. Cardiovasc. Dev. Dis. 2018, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Plotnikov, A.N.; Danilo, P.; Shlapakova, I.; Cohen, I.S.; Robinson, R.B.; Rosen, M.R. Expression and function of a biological pacemaker in canine heart. Circulation. 2003, 107, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jin, K.; Zhou, J.; Gu. J.; Gu, X.; Dong, L.; Sun, X. G9a inhibition promotes the formation of pacemaker-like cells by reducing the enrichment of H3K9me2 in the HCN4 promoter region. Mol. Med. Rep. 2023, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.; Lin, L.Y.; Tsai, T.F. Use of calcium channel blockers in dermatology: a narrative review. Expert. Rev. Clin. Pharmacol. 2021, 14, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kokilambigai, K.S.; Kavitha, J.; Seetharaman, R.; Lakshmi, K.S.; Sai Susmitha, A. Analytical and Bioanalytical Techniques for the Quantification of the Calcium Channel Blocker - Amlodipine: A Critical Review. Crit. Rev. Anal. Chem. 2021, 51, 754–786. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, D.A.; Ozer, L.Y.; Al Haj Zen, A. A Novel High Content Angiogenesis Assay Reveals That Lacidipine, L-Type Calcium Channel Blocker, Induces In Vitro Vascular Lumen Expansion. Int. J. Mol. Sci. 2022, 23, 4891. [Google Scholar] [CrossRef]
- Rabah, F.; El-Naggari, M.; Al-Nabhani, D. Amlodipine: The double edged sword. J. Paediatr. Child Health. 2017, 53, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Kung, J.Y.; Mitchelmore, B.; Cave, A.; Banh, H.L. Comparative peripheral edema for dihydropyridines calcium channel blockers treatment: A systematic review and network meta-analysis. J. Clin. Hypertens. (Greenwich). 2022, 24, 536–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, R.; Wang, K.; Zhang, W.; Zhang, M.; Jin, L.; Xie, P.; Zheng, W.; Shang, H.; Hu, Q.; Li, J.; Chen, G.; Wu, F.; Lan, F.; Wang, L.; Wang, S.Q.; Li, Y.; Zhang, Y.; Liu, J.; Lv, F.; Hu, X.; Xiao, R.P.; Lei, X.; Zhang, Y. Novel CaMKII-δ Inhibitor Hesperadin Exerts Dual Functions to Ameliorate Cardiac Ischemia/Reperfusion Injury and Inhibit Tumor Growth. Circulation. 2022, 145, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, F.; Zhang, M.; Jin, L.; Xie, P.; Liu, D.; Zhang, J.; Hu, X.; Lv, F.; Shang, H.; Zheng W, Sun X, Duanmu J, Wu F, Lan F, Xiao RP, Zhang Y. Targeting CaMKII-δ9 Ameliorates Cardiac Ischemia/Reperfusion Injury by Inhibiting Myocardial Inflammation. Circ. Res. 2022, 130, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zahra, A.; Jia, M.; Wang, Q.; Wang, Y.; Campbell, S.L.; Wu, J. Na+/H+ Exchanger 1, a Potential Therapeutic Drug Target for Cardiac Hypertrophy and Heart Failure. Pharmaceuticals (Basel). 2022, 15, 875. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat. Rev. Cardiol. 2023, 20, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, H.G.; Tafesse, T.B.; Bule, M.H. If Channel as an Emerging Therapeutic Target for Cardiovascular Diseases: A Review of Current Evidence and Controversies. Front. Pharmacol. 2017, 8, 874. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Y.; Huang, W.; Li, X.; Li, S. Role of Transient Receptor Potential Channels in Heart Transplantation: A Potential Novel Therapeutic Target for Cardiac Allograft Vasculopathy. Med. Sci Monit. 2017, 23, 2340–2347. [Google Scholar] [CrossRef]
- Cheraghi, M.; Negahdari, B.; Daraee, H.; Eatemadi, A. Heart targeted nanoliposomal/nanoparticles drug delivery: An updated review. Biomed. Pharmacother. 2017, 86, 316–323. [Google Scholar] [CrossRef] [PubMed]
| Са2+ channel | Gene | Channelopathy, syndromes | OMIM |
|---|---|---|---|
| Cav | |||
| Cav1.1 | CACNA1S | Hypokalemic Periodic Paralysis Type 1 Normokalemic Periodic Paralysis Malignant Hypothermia Susceptibility 5 |
170400 170600 601887 |
| Cav1.2 | CACNA1C | Timothy Syndrome Long QT Syndrome 8 (LQT8) Brugada Syndrome 3 |
601005 618447 611875 |
| Cav1.3 | CACNA1D | Sinoatrial Node Dysfunction and Deafness Syndrome Primary aldosteronism, seizures, and neurologic abnormalities Autism spectrum disorder (with or without more severe manifestations including intellectual disability, neurological abnormalities, primary aldosteronism and/or congenital hyperinsulinism |
614896 615474 Not listed in OMIM |
| Cav1.4 | CACNA1F | Aldosterone producing adenomas Congenital Stationary Night Blindness Type 2 X-linked Cone-Rod Dystrophy 3 Aland Island Eye Disease |
Not listed in OMIM 300071 300476 300600 |
| Cav2.1 | CACNA1A | Familial and Sporadic Hemiplegic Migraine Type 1 with or without progressive cerebellar ataxia Episodic Ataxia Type 2 Spinocerebellar Ataxia Type 6 Early Infantile Epileptic Encephalopathy 42 Congenital Ataxia |
141500 108500 183086 617106 Not listed in OMIM |
| CACNA1B | Neurodevelopmental disorder with seizures and non-epileptic hyperkinetic movements | 618497 | |
| Cav2.3 | CACNA1E | Early Infantile Epileptic Encephalopathy 69 | 618285 |
| Cav3.1 | CACNA1G | Spinocerebellar Ataxia Type 42 Spinocerebellar Ataxia Type 42 early-onset, with neurodevelopmental deficits (Childhood-Onset Cerebellar Atrophy) |
616795 618087 |
| Cav3.2 | CACNA1H | Familial Hyperaldosteronism type IV Aldosterone producing adenomas |
617027 Not listed in OMIM |
| Cav3.3 | CACNA1I | Neurodevelopmental disorder with epilepsy and intellectual disability | Not listed in OMIM |
| RyR | |||
| RyR2 | RYR2 | Arrhythmogenic right ventricular dysplasia/cardiomyopathy type 2 Stress-induced polymorphic ventricular tachycardia (Catecholaminergic polymorphic ventricular tachycardia 1) |
600996 604772 |
| TRP | |||
| TRPC3 | TRPC3 | Spinocerebellar ataxia | 602345 |
| TRPC6 | TRPC6 | Glomerulosclerosis, focal segmental, 2 | 603965 |
| TRPV3 | TRPV3 | Olmsted Syndrome | 614594 |
| TRPV4 | TRPV4 | Brachyolmia type 3 | 113500 |
| Digital arthropathy-brachydactyly, familial | 606835 | ||
| Hereditary motor and sensory neuropathy, type IIc | 606071 | ||
| Metatropic dysplasia | 156530 | ||
| Parastremmatic dwarfism | 168400 | ||
| Scapuloperoneal spinal muscular atrophy | 181405 | ||
| SED, Maroteaux type | 184095 | ||
| Spinal muscular atrophy, distal, congenital nonprogressive | 600175 | ||
| Spondylometaphyseal dysplasia, Kozlowski type | 184252 | ||
| TRPM1 | TRPM1 | Night blindness, congenital stationary (complete), 1C, autosomal recessive | 613216 |
| TRPM4 | TRPM4 | Progressive familial heart block, type IB | 604559 |
| TRPM6 | TRPM6 | Hypomagnesemia 1, intestinal | 602014 |
| TRPA1 | TRPA1 | Episodic pain syndrome, familial | 615040 |
| TRPML1 | TRPML1 | Mucolipidosis IV | 252650 |
| PKD2 (TRPP1) | PKD2 | Autosomal dominant polycystic kidney disease | 613095 |
| HCN | |||
| HCN1 | HCN1 | Dravet syndrome | Not listed in OMIM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




