1. Introduction
Bone morphogenic proteins, which are known as abbreviations (BMP), are a group of about 20 proteins that belong to the group of transforming growth factors (BMPs). They help mesenchyme to differentiate into osteoblasts and regulate many cellular activities such as migration, differentiation, adhesion, and proliferation in addition to programmed cell death.
The use of these proteins in oral and maxillofacial surgeries has increased dramatically over the past decade, but their roles in many intracellular pathways that intersect with oncogenic pathways have propelled researchers to study these roles in carcinogenic processes and neoplastic formations in many organs, as it has been proven that these proteins are involved in many tumors, especially those that include the invasion and colonization of bone tissue.
Since Urist discovered bone morphogenetic proteins (BMPs) as bone-inducing proteins (1), many researchers have demonstrated that BMPs induce stem and mesenchymal cells to differentiate into osteogenic cells that can produce bone, after binding to receptors found on various cell types’ plasma membrane (autocrine and paracrine effects), establishing cell and tissue organization (2). They alter the developmental process by diffusing via a gradient of concentration.
It is widely demonstrated that BMPs play a general role in the process of bone formation during the development and repair of fractures. In various animal models, BMPs could induce the formation of bone tissue in ectopic locations and in critical-sized bone lesions. Nowadays, the cellular and molecular mechanisms behind the ectopic ossification provoked by BMPs still not well identified.(3) BMPs are members of the superfamily transforming growth factor-β, and there have been at least 15 ones of BMPs identified to date. BMP-2, BMP-3 (osteogenic), BMP-4, and BMP-7 (osteogenic protein-1) are the most studied ones. Any recombination type of morphogenic proteins have been synthesized, such as - recombinant human BMPs (rh-BMP). The sequences of BMPs and transforming growth factor-β are similar. It is showed that BMPs are related to regulatory genes.(4, 5) And according to several articles, BMPs stimulate bone and cartilage formation (BMP-2) and initiate the endochondral bone formation (BMP-3, BMP-7). Also, BMP-3 plays a role in the development of the central nervous system. BMP-2 and BMP-4 in RNA transcripts are found to have a role in developing tooth buds, odontoblast layer, and other craniofacial structures. BMPs are involved in extracellular matrix during repair and regeneration also, they stimulate angiogenesis by extracellular matrix interactions (7-11).
Gerdes et al 1983 originally identified the KI-67 antigen using a monoclonal antibody. Generated by immunizing mice with the nuclei of Hodgkin’s lymphoma cell line. Detailed analyses showed that the Ki67 antigen was present in the nuclei of cells during all phases of the cell cycle, whereas it was not expressed in quiescent or resting cells in the G0 phase (6). This protein is considered as an excellent marker for determining the growth fraction of a given cell population due to its absence in quiescent cells and universal expression in all proliferating cells (normal and tumor cells) (7).
Regarding complications, some studies reported some after using BMP2, mainly in spine surgeries and bone repairing, being related to the concentration and dosage of BMP2 used. (12,13) However, reported adverse events using BMP2 in oral/dental surgery and GBR treatment were scarce in the literature probably because the long-term complications were not included as variable in conducted clinical studies and the quantity used was significantly lower than the one used in other general surgeries and therapeutic procedures (14)
In contrast to protein delivery, BMP-2 gene transfer into the defect site induces BMP-2 synthesis in vivo and leads to secretion for weeks to months, depending on the vector, at a concentration of nanograms per milliliter. BMP-2 gene delivery is advantageous for bone wound healing process in terms of dosage and duration. However, safety concerns related to viral vectors are one of the hurdles that need to be overcome for gene delivery to be used in clinical practice (15).
At the gingival level, several variables can be studied to evaluate the impact of recombinant human bone morphogenetic protein (rh-BMP2), for example: the vascular endothelial growth factors (VEGF) in the epithelium and connective tissue graft. as well, the index of the percentage of proliferation (Ki67Marker) (16- 20). Antibodies against Ki67 have been used as prognostic factors in the diagnosis of several types of neoplasms. However, despite its value as a clinical marker, its cellular function is not well identified (20,21).
Based on the strong correlation between the intensity of proliferation and the rate of gingival healing, this study aims to evaluate the effects of recombinant human bone morphogenetic protein (rh-BMP2) on gingival tissue proliferation in patients undergoing dental implant, also by evaluating histological (Ki67) related variables as proliferation of the gingival tissue. The null hypothesis supposed that we don’t have significant differences in gingival tissue healing on the site where dental implants are placed without using rh-BMP2. Ki67 Marker (index of the percentage of proliferation). (From Sigma Aldrich Chemical Pvt. ltd. Gillingham, Dorset, SP8,4XT, U.K Number: BSB-3767-01).
The literature review shows that most of the research is done about the effect of rh-BMP2 on the bone healing and it showed that rh-BMP2 is injected through the gingiva to the bone graft.
The aim of the study was to investigate the effect of rh-BMP2 injected in the gingiva and his effect on the gingival tissue knowing that a better and faster gingival cell proliferation guide us to a better gingival healing, reduce infection and provides a good environment for favorable wound healing. A faster gingival healing provides a good barrier for bone graft or implant done in the same region other than a better vascularization.
A strong correlation between the intensity of proliferation and the rate of gingival healing was revealed. Higher rates of proliferation are associated with faster rates of healing, while lower rates of proliferation are associated with slower rates of healing.
2. Materials and Methods
This scientific protocol has been approved by the ethical committee of the Lebanese University under the number CUER 27-2020 and the randomized control trial registration number: LU-DP-5.2.20. All subjects were initially interviewed and examined for eligibility after a written informed consent.
2.1. Study Design
This study was a clinical and histological investigation.
To measure proliferation, expressions of the marker Ki-67 were counted in cells on each side of the mouth, where a high Ki-67 index has a larger number of proliferating cells and are therefore likely to grow more quickly.
2.2. Study population
Twenty patients (n=20), of several systemically healthy subjects of mean age group ≥ 25 years irrespective of gender, were selected from those visiting the Faculty of Dental Medicine (FDM) at the Lebanese University seeking for dental treatment with the chief complaint to replace lower missing teeth (bilateral inferior posterior edentulous).
Patients followed the clinical screening policy at the FDM starting with a screening at the Oral Diagnostic Department that includes a general medical observation, dental screening, then X-Rays performed at the oral radiology department. Then based on the clinical and radiological data, the patients received the proposed diagnosis and treatment plan that was either placement of dental implants replacing missing posterior teeth or a conventional removable denture. Patients decided about the treatment plan according to their best convenience after having received a detailed explanation about the different advantages and disadvantages of each option.
Patients that decided to undergo the dental implant treatment option were referred to the Periodontology Department at FDM and re-examined to decide about the appropriate technique to assure success of the therapy. These patients had implants placed on both sides of the mouth: one side with rh-BMP2 and the other side without rh-BMP2. All withdrawals of subjects who gave informed consent to participate in this study were reported regardless of whether implant placement has taken place. All subjects scheduled for the study participated in the investigation in a consecutive order provided they fulfill the criteria stated in this protocol.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria for the study requires participants to be either men or women above 18 years of age, available for all study appointments over four months, and able to give written informed consent. Additionally, they must need bone augmentation in the posterior areas in both sides of the mandible, have a non-smoking habit (less than 5 cigarettes per day), and have a split mouth edentulous inferior posterior ridge with a bone width Bucco-lingually of 6mm or less calibrated with CBCT. On the other hand, the exclusion criteria include patients at risk of infection due to previous infectious diseases such as HIV, hepatitis, or infectious mononucleosis, patients with clinical signs of systemic disease or non-controlled diabetes, patients with endocarditis risk or under anti-thrombotic treatment (heparin, anti-vitamin K), and patients with severe communication difficulties in understanding written and spoken Arabic/English/French. Patients requiring chronic or intermittent use of antibiotics, those with a known hypersensitivity to BMPs, those who have participated in another intervention study, and those where both amoxicillin and clindamycin are contraindicated, or dental local anesthetic is contraindicated are also excluded. Furthermore, pregnant, or lactating patients or those with the intent to become pregnant, as well as smokers, are excluded from the study.
2.4. Implant placement
With 15c blade an intra-sulcular incision is done from the first existing teeth mesial to the edentulous ridge continuing with mid-crestal incision distally to the second molar position.
- -
A full thickness muco-periosteal flap elevation was done.
- -
Drilling sequence
A 4.0 INNO implant placed with a conventional implant placement technique:
- -
Starting with the point drill
- -
2.0mm Pilot Drill
- -
2.8mm Drill
- -
Final drill 3.5mm
- -
Inno Implant (4.0) in place by the implant holder using the hand piece with a torque of 30N/cm.
After placing an implant of 4.0mm diameter in a ridge of 6mm or less, a bone dehiscence occurs. GBR procedure is needed to cover the implants in place.
GBR procedure:
- -
Bovine bone graft with (Cytoplast membrane)
- -
Right posterior side using BMP (Cowell Medi R&D, Ref : BB1025, Korea ) injected with a syringe
- -
Left posterior side without BMP (controlled Group)
Second implant stage: 4 months later:
- -
By a periodontal probe we localize the implant in place
- -
Using a traditional punch technique, we uncover the implant by extruding the gingiva.
- -
The gingiva will be fixed in 10% buffered formalin and routinely processed and embedded with paraffin then we send it to the laboratory.
Patients underwent conventional dental implant procedure to replace missing teeth on both lower sides with GBR: one side received implants + GBR + 0.1mg of the Cowell product with a concentration of 0.5µg/ml of rh-BMP2 and the other side received dental implants + GBR. Both techniques were conventionally used in bone regeneration.
Forty gingival samples were collected 4 months later at the healing abutment placement stages using a tissue puncher. Gingival thickness and the type of attached gingiva was measured after 14 days to the healing abutment insertion. Gingival samples were collected from the two different treated sites (20 with and 20 without rh-BMP2).
Samples were stained with hematoxylin-eosin and immune-histochemical. The expression of Ki67 and VEGF was measured in two ways: 1) the number of the cells that express the marker and 2) the intensity of staining.
2.5. Histological procedures
Tissues were fixed in 10% buffered formalin and routinely processed and embedded with paraffin; Two or three serial sections 4μm thick were prepared and placed on salinized slides. The sections were then deparaffinized and rehydrated through xylene and descending grades of alcohol. Antigen retrieval was carried out in a pressure cooker in 10 mM citrate buffer (pH 6.0) for 2 to 5min. The sections were then incubated after covering them with 3% hydrogen peroxide for 15min to block any endogenous peroxidase activity, and then 20 slides incubated with primary anti Ki67 Rabbit polyclonal antibody (Abcam Inc., Cambridge, MA) for 4h at room temperature using an optimal dilution of 6 μg/ ml. After further incubation with the secondary antibody (45min) and streptavidin peroxidase (30min), visualization was performed using freshly prepared diaminobenzidine (DAB) chromogen for 10min. The slides were finally counter stained with Harris hematoxylin. Then the sections were examined under microscope under magnification of 40X to investigate Ki67 expression value and Ki67 was calculated by counting the percentage of the stained cells in addition to the expression degree of the whole slide.
2.6. Statistical analysis
The comparison between groups was done using the One-way ANOVA test in the numerical data SPSS 17.0 software. Differences were considered significant at p=0.05.
3. Results:
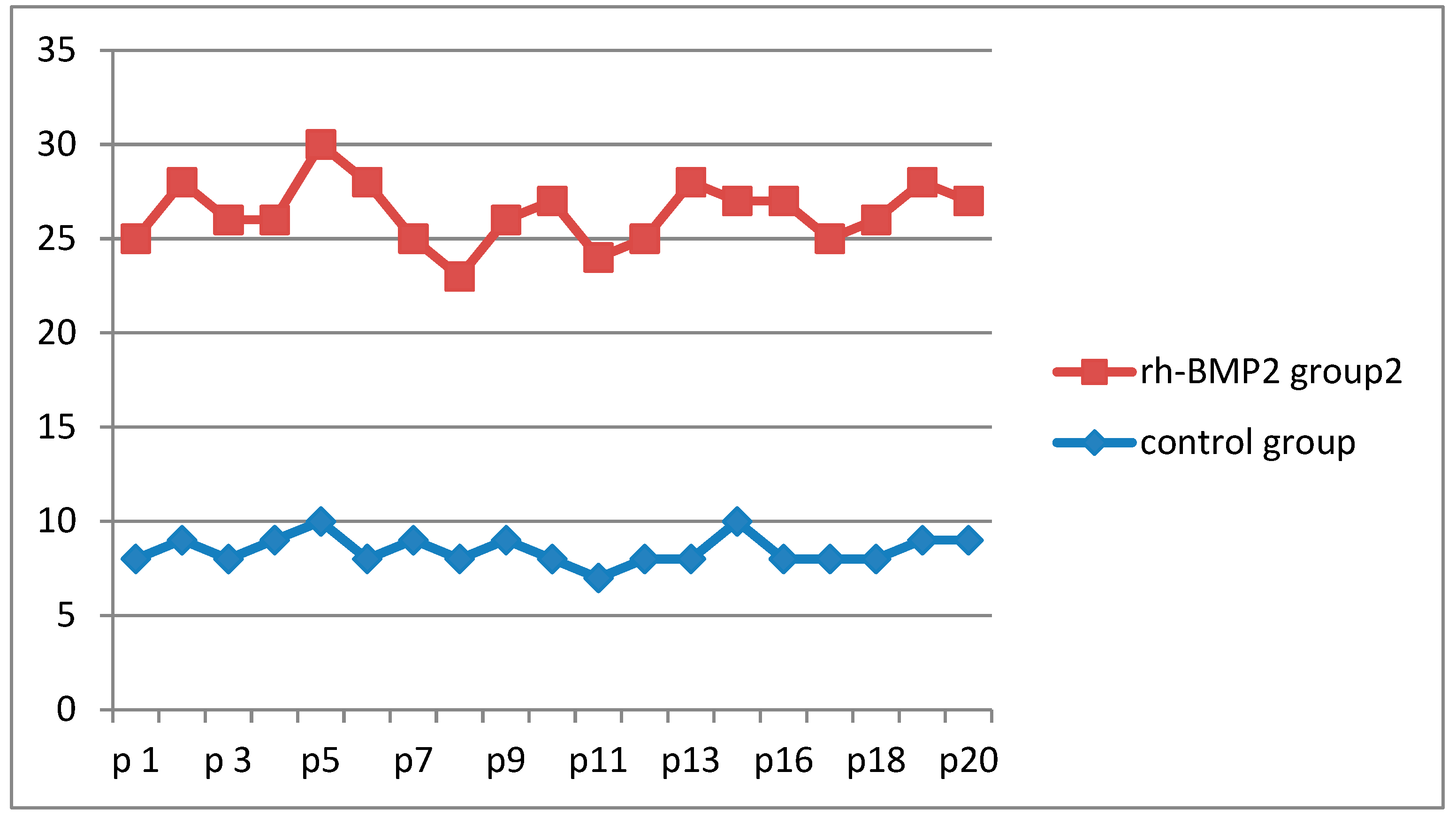
The results of the study showed that gingival tissue proliferation was significantly higher in the rh-BMP2 group than in the control group. The average number of Ki-67 expressing cells was higher in the rh-BMP2 group (23.7 cells per mm2) than the control group (13.5 cells per mm2).
The mean of ki-67 expression in the (RH-BMP2 group) was 27.11% of the cells and in the control, group was 8.19% of the cells. Statistically, we found a significant difference between the two groups (
Figure 1 and
Figure 2).
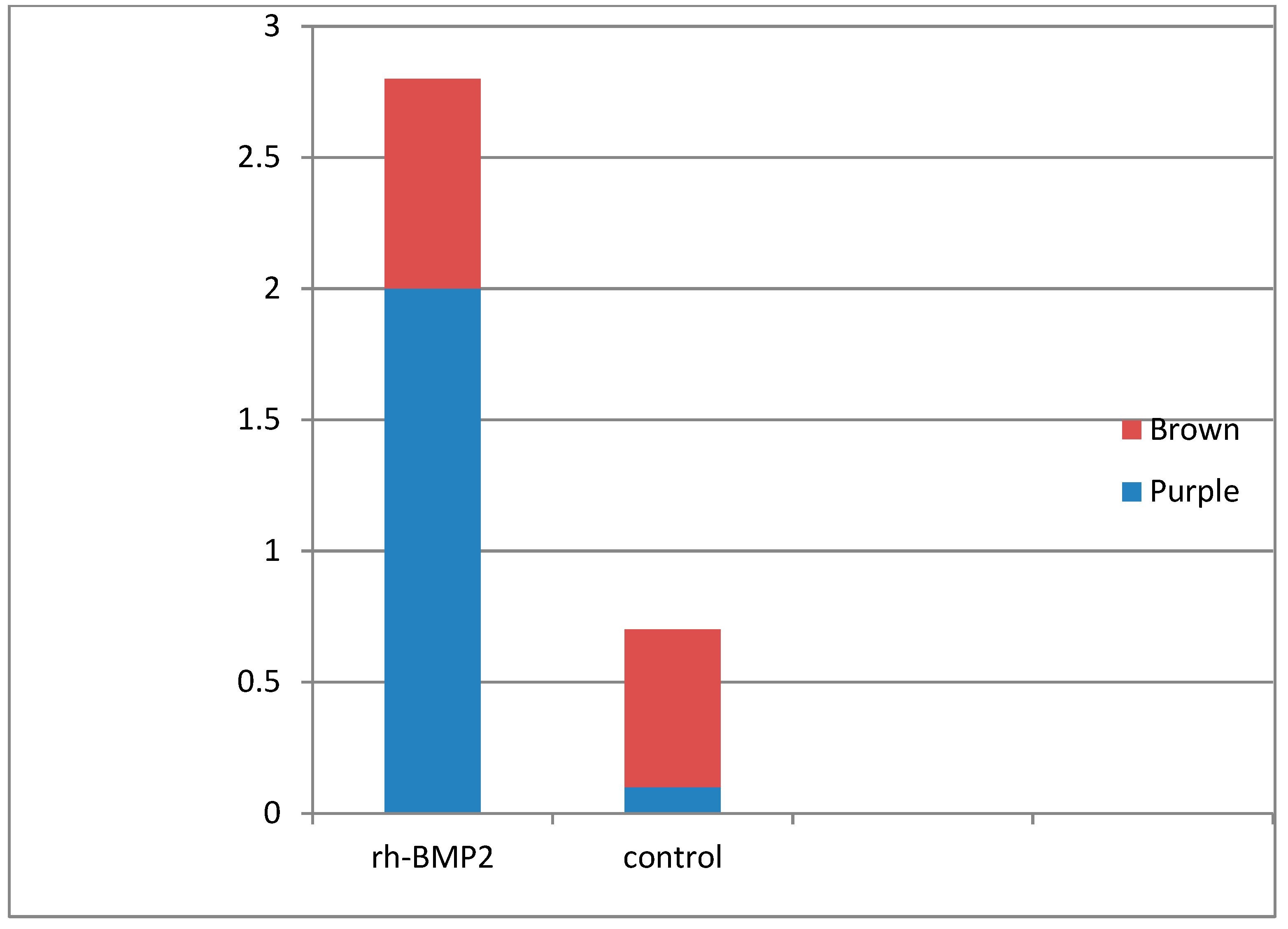
Ki-67 stains cells that are in the process of replicating or dividing. Cells-stained purple indicate a higher level of cell proliferation compared to cells stained brown, which indicates a lower level of cell proliferation. (
Figure 1 and
Figure 2).
According to the intensity of staining in the group treated with rh-bmp2 72% of the cell expression the ki-67 marker was colored in purple and 28% in brown. The control group we have 80% to 90% of the cells colored in brown. According to a visual scale, not only the percentage of the cell proliferated is higher but also the cell in the rh-BMP2 group indicate a higher level of cell proliferation than the one in the control group.
4. Discussion:
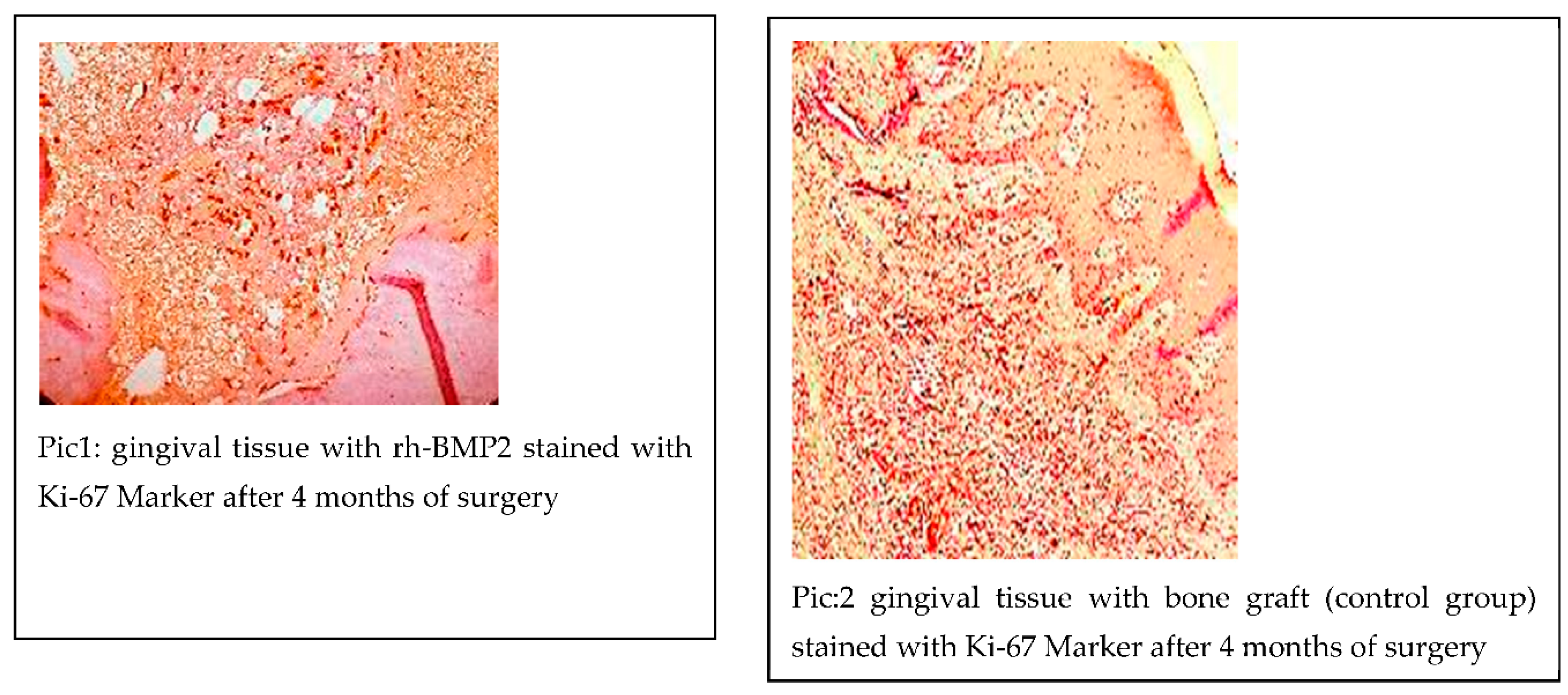
The staining of Ki-67 on gingival tissue is typically assessed by evaluating the percentage of cells that are stained in the tissue sample. The staining of Ki-67 is usually detected using immunohistochemistry (IHC), and the cells appear brown or purple in color with 20 Buccal pouches injected with rhBMP2 during the implant surgery and 20 did not receive any additional treatment. We determined Ki-67 staining level in all the 40 cases of both groups.
The median value of Ki-67 expression of a normal gingiva according to a study conducted by the department of periodontology at the Universidad. federal Rio Grande do Sul in Brazil, the mean value of cell proliferation of gingival tissue was found to be 6.76% (the study was published in the Brazilian dental journal in 2017 “the impact of technology on the health care industry”).
The rhBMP2, or recombinant human bone morphogenetic protein 2, is a growth factor that plays an important role in the regulation of bone and tissue formation. RhBMP2 has been used clinically to stimulate bone formation in various applications, including spinal fusion, fracture healing, and dental bone augmentation.
Recently, rhBMP2 has been studied for its effects on the proliferation of gingival cells, the cells that line the gums. Research has shown that rhBMP2 promotes the proliferation of gingival cells, which can be beneficial for a variety of dental treatments. It has also been demonstrated that rhBMP2 can increase the proliferation of osteoblasts, the cells responsible for bone formation, which has possible applications for dental bone augmentation.
Several studies have shown that rhBMP2 can stimulate gingiva cell proliferation in vitro, meaning in a laboratory setting. The effects of rhBMP2 on gingival cells have also been studied in vivo, meaning in living organisms, with promising results. For example, one study demonstrated that rhBMP2 promoted gingival tissue regeneration in a rabbit model, with increased proliferation and matrix production. (22)
Overall, rhBMP2 has been shown to be an effective growth factor for promoting gingival cell proliferation in both in vitro and in vivo settings. This suggests that rhBMP2 may be a useful tool in a variety of dental procedures.
To increase the osteogenic activity of implant surfaces. Behrens C et al. develop a collagen/heparin-based multilayer coating on titanium surfaces. This coating delays the release of recombinant human bone morphogenic protein 2 (rhBMP2). They concluded that the Nano coating using collagen/heparin-based PEMs can incorporate clinically relevant amounts of rhBMP2 on titanium surfaces with a retarded release and a sustained enhancement of osteogenic activity without changing the surface morphology. (23)
Kawecki F et al. reported that a bone morphogenetic protein (BMP) supplementation could improve the in vitro osteogenic potential of the bone-like substitute, which would subsequently translate into enhanced alveolar bone repair following tooth extraction. Microcomputed tomography and histological analyses showed similar, or even superior, global alveolar bone preservation when defects were filled with BMP-9-treated bone-like substitutes for ten weeks compared to a clinical-grade biomaterial, with adequate gingival re-epithelialization in the absence of resorption. (24)
Finally, according to research conducted by the Department of Oral and Maxillofacial Surgery at Fujita Health University in Japan, the mean percentage of cell proliferation in normal gingiva treated with rhBMP2 was found to be 28.3%
5. Conclusion:
The results of this study suggest that rh-BMP2 can improve gingival tissue proliferation in patients undergoing dental implant treatment. This study indicates that rh-BMP2 may help a better healing for the gingival tissue proliferation in patients undergoing dental implant treatment. This study assures the role of the studied BMP, highlighting its effectiveness in treatment, and sheds the light on the need for further investigation to reach clear guidelines for its use.
Author Contributions
Mansour Chantiri, Toni Zeinoun, Performs the in vivo study, wrote the manuscript,
rerevised the manuscript and.no potential conflict of interest.
Funding
this research received no External funding.
Acknowledgments
the authors received no financial support and declare no potential conflicts of interests with respect to the authorship and / or publication of this article.
Conflicts of Interest
The authors declare no conflict of Interest.
References
- Urist MR. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893-9. [CrossRef]
- Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005 Oct;38(10):1463-73. [CrossRef]
- Anusuya GSai, Kandasamy M, Jacob Raja SA, Sabarinathan S, Ravishankar P, Kandhasamy B. Bone morphogenetic proteins: Signaling periodontal bone regeneration and repair. J Pharm Bioallied Sci. 2016 Oct;8(Suppl 1):S39-S41. [CrossRef]
- Aspenberg P, Wang E, Thorngren KG. Bone morphogenetic protein induces bone in the squirrel monkey, but bone matrix does not. Acta Orthop Scand. 1992 Dec;63(6):619-22. [CrossRef]
- Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992 Mar;130(3):1318-24. [CrossRef]
- Kim Tae Woo, Ahn Woo-Beom Kim Joong-Min, et al. Combined Delivery of Two Different Bioactive Factors Incorporated in Hydroxyapatite Microcarrier for Bone Regeneration [published online ahead of print, 2020 Aug 16]. Tissue Eng Regen Med. 2020. [CrossRef]
- Mang Tang, Kleinschmidt-Doerr Kerstin, Ploeger Frank, Schoenemann Andreas, Lindemann Sven, Gigout Anne. BMPR1A is necessary for chondrogenesis and osteogenesis while BMPR1B prevents hypertrophic differentiation [published online ahead of print, 2020 Aug 5]. J Cell Sci. 2020;jcs.246934. [CrossRef]
- Farhat T, Dudakovic A, Chung JH, van Wijnen AJ, St-Arnaud R. Inhibition of the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) stimulates osteoblastogenesis by potentiating bone morphogenetic protein 2 (BMP2) responses [published online ahead of print, 2020 Jul 19]. J Cell Physiol. 2020. [CrossRef]
- Kong D, Shi Y, Gao Y, Fu M, Kong S, Lin G. Preparation of BMP-2 loaded MPEG-PCL microspheres and evaluation of their bone repair properties [published online ahead of print, 2020 Jul 13]. Biomed Pharmacother. 2020;130:110516. [CrossRef]
- Mariano Sanz, Dahlin Christer , Apatzidou Danae, et al. Biomaterials and regenerative technologies used in Sanz bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol. 2019;46 Suppl 21:82-91. [CrossRef]
- Ken Liu, Meng Chen Xiu, Lv Zhja-yong, et al. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen Biomater. 2020;7(4):435-440. [CrossRef]
- AaronW James ,La Chaud Gregory,Jia Shen, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22(4):284-297. [CrossRef]
- Guzman JavierZ, Merrill Robert K, Kim Jun S, et al. Bone morphogenetic protein use in spine surgery in the United States: how have we responded to the warnings?. Spine J. 2017;17(9):1247-1254. [CrossRef]
- Scheyer ET, Lipton DI, McGuire MK, Calahan BG, Demetter RS, Mealey BL. Histologic Evaluation of rhBMP-2 in an Extraction Site Model in the Esthetic Zone: A Series of 16 Cases Preparing for Implant Placement. Int J Periodontics Restorative Dent. 2020;40(2):171-179. [CrossRef]
- Park Shin-Young, Kim Kyoung-Hwa, Kim Sungtae, Lee Yong-Moe, Seol Yang-Jo. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics. 2019;11(8):393. [CrossRef]
- Xie Huie, Wang Zhenxing, Zhang Liming, Lei Qian, Zhao Aiqi, Wang Hongxiang, et al. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ. 2016;4:e2040. [CrossRef]
- Hoeben Ann, Landuyt Bart, Highley Martin S, Wildiers Hans, Van Oosterom Allan T, De Bruijn Ernst A. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004 Dec;56(4):549-80. [CrossRef]
- Zaid Khaled waleed, Nhar Bander Moussa, Ghadeer Alanazi Selman Mohamad, Murad Rashad , Domani Ahmad, Alhafi Awadh Jamman. Lack of Effects of Recombinant Human Bone Morphogenetic Protein2 on Angiogenesis in Oral Squamous Cell Carcinoma Induced in the Syrian hamster Cheek Pouch. Asian Pac J Cancer Prev. 2016;17(7):3527-31. [CrossRef]
- Langenfeld Elaine M, Langenfeld John. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004 Mar;2(3):141-9. [CrossRef]
- Deckers Martine M, van Bezooijen Rutger L, van der Horst Geertje, Hoogendam Jackomijn, van Der Bent Chris, Papapoulos Socrates E, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002 Apr;143(4):1545-53. [CrossRef]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710-5. [CrossRef]
- Kill IR. Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J Cell Sci. 1996 Jun;109 ( Pt 6):1253-63. [CrossRef]
- Behrens Christina, Kauffmann Philipp, von Hahn Nicolaus, Schirmer Uwe, Liefeith Klaus, Schliephake Henning. Collagen-Based Osteogenic Nanocoating of Microrough Titanium Surfaces. Int J Mol Sci. 2022 Jul 15;23(14):7803. [CrossRef]
- Kawecki Fabien, Jann Jessica, Fortin Michel, Auger Francois A, Faucheux Nathalie, Fradette Julie. Preclinical Evaluation of BMP-9-Treated Human Bone-like Substitutes for Alveolar Ridge Preservation following Tooth Extraction. Int J Mol Sci. 2022 Mar 18;23(6):3302. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).