1. Introduction
Necrotizing fasciitis (NF) is a potentially fatal infection characterized by rapidly progressive necrosis of the superficial fascia and overlying subcutaneous tissue. It is often associated with sepsis and multisystem organ failure. The term “necrotizing fasciitis” was first used by Wilson in 1952[
1] but Hippocrates already described a condition resulting in “great falling off of the flesh, tendons and bones; and the defluxion which seated in the parts was not like pus, but a sort of putrefaction” in 500BC[
2,
3]. Abdominal wall/perineum, thorax or lower limbs are most commonly affected. The incidence of necrotizing fasciitis ranges from 4 to 15.5 cases per 100,000 population worldwide[
4]. According to the Center for Disease Control, since 2010, approximately 700 to 1,150 cases of NF caused by group A Streptococcus alone occur each year in the United States[
5] and UK has a reported incidence of NF of 500 cases per year[
6]. However, necrotizing fasciitis of the head and neck is reported to be rare (1-10%)[
2]. This is probably due to the increased blood supply of the head and neck region.
2. Pathogenesis and Clinical Features
2.1. Pathogenesis
NF is an invasive infection of the subcutaneous tissue, disseminated through the fascial planes due to its relatively poor blood supply, causing thrombosis of the affected blood vessels, skin devascularization and fascial necrosis. The skin surface appears relatively normal at the onset while the fascial necrosis is more extensive[
2,
7]. The infection of subcutaneous tissue leads to subdermal venous thrombosis and inflammatory cell infiltration with abscess formation, followed by arterial compromise due to endarteritis obliterans. This causes critical skin ischemia, with blisters or bullae formation. Necrosis of the skin and coliquative gangrene follow[
8,
9].
The most common causes of CNF are odontogenic (47%) or pharyngeal (28%), tonsillar/peritonsillar infections (6%), the major salivary glands (2.5%), the skin (1.7%), otitis media, and mastoiditis. In 10% of the cases no source is identified. [
10] Other causes of NF include skin disruption as in surgical wounds, animal bites, lacerations and scratches or injection (i.e., intravenous drug use). Blunt trauma without laceration of the skin or radiotherapy have also been described[
4,
11].
According to a systematic review conducted by Gunaratne et al.[
10], the most common organisms were
Streptococcus spp. (61% of the patients) and
Staphylococcus spp. (18%).
Prevotella spp., Peptostreptococcus spp., Bacteroides spp., Fusobacterium spp., Enterobacter spp., Klebsiella spp., Escherichia coli, Pseudomonas spp. and
Candida spp. were also reported. A mean of 2 ± 0.98 organisms were identified per patient in this study.
An association between nonsteroidal anti-inflammatory drugs and necrotizing soft-tissue infections, particularly those caused by group A streptococcus has been previously described. Ketorolac and ibuprofen have been reported to accelerate the disease course and worsen outcomes[
12,
13].
2.2. Classification
Necrotizing fasciitis may be divided into two microbiologic categories. Their relative incidences varies considerably depending on the studies[
14]:
Type I is characterized by a polymicrobial infection with aerobic and anaerobic bacteria. Typically, at least one anaerobic species (most commonly
Bacteroides spp.,
Clostridium spp. or
Peptostreptococcus spp.) is isolated in combination with Enterobacteriaceae (
E. coli,
Enterobacter spp., Klebsiella spp., Proteus spp.) and one or more facultative anaerobic streptococci (other than group A Streptococcus)[
15]. In the head and neck, usually mouth anaerobes (
Fusobacterium spp., anaerobic Streptococci,
Bacteroides spp. and
Spirochetes spp.) are found.
Pseudomonas aeruginosa and
Candida spp. can be found rarely in these mixed infections. This is the most frequent type in immunodeficient patients or with comorbidities such as diabetes mellitus. These polymicrobial infections tend to have longer incubation periods than monomicrobial (type II) infections[
16].
Type II are monomicrobial infections, usually caused by group A Streptococcus, other beta-hemolytic Streptococci or
Staphylococcus aureus[
17]. It may occur in any age group and in individuals with no underlying comorbidities. In half of the cases, a clear portal of entry is not found, and the pathogenesis likely consists of hematogenous translocation of group A Streptococcus from the throat (pharyngitis, either symptomatic or asymptomatic) to a site of blunt trauma or muscle strain[
4,
18]. Necrotizing infections caused by group A Streptococcus strains with M protein types 1 and 3 are associated with toxic shock syndrome in about 50% of cases[
19,
20]. Pyrogenic exotoxins produced by these strains induce cytokine release, contributing to shock, tissue destruction and organ failure[
21]. In the last decades, an increasing incidence of methicillin-resistant
S. aureus soft tissue infections has been reported, being cultured in up to 40% of necrotic wounds[
17]. Other microorganisms can cause type II necrotizing infections less frequently, for example
Vibrio vulnificus and
Aeromonas hydrophila, which are associated to traumatic injury in sea water or fresh water respectively.
Some authors advocate for a 4-type classification, where type III includes
Clostridium spp., Gram-negatives and
Vibrio spp. and has a fulminant progression and a type IV would be
Candida spp. or Zygomycetes infections[
22,
23].
2.3. Clinical features
The main clinical feature is the rapidly progressive necrotizing infection. The patient usually has a history of dental infection left untreated or unresponding to antibiotics. In some of these cases, nonsteroidal anti-inflammatory drugs and oral antibiotic treatment may mask signs and symptoms, delaying diagnosis. Fever is not always present and often patients can appear systemically quite well in the initial phases, especially immunocompromised patients such as diabetics. Swelling, erythema and edema of the cervical/submandibular region usually follow as the patient’s general condition worsens. The margins of tissue involvement are often poorly defined. Disproportionate pain and tenderness extending beyond the involved area is characteristic. Hypoesthesia or anesthesia have also been described in a later stage, secondary to dermal necrosis[
22]. Crepitus on physical examination is also a typical local sign, revealing the presence of gas-forming organisms. Wang et al.[
24] described the consecutive dermatological findings as the infection progresses: 1) tenderness, erythema, warmth and swelling; 2) blistering and bullae; 3) skin crepitus, necrosis and anesthesia. Tachycardia or hypotension are typical as the disease progresses [
2].
3. Diagnosis
The diagnosis of CNF is based on clinical examination, imaging and laboratory tests. Surgical exploration is the most sensitive and specific option to confirm or exclude NF[
16] and should be performed in case of doubt. Final diagnosis is confirmed by histopathological findings, which typically show necrosis of the fascia and a polymorphonuclear infiltration. Angiothrombosis of the medium and small vessels is also characteristic.
3.1. Clinical diagnosis
CNF should be suspected in patients with facial or cervical/submandibular swelling, edema and erythema (
Figure 1) and systemic manifestations such as fever and hemodynamic instability. Association with crepitus, rapid progression of clinical manifestations and severe, disproportionate pain should raise alarms. Since CNF is rare, many surgeons are not familiar with this condition, therefore making an early diagnosis is challenging. Moreover, differential diagnosis of CNF from non-necrotizing deep neck infections may be difficult at early stages, since these two entities are clinically similar initially[
8,
9]. An accurate diagnosis at the time of presentation is reached in less than 40% of the patients[
25]. The presence of blistering and bullae has been signaled as an important distinguishing feature from other non-necrotizing infections such as erysipelas or cellulitis. Crepitus with skin necrosis are pathognomonic, but late signs in the evolution of the disease[
24].
If the clinical features are suggestive of necrotizing fasciitis but doubts remain, exploratory surgery should be considered. Surgical inspection of the wound demonstrates dull grey necrotic tissue and fascia, lack of bleeding, loss of structural integrity of the subcutaneous fat and little resistance of the tissues[
2,
26]. A foul, penetrating odor is also characteristic, usually revealing the presence of anaerobes. Biopsies should be taken from non-necrotic tissue; specimens should also be sent to microbiology for Gram’s stain, sensitivity and culture.
3.2. Laboratory tests
Laboratory findings are generally nonspecific. A marked leukocytosis with left shift is common, but 20% of patients have a normal white cell count. Acidosis, coagulopathy, hyponatremia, elevated inflammatory markers as C-reactive protein and elevation in serum creatinine and lactate can be present. Elevations in serum creatin kinase or aspartate aminotransferase suggest deep infection involving muscle or fascia (as opposed to cellulitis)[
14].
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) is a scoring system developed by Wong et al.[
27] to help distinguish NF from other soft tissue infections based on laboratory parameters (white cell count, hemoglobin, sodium, glucose, creatinine and C-reactive protein). The scoring varies from 0 to 13, where 6–7 indicates a risk of NF of 50–75%, and 8-13 a risk >75%. Its positive predictive value ranges from 57 to 92% and negative predictive value ranges from 86 to 96%, in adults with a score of 6 or higher. Different studies have assessed its applicability to the cervical region[
8,
9,
28,
29]. A modified LRINEC has also been developed[
30] adding coexisting diabetes and renal disease, and high-sensitivity C-reactive protein instead of C-reactive protein. A sensitivity of 93.2% and specificity of 86.9% has been reported by the authors. Of the LRINEC parameters, glucose and C-reactive protein are considered predictors of mortality in critically ill patients, in relation to sepsis, hyperglycemia and end-stage acute renal failure[
16]. Some authors state that the LRINEC should not be used to rule out NF due to the variable sensitivity of this tool[
31,
32].
3.3. Imaging studies
Computed tomography (CT) scan findings include diffuse thickening of the subcutaneous tissue and the cervical fascia, fluid collections in neck compartments and gas collections. Presence of gas is not a specific sign of necrotizing fasciitis, being present in other musculoskeletal infections[
33] and its absence should not exclude the diagnosis if clinically suspected. Gas within fluid collections along subfascial planes is the hallmark of NF[
34]. Other signs that support a diagnosis of necrotizing fasciitis include extensive involvement of the deep intermuscular fascia (high sensitivity but low specificity), thickening to more than 3mm, and partial or complete absence of signal enhancement of the thickened fasciae on post-gadolinium images (fairly high sensitivity and specificity)[
35].
Becker et al.[
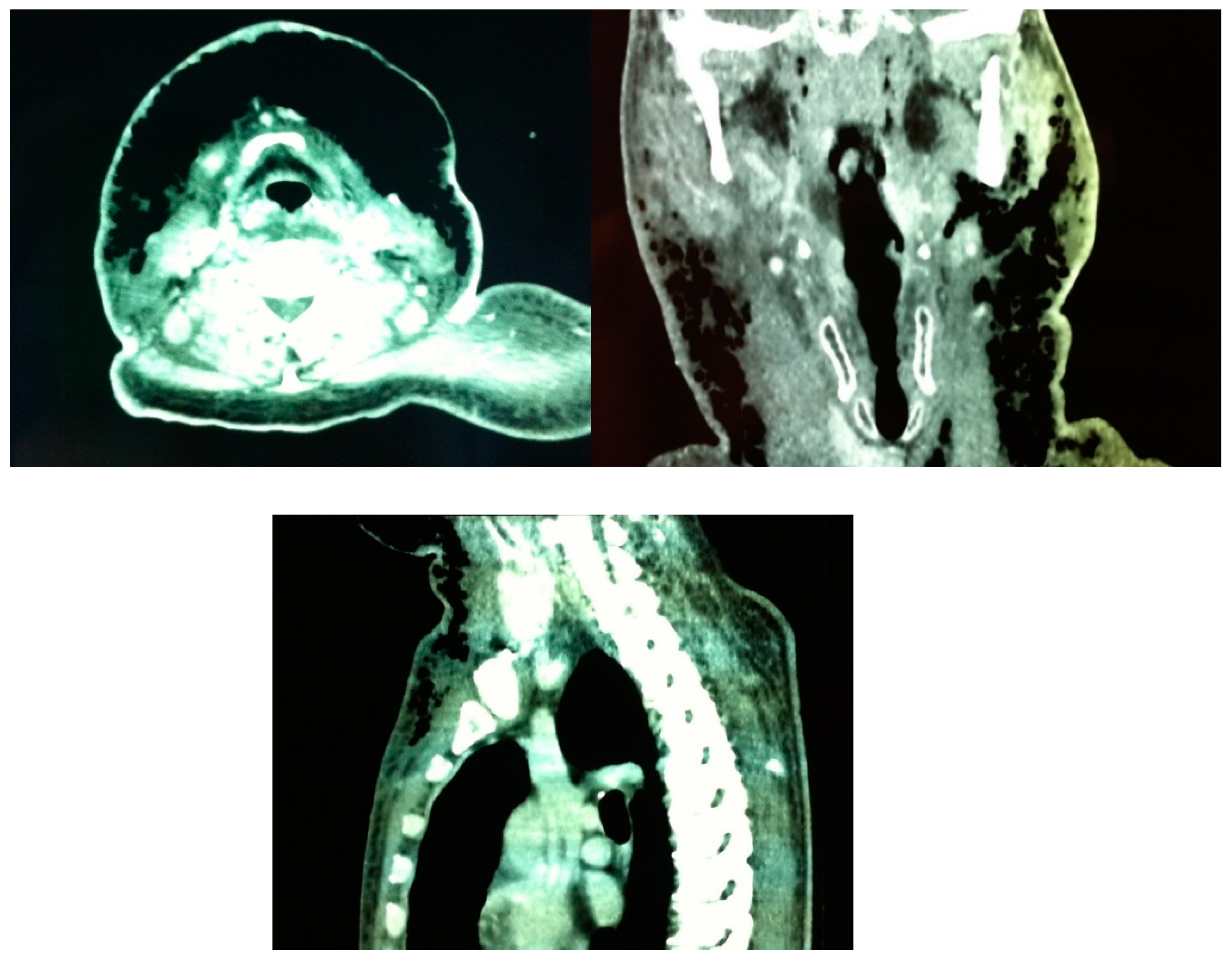
36] defined constant diagnostic features found on CT scans of patients with histologically confirmed NF: 1) cellulitis (diffuse thickening of cutaneous and subcutaneous tissue and reticular enhancement of the subcutaneous fat); 2) fasciitis (thickening and/or enhancement of cervical fascia); 3) myositis (asymmetric thickening or enhancement of cervical muscles) and 4) fluid collections in multiple neck spaces. Gas collections and involvement of the mediastinum were inconstant findings. Involvement of the superficial cervical fascia and thickening and/or enhancement of sternocleidomastoid muscle were present in all patients. CT scan of our first patient showed abundant and continuous gas collections, descending from the mandibular region to the upper portion of the anterior thoracic wall (
Figure 2), in contrast with Becker et al.[
36] findings where all gas collections were located within fluid collections.
Since the infection can descend to the mediastinum, this region must be routinely explored with a thoracic CT scan. CT scan is also useful for re-evaluation of new/remaining collections or areas of progression when recovery is not as expected after surgical debridement.
Sensitivity of CT has been reported to be 80%, with low specificity[
8,
9], although a systematic review from 2019[
32] found a sensitivity of 94.3% for the composite findings of fascial enhancement, fascial edema or fascial gas, and a specificity of 93.3% for fascial gas. In case of doubt, clinical suspicion should prevail. Martinez et al.[
37] described a sensitivity of 100% with 98% specificity and a positive predictive value of 76% and negative predictive value of 100% after assessing CT scans and medical records of 184 patients with suspicion of necrotizing soft tissue infection. According to their findings, they stated that a negative IV contrast-enhanced CT scan can reliably rule out the need for surgical intervention in patients with clinical suspicion of necrotizing soft tissue infection.
Magnetic resonance imaging is superior to CT scan[
33] and can differentiate from a non-necrotizing cellulitis[
38] (that can be treated medically) but it is usually not available in an emergency setting.
4. Management
Early and aggressive surgical debridement of all necrotic tissues is the cornerstone of management. Re-interventions are usually necessary as the infection progresses. Some authors even recommend routine second-look surgery[
39]. When deciding the extension of the area to resect, the surgeon should bear in mind that adjacent, normal-appearing tissues probably have extensive early vascular thrombosis and vasculitis[
40]. Specimens must be sent to both pathology and microbiology for histological confirmation and Gram’s stain, culture and antibiotic sensitivity.
For our first patient, a more conservative approach was chosen. Since neither the skin nor the fascia showed signs of necrosis at the moment of first surgical exploration, only cutaneous incisions and fasciotomies were performed initially to drain the purulent exudate and profusely irrigate the tissues. The wounds and skin were closely monitored and patient was taken back to theater as soon as the first signs of necrosis were noticed (
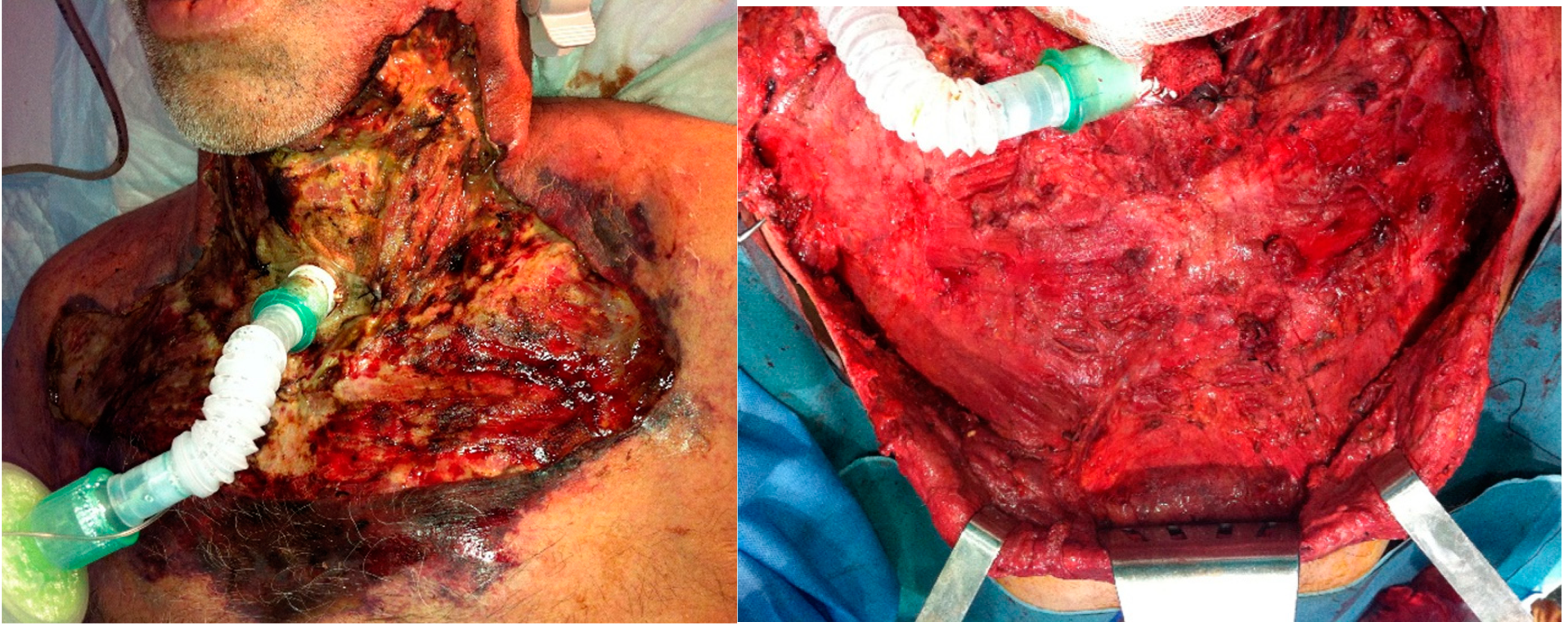
Figure 1B). For our second patient, who presented at the emergency department with a necrotic patch of skin and bullae, an aggressive surgical debridement of all skin, fascia and cervical fat was performed straight away (
Figure 4).
A temporary tracheostomy is routinely performed to secure the airway in patients that need ventilatory support or in which several interventions under general anesthesia are anticipated.
Broad-spectrum empiric antibiotic therapy should cover gram-positive, gram-negative and anaerobic organisms:
- -
A carbapenem (imipenem 1 gr every 6 to 8 hours or meropenem 2 g iv every 8 hours -extended infusion) or piperacillin-tazobactam 4.5 g every 6 h
- -
plus an agent with activity against methicillin-resistant Staphylococcus aureus (vancomycin (20 mg/kg initially -and monitor levels-) or daptomycin (10 mg/kg every 24 h)
- -
plus clindamycin 600 to 900 mg iv every 8 hours or linezolid 600mg iv every 8 hours initially -and monitor levels- if resistance to clindamycin (for its antitoxin effects against toxin-producing strains of beta-hemolytic streptococci and S. aureus).
Once Gram’s stain, culture and sensitivity results are available, antibiotic treatment should be updated.
Hemodynamic support in the intensive care unit should treat hemodynamic instability with fluids and vasopressors. These patients may have increased fluid requirements and profound hypoalbuminemia, so albumin replacement (colloid) may be necessary. Hematocrit should be checked to assess the need for transfusion (better indicator than hemoglobin level[
14]). Acidosis and hyperglycemia, if present, must be addressed as well.
The role of hyperbaric oxygen remains controversial[
14,
41]. On the one hand, its availability may be limited in many centers, and transferring a systemically unstable patient to pursue hyperbaric oxygen treatment seems logistically complicated. On the other, several studies concluded that the application of hyperbaric oxygen treatment to necrotizing fasciitis is not useful[
42]. Some studies found, however, a significant survival benefit[
41,
43].
Adjuvant treatment with intravenous immune globulin is based on its ability to bind and neutralize circulating toxins produced by group A streptococcus. A meta-analysis from 2018[
44] found a drop in mortality (from 33.7% to 15.7%) when administered, and therefore its addition to the treatment of patients with toxic shock syndrome due to beta-hemolytic streptococci has been suggested. A study with 4127 patients in 130 hospitals in the United States found, however, no effect on mortality or length of hospital stay with the immune globulin treatment[
45] and guidelines of the Infectious Disease Society of America do not recommend this treatment[
18].
Finally, due to the rarity of these severe infections, treatment of NF in centers with a high annual caseload of these entities enhances the chances of favorable outcome[
41].
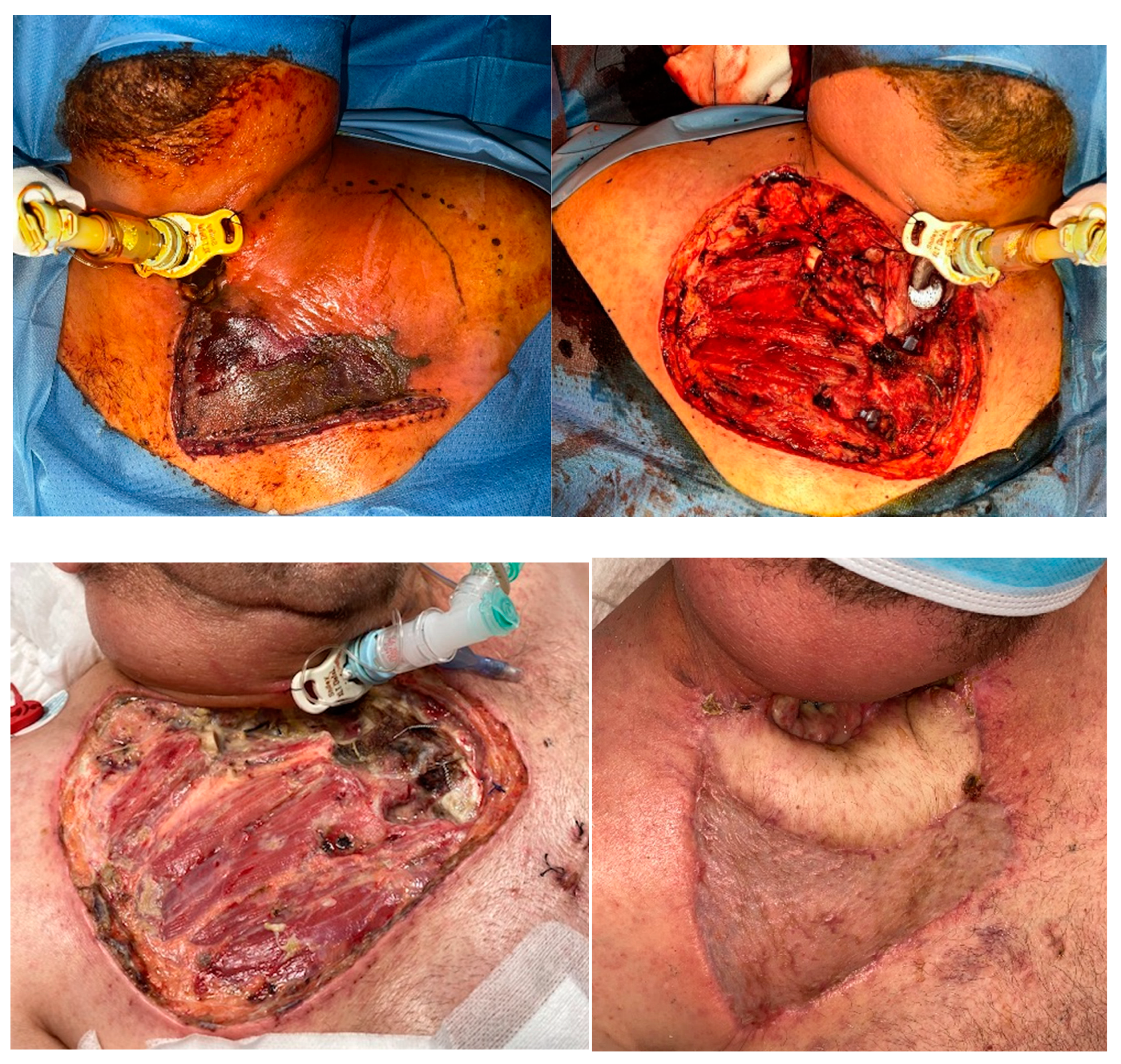
Wound dressings should be changed every 8 hours in order to monitor the appearance of new necrotic tissue and to keep the wound bed clean and moisture. Saline irrigation and gauze with topical antibiotics are applied. Once the infection is controlled and the patient is stable, skin grafting or compound flaps are required to reconstruct the defect, depending on the extension of the previous debridement. In our third patient, a resection of the sternoclavicular joint and part of the pectoralis major muscle was performed together with the compromised skin and fascia, and a latissimus dorsi pedicled flap was used to cover the defect (
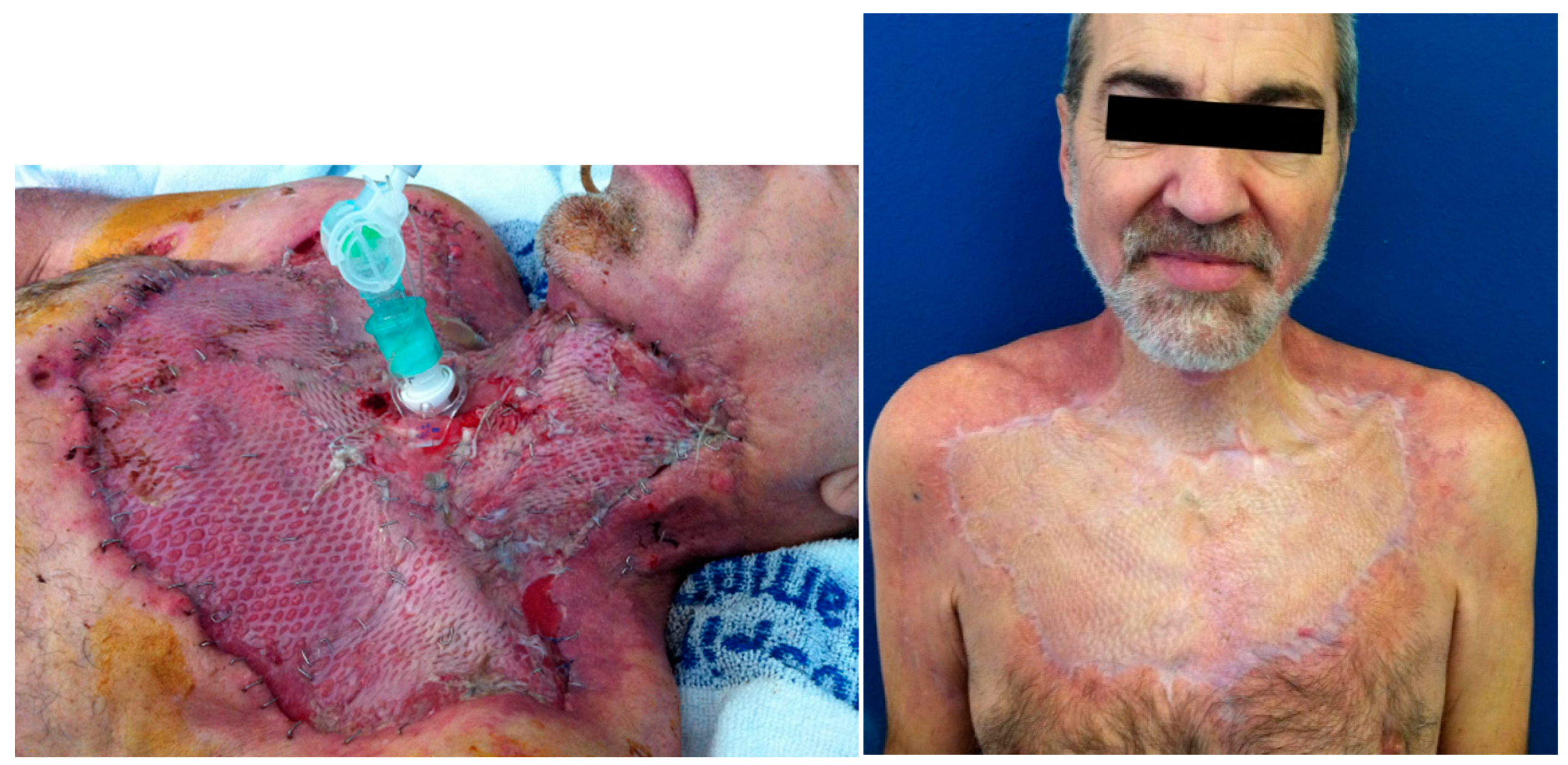
Figure 5). In order to allow for primary closure of the donor site, a small skin paddle was harvested and the remaining latissimus dorsi muscle was skin grafted in the same procedure. Split-thickness skin grafts harvested from the thigh were used for our first patient (
Figure 6).
Management summary:
- -
Diagnosis should be made promptly. If sufficient data supports the diagnosis of NF, surgical exploration is preferred to other laboratory or imaging tests that may delay surgery.
- -
Surgical debridement without delay (not waiting for microbiological results), removing all necrotic tissues (skin, fascia, muscle and fat).
- -
Tracheostomy is routinely performed to secure the airway and when a prolonged stay is anticipated.
- -
Aggressive resuscitation measures by intensive care physicians are also key in the management of NF, together with broad spectrum antibiotics covering most frequent pathogens until culture results and Gram’s stain are available.
- -
Re-interventions are usually necessary: wounds should be closely monitored looking for signs of progression, and laboratory results and vital signs continuously assessed. When in doubt, repeat CT-scan looking for new collections/ progression to descending mediastinitis.
- -
Treatment with hyperbaric oxygen is logistically complicated and should not delay surgical debridement. Its benefits, as well as those of immune globulin treatment are controversial according to literature evidence, and therefore they cannot be routinely recommended.
5. Prognosis
Despite adequate and early management, the mortality rate of CNF is high. A review of 146 cases performed in 1982 reported a mortality rate of 38%[
2,
46]. Depending on the literature, data varies from 7-20 to up to 50%[
8,
47]. In a study analyzing causes of death from a US database, a crude mortality rate of 4.8 deaths per 1 million person-years was attributed to NF of all locations (a total of 9871 necrotizing fasciitis-related deaths in the US from 2003 to 2013)[
48].
Mortality is higher among patients who develop streptococcal toxic shock syndrome or septic shock (38 and 45% respectively)[
14]. As the infection progresses, necrosis can easily propagate to mediastinum through the fascial planes. Descending necrotizing mediastinitis increases the rate of sepsis from 7 to 22% and the mortality rate from 31% to 41%. When descending necrotizing mediastinitis and sepsis occur, the mortality rate of CNF can reach 64%[
49].
Immunocompromised patients and those with chronic illness are at higher risk of developing CNF. Diabetes mellitus is the most common comorbidity[
10,
47,
48,
50,
51]; alcoholism or drug abuse, presence of malignancies, corticosteroid treatment or HIV infection[
10], renal failure and obesity[
48] are also associated.
Other factors have been associated with increased mortality: white blood cell count > 30000/microL; serum creatinine > 2 mg/dl, age >60 years, streptococcal toxic shock syndrome, clostridial infection, delay in surgery for more than 24 hours, infection involving the head, neck thorax or abdomen[
47,
52]. A study with 89 patients found that delay of surgery more than 24 hours from admission was the only independent predictor of mortality, in the multivariate logistic regression analysis[
47]. Advanced age and the presence of two or more associated comorbidities were also found to affect survival in the univariate analysis. Another study including 472 patients reported an overall mortality of 12% and found 8 independent predictors of mortality for NF in the multivariate analysis: liver cirrhosis, soft tissue air, infection by
Aeromonas spp., age older than 60 years, band polymorphonuclear neutrophils > 10%, activated partial thromboplastin time > 60 s, bacteremia, and serum creatinine > 2 mg/dL[
52].
5.2. Complications.
Descending necrotizing mediastinitis was reported in 255 cases out of 808 (31,56%) in the systematic review by Gunaratne et al.[
10] and had an odontogenic origin in 50% of the cases as opposed to the results of the systematic review published by Prado-Calleros et al.[
53], where the most common origin was pharyngeal (45%), followed by odontogenic (36%). Established DNM can further give rise to septic shock and organ failure (if not present already) pneumonia, obstruction of the airway, severe bleeding, cranial nerve paralysis, empyema or bronchocavitary fistula[
53,
54].
Major vascular complications include internal jugular vein thrombosis, suppurative jugular vein thrombophlebitis (Lemierre’s syndrome), carotid sheath necrosis and rupture and carotid artery aneurysm.
Additionally, patients with comorbidities such as uncontrolled diabetes may develop important functional deficits that require prolonged therapy if they survive. Our first patient suffered an important loss of visual acuity and a peripheral neuropathy (both related to the uncontrolled diabetes) that needed rehabilitation after being discharged. Other important complications are those related to the critically ill patient condition and the long stay in the intensive care unit. Functional impairment originated from scarring can also be of concern.
6. Conclusions
Cervical necrotizing fasciitis is a rare but severe surgical emergency. Although clinical diagnosis is straightforward at an advanced stage, differential diagnosis with other, more benign, deep neck infections can be challenging at initial presentation. Skin manifestations are late signs and do not accurately reflect the severity of the infection. Therefore, even though crepitus and skin necrosis are pathognomonic, these and other systemic signs can be absent. A CT scan can help to establish an early diagnosis and it is also essential to reassess progression of the infection. Mediastinum should be explored routinely. Early and aggressive surgical debridement, broad-spectrum antibiotics and life-support measures are the cornerstones of treatment of CNF. Despite appropriate management, mortality remains high.
Author Contributions
Conceptualization, P.L. and C.C.; methodology, P.L.; investigation, P.L., C.C., P.D.; writing—original draft preparation, P.L., C.C., P.D.; writing—review and editing, A.R., F.A., E.S.; supervision, J.A.; funding acquisition, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding; The APC was funded by Ramón y Cajal Research Foundation (FIBioHRC).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilson, B. Necrotizing fasciitis. Am Surg 1952; 18: 416–431.
- McGurk, M. Diagnosis and treatment of necrotizing fasciitis in the head and neck region. Oral Maxillofac. Surg. Clin. North Am. 2003, 15, 59–67. [Google Scholar] [CrossRef]
- Adams, F. The Genuine Works of Hippocrates. South. Med J. 1922, 15, 519. [Google Scholar] [CrossRef]
- Stevens DL, Bryant AE, Goldstein EJ. Necrotizing Soft Tissue Infections. Infect Dis Clin North Am 2021; 35: 135–155.
- Type II Necrotizing Fasciitis: Information For Clinicians | CDC, https://www.cdc.gov/groupastrep/diseases-hcp/necrotizing-fasciitis.html (2023, accessed ). 15 May.
- Hasham S, Matteucci P, Stanley PRW, et al. Necrotising fasciitis. BMJ 2005; 330: 830–833.
- Sepúlveda A, Sastre N. Necrotizing fasciitis of the face and neck. Plast Reconstr Surg 1998; 102: 814–817.
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Application of the laboratory risk indicator for necrotizing fasciitis score to the head and neck: a systematic review and meta-analysis. ANZ J. Surg. 2022, 92, 1631–1637. [Google Scholar] [CrossRef]
- Sideris, G.; Sapountzi, M.; Malamas, V.; Papadimitriou, N.; Maragkoudakis, P.; Delides, A. Early detecting cervical necrotizing fasciitis from deep neck infections: a study of 550 patients. Eur. Arch. Oto-Rhino-Laryngology 2021, 278, 4587–4592. [Google Scholar] [CrossRef]
- Gunaratne, D.A.; Tseros, E.A.; Hasan, Z.; Kudpaje, A.S.; Suruliraj, A.; Smith, M.C.; Riffat, F.; Palme, C.E. Cervical necrotizing fasciitis: Systematic review and analysis of 1235 reported cases from the literature. Head Neck 2018, 40, 2094–2102. [Google Scholar] [CrossRef]
- Nuwayhid, Z.B.; Aronoff, D.M.; Mulla, Z.D. Blunt Trauma as a Risk Factor for Group A Streptococcal Necrotizing Fasciitis. Ann. Epidemiology 2007, 17, 878–881. [Google Scholar] [CrossRef]
- Hamilton, S.M.; Bayer, C.R.; Stevens, D.L.; Bryant, A.E. Effects of Selective and Nonselective Nonsteroidal Anti-inflammatory Drugs on Antibiotic Efficacy of Experimental Group A Streptococcal Myonecrosis. J. Infect. Dis. 2013, 209, 1429–1435. [Google Scholar] [CrossRef]
- Weng, T.-C.; Chen, C.-C.; Toh, H.-S.; Tang, H.-J. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J. Microbiol. Immunol. Infect. 2011, 44, 418–423. [Google Scholar] [CrossRef]
- Stevens DL, Bryant AE. Necrotizing Soft-Tissue Infections. N Engl J Med 2017; 377: 2253–2265. [CrossRef]
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis Off Publ Infect Dis Soc Am 2007; 44: 705–710. [CrossRef]
- Lancerotto L, Tocco I, Salmaso R, et al. Necrotizing fasciitis: classification, diagnosis, and management. J Trauma Acute Care Surg 2012; 72: 560–566. [CrossRef]
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352: 1445–1453. [CrossRef]
- Stevens DL, Bryant AE. Necrotizing Soft-Tissue Infections. N Engl J Med 2018; 378: 971. [CrossRef]
- Kaul, R.; McGeer, A.; E Low, D.; Green, K.; Schwartz, B.; E Simor, A. Population-Based Surveillance for Group A Streptococcal Necrotizing Fasciitis: Clinical Features, Prognostic Indicators, and Microbiologic Analysis of Seventy-Seven Cases. Am. J. Med. 1997, 103, 18–24. [Google Scholar] [CrossRef]
- Darenberg, J.; Luca-Harari, B.; Jasir, A.; Sandgren, A.; Pettersson, H.; Schalén, C.; Norgren, M.; Romanus, V.; Norrby-Teglund, A.; Normark, B.H. Molecular and Clinical Characteristics of Invasive Group A Streptococcal Infection in Sweden. Clin. Infect. Dis. 2007, 45, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E.; Hackett, S.P.; Chang, A.; Peer, G.; Kosanke, S.; Emerson, T.; Hinshaw, L. Group A Streptococcal Bacteremia: The Role of Tumor Necrosis Factor in Shock and Organ Failure. J. Infect. Dis. 1996, 173, 619–626. [Google Scholar] [CrossRef]
- Leiblein M, Marzi I, Sander AL, et al. Necrotizing fasciitis: treatment concepts and clinical results. Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc 2018; 44: 279–290. [CrossRef]
- Yahav, D.; Duskin-Bitan, H.; Eliakim-Raz, N.; Ben-Zvi, H.; Shaked, H.; Goldberg, E.; Bishara, J. Monomicrobial necrotizing fasciitis in a single center: the emergence of Gram-negative bacteria as a common pathogen. Int. J. Infect. Dis. 2014, 28, 13–16. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, C.; Tay, Y. Staging of necrotizing fasciitis based on the evolving cutaneous features. Int. J. Dermatol. 2007, 46, 1036–1041. [Google Scholar] [CrossRef]
- Salati, SA. Necrotizing fasciitis a review. Pol Przegl Chir 2022; 95: 1–8.
- Hua, J.; Friedlander, P. Cervical Necrotizing Fasciitis, Diagnosis and Treatment of a Rare Life-Threatening Infection. Ear, Nose Throat J. 2021, 102, NP109–NP113. [Google Scholar] [CrossRef]
- Wong, C.-H.; Khin, L.-W.; Heng, K.-S.; Tan, K.-C.; Low, C.-O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections*. Crit. Care Med. 2004, 32, 1535–1541. [Google Scholar] [CrossRef]
- Ogawa, M.; Yokoo, S.; Takayama, Y.; Kurihara, J.; Makiguchi, T.; Shimizu, T. Laboratory Risk Indicator for Necrotizing Fasciitis of the Oro-Cervical Region (LRINEC-OC): A Possible Diagnostic Tool for Emergencies of the Oro-Cervical Region. Emerg. Med. Int. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Sandner, A.; Moritz, S.; Unverzagt, S.; Plontke, S.K.; Metz, D. Cervical Necrotizing Fasciitis—The Value of the Laboratory Risk Indicator for Necrotizing Fasciitis Score as an Indicative Parameter. J. Oral Maxillofac. Surg. 2015, 73, 2319–2333. [Google Scholar] [CrossRef]
- Wu, H. ; Liu, /.S.; Li, /.C., Ed.; Song, Z. Modified Laboratory Risk Indicator for Necrotizing Fasciitis (m-LRINEC) Score System in Diagnosing Necrotizing Fasciitis: A Nested Case–Control Study. Infect. Drug Resist. 2021, ume 14, 2105–2112. [Google Scholar] [CrossRef]
- Wilson, M.P.; Schneir, A.B. A Case of Necrotizing Fasciitis with a LRINEC Score of Zero: Clinical Suspicion Should Trump Scoring Systems. J. Emerg. Med. 2013, 44, 928–931. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Kyeremanteng, K.; Seely, A.J.E.; Inaba, K.; Perry, J.J. Necrotizing Soft Tissue Infection: Diagnostic Accuracy of Physical Examination, Imaging, and LRINEC Score. Ann. Surg. 2019, 269, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, F.; Cremona, A.; Carusi, V.; Guidi, M.; Iannicelli, E.; Di Girolamo, M.; Sergi, D.; Clarioni, A.; Baio, G.; Antonelli, G.; et al. The role of contrast enhanced computed tomography in the diagnosis of necrotizing fasciitis and comparison with the laboratory risk indicator for necrotizing fasciitis (LRINEC). La Radiol. medica 2015, 121, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Tso, D.K.; Singh, A.K. Necrotizing fasciitis of the lower extremity: imaging pearls and pitfalls. Br. J. Radiol. 2018, 91, 20180093. [Google Scholar] [CrossRef] [PubMed]
- Malghem, J.; Lecouvet, F.E.; Omoumi, P.; Maldague, B.E.; Berg, B.C.V. Necrotizing fasciitis: Contribution and limitations of diagnostic imaging. Jt. Bone Spine 2013, 80, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Zbären, P.; Hermans, R.; Becker, C.D.; Marchal, F.; Kurt, A.M.; Marré, S.; A Rüfenacht, D.; Terrier, F. Necrotizing fasciitis of the head and neck: role of CT in diagnosis and management. . 1997, 202, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Peponis, T.; Hage, A.; Yeh, D.D.; Kaafarani, H.M.A.; Fagenholz, P.J.; King, D.R.; de Moya, M.A.; Velmahos, G.C. The Role of Computed Tomography in the Diagnosis of Necrotizing Soft Tissue Infections. Mol. Med. 2017, 42, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Kim, S.; Cho, E.B.; Lee, G.Y.; Choi, S.-H.; Kim, S.O.; Chung, J.-W. Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm. J. Clin. Med. 2020, 9, 3040. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Andreasen TJ, Green SD, Childers BJ. Massive infectious soft-tissue injury: diagnosis and management of necrotizing fasciitis and purpura fulminans. Plast Reconstr Surg 2001; 107: 1025–1035.
- Mladenov, A.; Diehl, K.; Müller, O.; von Heymann, C.; Kopp, S.; Peitsch, W.K. Outcome of necrotizing fasciitis and Fournier’s gangrene with and without hyperbaric oxygen therapy: a retrospective analysis over 10 years. World J. Emerg. Surg. 2022, 17, 1–16. [Google Scholar] [CrossRef]
- Wang C, Schwaitzberg S, Berliner E, et al. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch Surg Chic Ill 1960 2003; 138: 272–279; discussion 280.
- A Riseman, J.; A Zamboni, W.; Curtis, A.; Graham, D.R.; Konrad, H.R.; Ross, D.S. Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for debridements. . 1990, 108, 847–50. [Google Scholar]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific Intravenous Immunoglobulin in Clindamycin-treated Patients With Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef]
- Kadri, S.S.; Swihart, B.J.; Bonne, S.L.; Hohmann, S.F.; Hennessy, L.V.; Louras, P.; Evans, H.L.; Rhee, C.; Suffredini, A.F.; Hooper, D.C.; et al. Impact of Intravenous Immunoglobulin on Survival in Necrotizing Fasciitis With Vasopressor-Dependent Shock: A Propensity Score-Matched Analysis From 130 US Hospitals. Clin. Infect. Dis. 2016, 64, 877–885. [Google Scholar] [CrossRef]
- Janevicius RV, Hann SE, Batt MD. Necrotizing fasciitis. Surg Gynecol Obstet 1982; 154: 97–102.
- Wong, C.-H.; Chang, H.-C.; Pasupathy, S.; Khin, L.-W.; Tan, J.-L.; Low, C.-O. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. . 2003, 85, 1454–60. [Google Scholar] [CrossRef] [PubMed]
- Arif, N.; Yousfi, S.; Vinnard, C. Deaths from necrotizing fasciitis in the United States, 2003–2013. Epidemiology Infect. 2015, 144, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T.; Sengupta, T.; Miloro, M.; Kolokythas, A. Cervical Necrotizing Fasciitis With Descending Mediastinitis: Literature Review and Case Report. J. Oral Maxillofac. Surg. 2012, 70, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Leyva, P.; Herrero, M.; Eslava, J.; Acero, J. Cervical necrotizing fasciitis and diabetic ketoacidosis: Literature review and case report. Int. J. Oral Maxillofac. Surg. 2013, 42, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Gottlieb, M.; Long, B.; Perkins, J.C. Necrotizing Soft Tissue Infections (NSTI): Pearls and Pitfalls for the Emergency Clinician. J. Emerg. Med. 2022, 62, 480–491. [Google Scholar] [CrossRef]
- Huang, K.-F.; Hung, M.-H.; Lin, Y.-S.; Lu, C.-L.; Liu, C.; Chen, C.-C.; Lee, Y.-H. Independent Predictors of Mortality for Necrotizing Fasciitis: A Retrospective Analysis in a Single Institution. J. Trauma: Inj. Infect. Crit. Care 2011, 71, 467–473. [Google Scholar] [CrossRef]
- Prado–Calleros, H.M.; Jiménez–Fuentes, E.; Jiménez–Escobar, I. Descending necrotizing mediastinitis: Systematic review on its treatment in the last 6 years, 75 years after its description. Head Neck 2016, 38, E2275–83. [Google Scholar] [CrossRef]
- Sumi, Y. Descending necrotizing mediastinitis: 5 years of published data in Japan. Acute Med. Surg. 2014, 2, 1–12. [Google Scholar] [CrossRef]
Figure 1.
Patient A. A) Necrotizing fasciitis of odontogenic origin in a 51-year-old male with undiagnosed diabetes mellitus and smoker of 30 cigarettes/day. He received treatment at home with amoxicillin/clavulanic acid and ibuprofen and only consulted once the disease had seriously progressed. He presented a poor general status with submandibular swelling and erythema extending to the clavicles and subcutaneous crepitus in the anterior aspect of the neck and supraclavicular region. He was febrile (37º), tachycardic and hypotensive. The white blood cell count was 14.8 per mm3, creatinine 2.29 mg/dl, urea 170mg/dl and sodium 121 mmol/l and glucose >800 mg/dl. Surgery was performed within 5 hours from admission. No necrotic skin was present on admission nor after first surgery, therefore a conservative approach was chosen initially with drainage and irrigation of the tissues. He was transferred to the intensive care unit after surgery. His condition worsened to septic shock with acute renal failure and diabetic ketoacidosis. B) Twelve hours after surgical exploration and drainage, skin necrosis appeared, and patient was taken back to surgery. Three more surgeries with extensive debridement of all necrotic tissue were performed. Necrotizing fasciitis was histopathologically confirmed and Streptococcus sp. and Candida albicans were isolated. This clinical case was published in Int J Oral Maxillofac Surg, 42, Leyva P, Herrero M, Eslava JM, Acero J, Cervical necrotizing fasciitis and diabetic ketoacidosis: literature review and case report, 1592–1595, Copyright Elsevier (2013).
Figure 1.
Patient A. A) Necrotizing fasciitis of odontogenic origin in a 51-year-old male with undiagnosed diabetes mellitus and smoker of 30 cigarettes/day. He received treatment at home with amoxicillin/clavulanic acid and ibuprofen and only consulted once the disease had seriously progressed. He presented a poor general status with submandibular swelling and erythema extending to the clavicles and subcutaneous crepitus in the anterior aspect of the neck and supraclavicular region. He was febrile (37º), tachycardic and hypotensive. The white blood cell count was 14.8 per mm3, creatinine 2.29 mg/dl, urea 170mg/dl and sodium 121 mmol/l and glucose >800 mg/dl. Surgery was performed within 5 hours from admission. No necrotic skin was present on admission nor after first surgery, therefore a conservative approach was chosen initially with drainage and irrigation of the tissues. He was transferred to the intensive care unit after surgery. His condition worsened to septic shock with acute renal failure and diabetic ketoacidosis. B) Twelve hours after surgical exploration and drainage, skin necrosis appeared, and patient was taken back to surgery. Three more surgeries with extensive debridement of all necrotic tissue were performed. Necrotizing fasciitis was histopathologically confirmed and Streptococcus sp. and Candida albicans were isolated. This clinical case was published in Int J Oral Maxillofac Surg, 42, Leyva P, Herrero M, Eslava JM, Acero J, Cervical necrotizing fasciitis and diabetic ketoacidosis: literature review and case report, 1592–1595, Copyright Elsevier (2013).
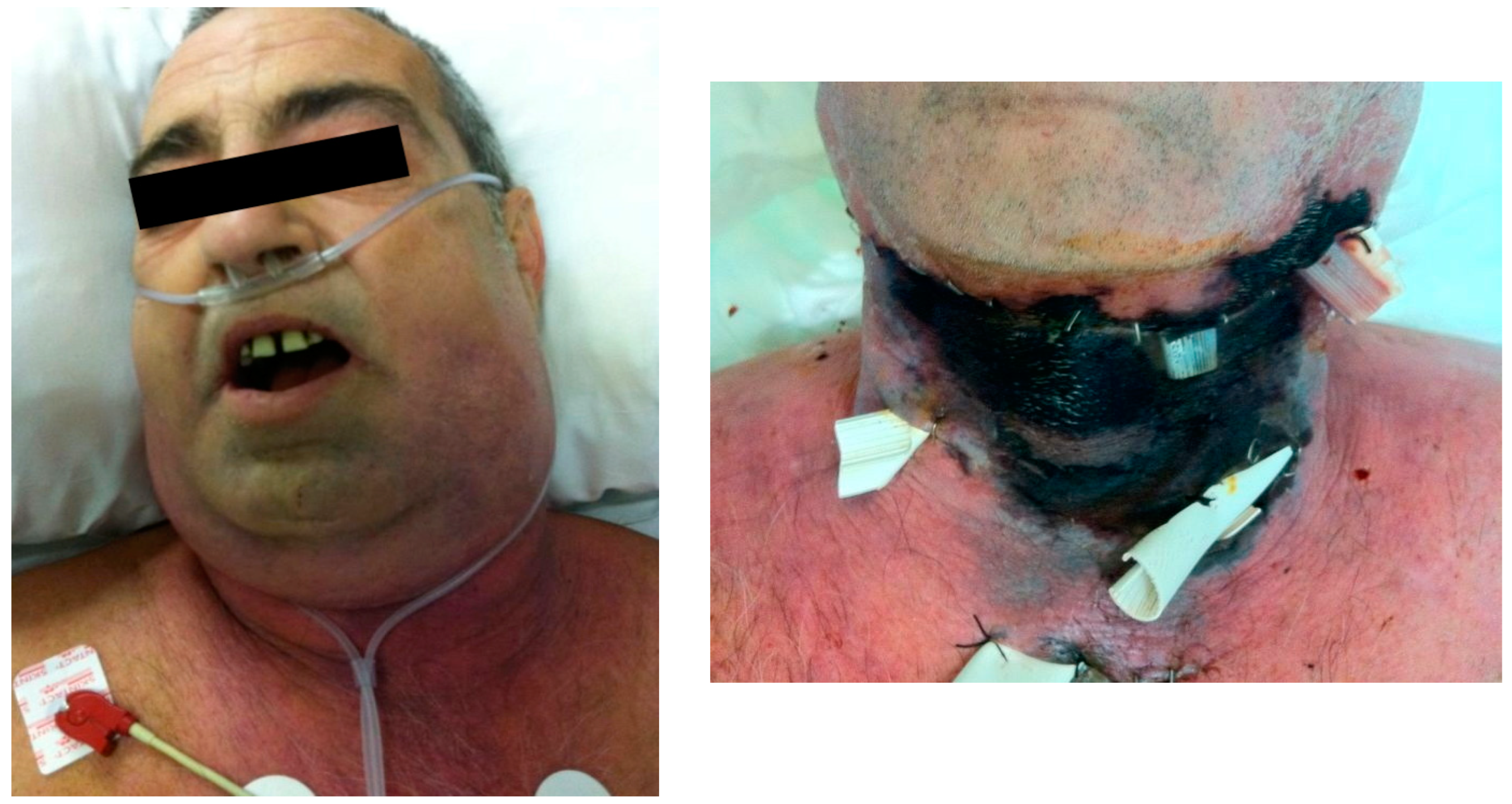
Figure 2.
Patient A. Preoperative CT scan showing abundant gas extending subcutaneously from the upper anterior chest wall and the anterolateral neck bilaterally up to the paramandibular region.
Figure 2.
Patient A. Preoperative CT scan showing abundant gas extending subcutaneously from the upper anterior chest wall and the anterolateral neck bilaterally up to the paramandibular region.
Figure 3.
Patient A. A) Progression of skin and muscle necrosis after second surgery. B) Final extension of debridement before skin grafting.
Figure 3.
Patient A. A) Progression of skin and muscle necrosis after second surgery. B) Final extension of debridement before skin grafting.
Figure 4.
Patient B. A) Sixty-two-year-old man with chronic ischemic cardiopathy and poorly controlled diabetes mellitus despite treatment with 4 drugs. Smoker. Odontogenic infection of a left-upper tooth treated with amoxicillin/clavulanic acid on the previous 14 days. Presented to the emergency department with crepitus, swelling and erythema on the anterolateral neck and upper thorax, a necrotic patch of skin and bullae with purulent exudate. Laboratory results were as follows: glucose 125 mg/dl, WBC 27 per mm3, hemoglobin was normal, creatinine 1.81 mg/dL, C-Reactive protein 385 mg/l, sodium 126 mmol/l; urea was 3 times higher than normal and liver function parameters were also high. Intravenous broad-spectrum antibiotics were started and patient was taken to surgery within 3 hours from admittance. B) Extensive debridement was performed as shown in picture. Tracheostomy was performed to secure the airway. Patient was acidotic and septic in intensive care unit and required increasing doses of vasoactive drugs. Intravenous immunoglobulin was administered. Patient died 16 hours after admittance. Histopathology confirmed the diagnosis of NF. Microbiology report showed Stenotrophomonas maltophilia and Enterobacter cloacae; Staphylococcus epidermidis and Streptococcus constellatus grew in the aerobic culture and Eggerthia catenaformis and Prevotella nigrescens in the anaerobic culture.
Figure 4.
Patient B. A) Sixty-two-year-old man with chronic ischemic cardiopathy and poorly controlled diabetes mellitus despite treatment with 4 drugs. Smoker. Odontogenic infection of a left-upper tooth treated with amoxicillin/clavulanic acid on the previous 14 days. Presented to the emergency department with crepitus, swelling and erythema on the anterolateral neck and upper thorax, a necrotic patch of skin and bullae with purulent exudate. Laboratory results were as follows: glucose 125 mg/dl, WBC 27 per mm3, hemoglobin was normal, creatinine 1.81 mg/dL, C-Reactive protein 385 mg/l, sodium 126 mmol/l; urea was 3 times higher than normal and liver function parameters were also high. Intravenous broad-spectrum antibiotics were started and patient was taken to surgery within 3 hours from admittance. B) Extensive debridement was performed as shown in picture. Tracheostomy was performed to secure the airway. Patient was acidotic and septic in intensive care unit and required increasing doses of vasoactive drugs. Intravenous immunoglobulin was administered. Patient died 16 hours after admittance. Histopathology confirmed the diagnosis of NF. Microbiology report showed Stenotrophomonas maltophilia and Enterobacter cloacae; Staphylococcus epidermidis and Streptococcus constellatus grew in the aerobic culture and Eggerthia catenaformis and Prevotella nigrescens in the anaerobic culture.
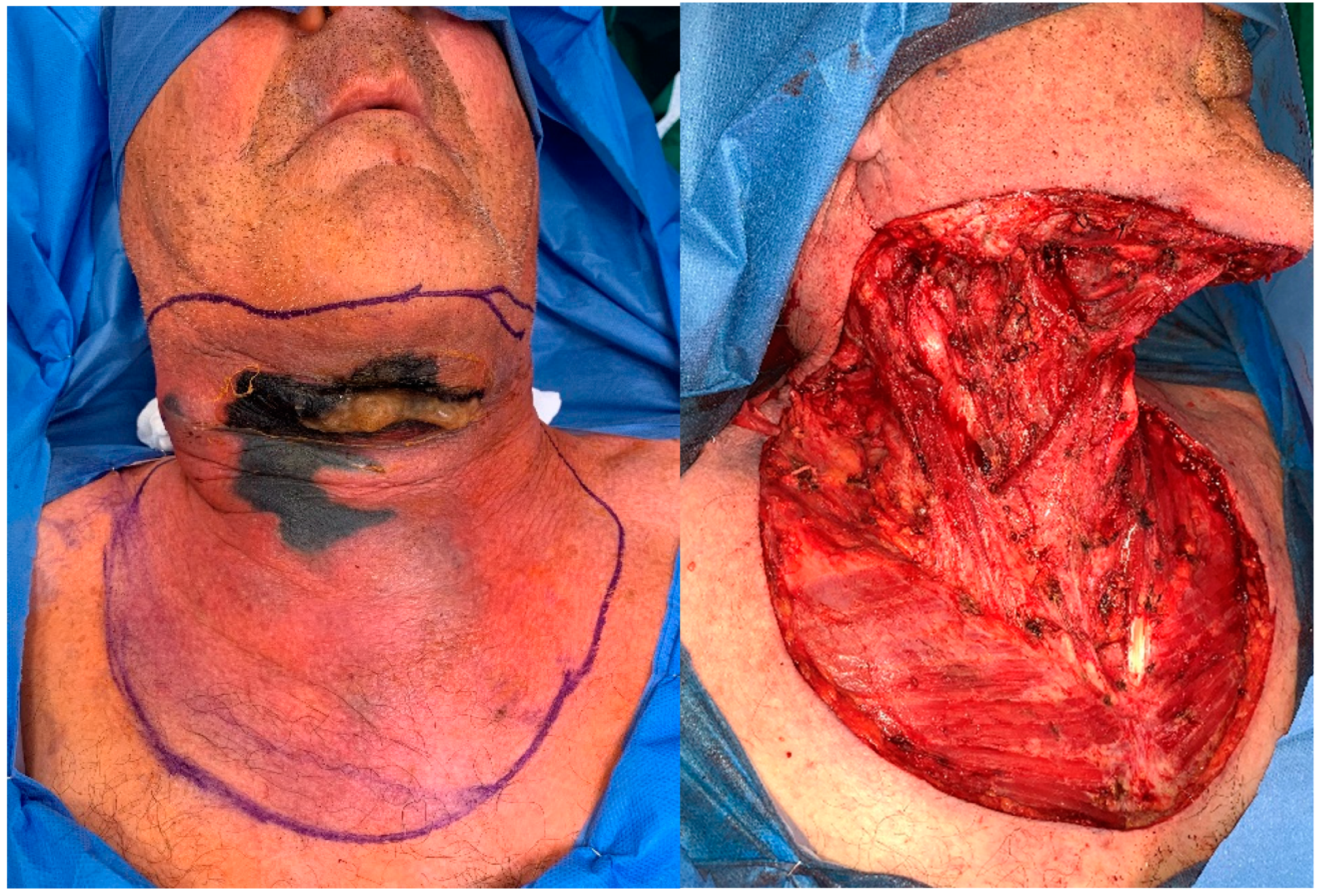
Figure 5.
Patient C was a 54-year-old male transferred from another hospital with cellulitis and abscessification of thoracic wall and mediastinitis needing thoracic surgery. Patient had already a tracheostomy due to supraglottic cancer, treated with chemo-radiotherapy. S. maltophilia, C. albicans, P. aeruginosa, S. constellatus, Serratia liquefaciens, Prevotella spp. and Chryseobacterium indologenes were isolated. B) The pectoralis mayor muscle was partially resected together with the medial portion of the right clavicle (white arrow), the superolateral portion of sternum and the sternocostal joints of first and second right ribs, in 3 different surgeries. C) Appearance before reconstruction. D) A latissimus dorsi pedicled flap + skin graft were harvested for reconstruction 2 weeks after admission. Necrotizing fasciitis and mediastinitis were confirmed by histopathology report. .
Figure 5.
Patient C was a 54-year-old male transferred from another hospital with cellulitis and abscessification of thoracic wall and mediastinitis needing thoracic surgery. Patient had already a tracheostomy due to supraglottic cancer, treated with chemo-radiotherapy. S. maltophilia, C. albicans, P. aeruginosa, S. constellatus, Serratia liquefaciens, Prevotella spp. and Chryseobacterium indologenes were isolated. B) The pectoralis mayor muscle was partially resected together with the medial portion of the right clavicle (white arrow), the superolateral portion of sternum and the sternocostal joints of first and second right ribs, in 3 different surgeries. C) Appearance before reconstruction. D) A latissimus dorsi pedicled flap + skin graft were harvested for reconstruction 2 weeks after admission. Necrotizing fasciitis and mediastinitis were confirmed by histopathology report. .
Figure 6.
Patient A. A) Split-thickness skin grafts were harvested to reconstruct the defect 39 days after first surgery. B) Outcome 6 months after reconstruction. This clinical case was published in Int J Oral Maxillofac Surg, 42, Leyva P, Herrero M, Eslava JM, Acero J, Cervical necrotizing fasciitis and diabetic ketoacidosis: literature review and case report, 1592–1595, Copyright Elsevier (2013).
Figure 6.
Patient A. A) Split-thickness skin grafts were harvested to reconstruct the defect 39 days after first surgery. B) Outcome 6 months after reconstruction. This clinical case was published in Int J Oral Maxillofac Surg, 42, Leyva P, Herrero M, Eslava JM, Acero J, Cervical necrotizing fasciitis and diabetic ketoacidosis: literature review and case report, 1592–1595, Copyright Elsevier (2013).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).