Submitted:
21 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of Long COVID and risk factors
3. Clinical manifestations of long COVID
4. Are clinical manifestations and pathogenesis unique to SARS-CoV-2 infection?
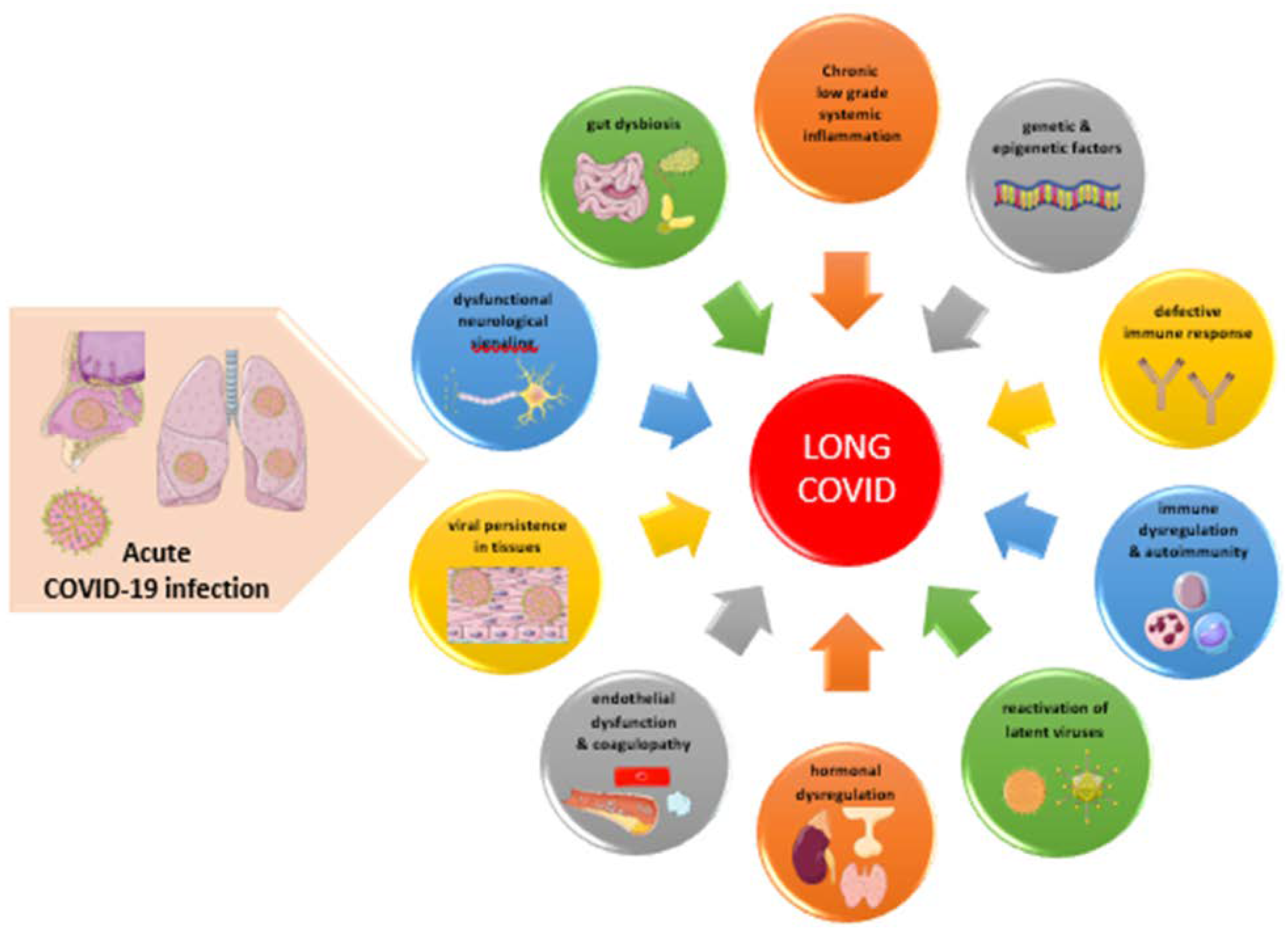
5. Pathogenesis of Long COVID
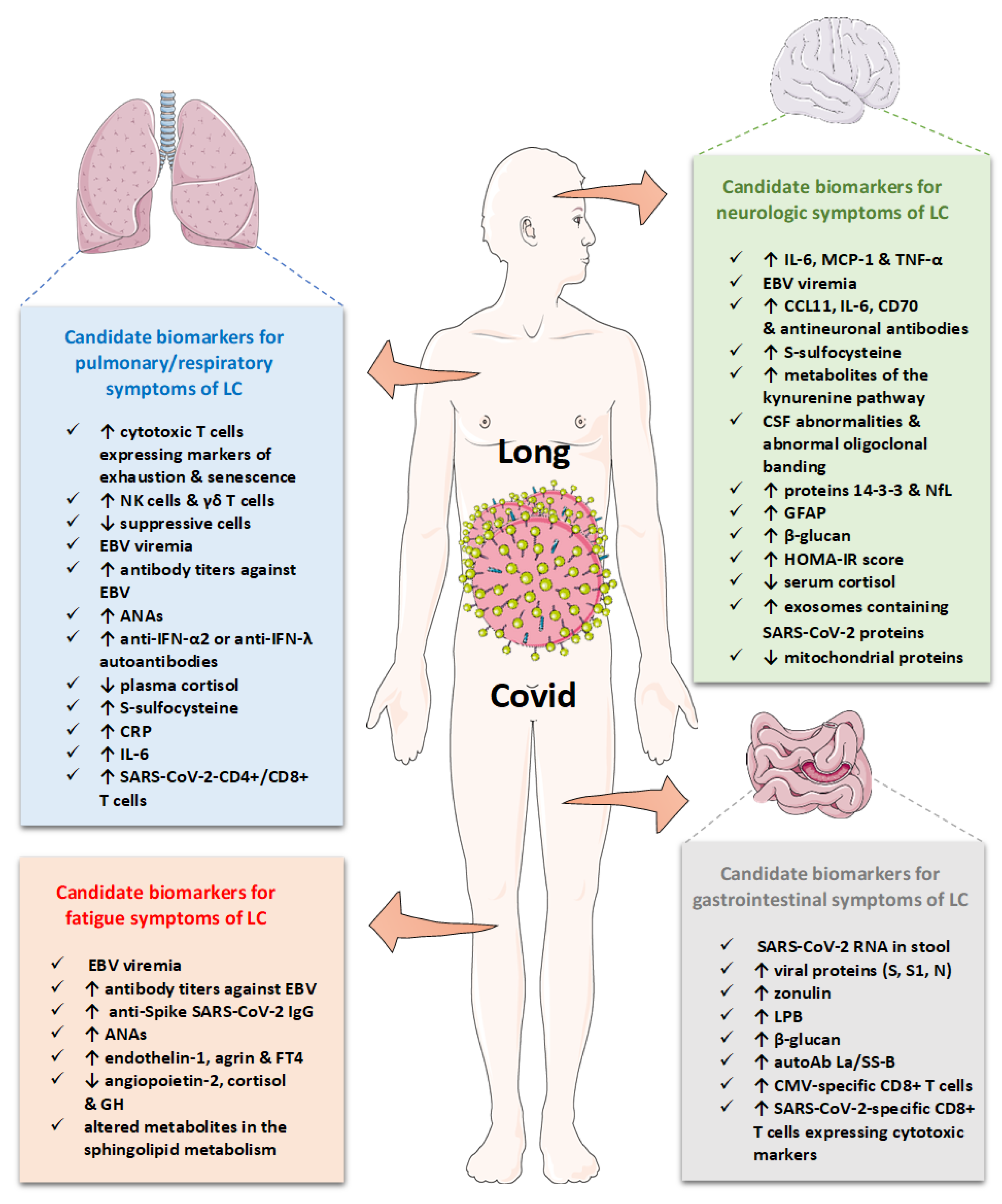
6. Laboratory findings and biomarkers in long COVID
6.1. Biomarkers of systemic inflammation
6.2. Immune profiling in long COVID
6.3. Biomarkers reflecting SARS-CoV-2 persistence
6.4. Humoral and cellular response against SARS-CoV-2 in long COVID
6.5. Biomarkers reflecting reactivation of latent viruses
6.6. Biomarkers reflecting autoimmunity
6.7. Endothelial or vascular biomarkers
6.8. Biomarkers of coagulation and fibrinolysis
6.9. Hormonal and metabolic biomarkers
6.10. Various proteins as biomarkers
6.11. Metabolites as biomarkers
6.12. Microbiota alterations in long COVID
6.13. Cerebrospinal fluid biomarkers
7. Biomarkers classifying clinical manifestations in long COVID
7.1. Candidate biomarkers for general symptoms and fatigue
7.2. Candidate biomarkers for neurological symptoms
7.3. Candidate biomarkers for respiratory symptoms
7.4. Candidate biomarkers for gastrointestinal and other specific symptoms
8. Limitations of studies and challenges
9. Therapeutic perspectives and challenges
10. Concluding remarks- Quo vadis?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 17 May 2023).
- Lee, W.E.; Woo Park, S.; Weinberger, D.M.; Olson, D.; Simonsen, L.; Grenfell, B.T.; Viboud, C. Direct and indirect mortality impacts of the COVID-19 pandemic in the United States, March 1, 2020 to January 1, 2022. Elife 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Liu, J.; Dalamaga, M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabol Open 2022, 13, 100159. [Google Scholar] [CrossRef] [PubMed]
- Marzianο, V.; Guzzetta, G.; Menegale, F.; Sacco, C.; Petrone, D.; Urdiales, A.; del Manso, M.; Bella, A.; Fabiani, M.; Vescio, M.; et al. The decline of COVID-19 severity and lethality over two years of pandemic. 2022. [Google Scholar] [CrossRef]
- Sigal, A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol 2022, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: What do we know about XBB.1.5 and should we be worried? Bmj 2023, 380, 153. [Google Scholar] [CrossRef] [PubMed]
- WHO. XBB.1.5 Updated Risk Assessment, 24 February 2023. Available online: https://www.who.int/docs/default-source/coronaviruse/22022024xbb.1.5ra.pdf (accessed on 15 May 2023).
- Siddiqui, S.; Alhamdi, H.W.S.; Alghamdi, H.A. Recent Chronology of COVID-19 Pandemic. Front Public Health 2022, 10, 778037. [Google Scholar] [CrossRef] [PubMed]
- Líška, D.; Liptaková, E.; Babičová, A.; Batalik, L.; Baňárová, P.S.; Dobrodenková, S. What is the quality of life in patients with long COVID compared to a healthy control group? Front Public Health 2022, 10, 975992. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Jennings, G.; Monaghan, A.; Xue, F.; Mockler, D.; Romero-Ortuño, R. A Systematic Review of Persistent Symptoms and Residual Abnormal Functioning following Acute COVID-19: Ongoing Symptomatic Phase vs. Post-COVID-19 Syndrome. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 15 May 2023).
- Roe, K. The Symptoms and Clinical Manifestations Observed in COVID-19 Patients/Long COVID-19 Symptoms that Parallel Toxoplasma gondii Infections. J Neuroimmune Pharmacol 2021, 16, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021, 11, 16144. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022, 54, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Karmaniolas, K.; Matekovits, A.; Migdalis, I.; Papadavid, E. Cutaneous manifestations in relation to immunologic parameters in a cohort of primary myelodysplastic syndrome patients. J Eur Acad Dermatol Venereol 2008, 22, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.A.; Dalamaga, M.; Lu, C.; Polissiou, M.G. Saffron for “toning down” COVID-19-related cytokine storm: Hype or hope? A mini-review of current evidence. Metabol Open 2021, 11, 100111. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Sandmann, S.; Ochs, K.; Schrempf, I.M.; Frömmel, C.; Dugas, M.; Schmidt, H.H.; Vollenberg, R.; Tepasse, P.R. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep 2021, 11, 12775. [Google Scholar] [CrossRef] [PubMed]
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long COVID: a scoping review. EBioMedicine 2023, 91, 104552. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front Med (Lausanne) 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw Open 2021, 4, e2111417. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. Bmj 2023, 380, e072529. [Google Scholar] [CrossRef]
- UK Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/alldatarelatingtoprevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk (accessed on 17 May 2023).
- Ahmad, F, B.; Anderson, R.N.; Cisewski, J.A.; Sutton, P.D. Identification of deaths with post-acute sequelae of COVID-19 from death certificate literal text: United States, January 1, 2020–June 30, 2022. NCHS Vital Statistics Rapid Release; no 25. December 2022. [CrossRef]
- Du, M.; Ma, Y.; Deng, J.; Liu, M.; Liu, J. Comparison of Long COVID-19 Caused by Different SARS-CoV-2 Strains: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Vetter, P.; Chappuis, F.; Kaiser, L.; Guessous, I. Prevalence of Post-Coronavirus Disease Condition 12 Weeks After Omicron Infection Compared With Negative Controls and Association With Vaccination Status. Clin Infect Dis 2023, 76, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Ballouz, T.; Menges, D.; Kaufmann, M.; Amati, R.; Frei, A.; von Wyl, V.; Fehr, J.S.; Albanese, E.; Puhan, M.A. Post COVID-19 condition after Wildtype, Delta, and Omicron SARS-CoV-2 infection and prior vaccination: Pooled analysis of two population-based cohorts. PLoS One 2023, 18, e0281429. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. Jama 2022, 327, 2452–2454. [Google Scholar] [CrossRef] [PubMed]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol Open 2022, 14, 100180. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Nasiri-Ansari, N.; Spyrou, N. Perspectives and Challenges of COVID-19 with Obesity-Related Cancers. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Nasiri-Ansari, N.; Spyrou, N.; Magkos, F.; Dalamaga, M. Management of Hematologic Malignancies in the Era of COVID-19 Pandemic: Pathogenetic Mechanisms, Impact of Obesity, Perspectives, and Challenges. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Syriga, M.; Karampela, I.; Dalamaga, M.; Karampelas, M. The effect of COVID-19 pandemic on the attendance and clinical outcomes of patients with ophthalmic disease: A mini-review. Metabol Open 2021, 12, 100131. [Google Scholar] [CrossRef]
- Kikkenborg Berg, S.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health 2022, 6, 614–623. [Google Scholar] [CrossRef]
- Sørensen, A.I.V.; Spiliopoulos, L.; Bager, P.; Nielsen, N.M.; Hansen, J.V.; Koch, A.; Meder, I.K.; Ethelberg, S.; Hviid, A. A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark. Nat Commun 2022, 13, 4213. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw Open 2022, 5, e2215787. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.A.; Rowley, A.H. Current Insights Into the Pathophysiology of Multisystem Inflammatory Syndrome in Children. Curr Pediatr Rep 2021, 9, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Lee, E.H.; Miller, A.D.; Lim, S.; Brown, C.M.; Yousaf, A.R.; Zambrano, L.D.; Belay, E.D.; Godfred-Cato, S.; Abrams, J.Y.; et al. Council of State and Territorial Epidemiologists/CDC Surveillance Case Definition for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection - United States. MMWR Recomm Rep 2022, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern Med 2023. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iran J Med Sci 2021, 46, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lee, G.M.; Razzaghi, H.; Lorman, V.; Mejias, A.; Pajor, N.M.; Thacker, D.; Webb, R.; Dickinson, K.; Bailey, L.C.; et al. Clinical Features and Burden of Postacute Sequelae of SARS-CoV-2 Infection in Children and Adolescents. JAMA Pediatr 2022, 176, 1000–1009. [Google Scholar] [CrossRef]
- Merzon, E.; Weiss, M.; Krone, B.; Cohen, S.; Ilani, G.; Vinker, S.; Cohen-Golan, A.; Green, I.; Israel, A.; Schneider, T.; et al. Clinical and Socio-Demographic Variables Associated with the Diagnosis of Long COVID Syndrome in Youth: A Population-Based Study. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long COVID in People Infected With Severe Acute Respiratory Syndrome Coronavirus 2 After 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect Dis 2022, 9, ofac464. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Antar, A.A.R.; Yu, T.; Demko, Z.O.; Hu, C.; Tornheim, J.A.; Blair, P.W.; Thomas, D.L.; Manabe, Y.C. Long COVID brain fog and muscle pain are associated with longer time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute infection. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Hirose, M.; Inoue, Y.; Kunishima, H.; Otsubo, T.; Matsuda, T. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J Med Virol 2022, 94, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Nguyen, Y.N.; Hoang, V.T.; Million, M.; Gautret, P. SARS-CoV-2 Reinfection and Severity of the Disease: A Systematic Review and Meta-Analysis. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 2022, 28, 2398–2405. [Google Scholar] [CrossRef]
- Fonseca, A.; Lima, R.; Ladeira, I.; Guimarães, M. Evaluation of pulmonary function in post-COVID-19 patients - when and how should we do it? J Bras Pneumol 2021, 47, e20210065. [Google Scholar] [CrossRef]
- Fortini, A.; Rosso, A.; Cecchini, P.; Torrigiani, A.; Lo Forte, A.; Carrai, P.; Alessi, C.; Fabbrizzi, F.; Lovicu, E.; Sbaragli, S.; et al. One-year evolution of DLCO changes and respiratory symptoms in patients with post COVID-19 respiratory syndrome. Infection 2022, 50, 513–517. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology 2023, 306, e221806. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021, 6. [Google Scholar] [CrossRef]
- Canas, L.S.; Molteni, E.; Deng, J.; Sudre, C.H.; Murray, B.; Kerfoot, E.; Antonelli, M.; Chen, L.; Rjoob, K.; Pujol, J.C.; et al. Profiling post-COVID syndrome across different variants of SARS-CoV-2. 2022. [Google Scholar] [CrossRef]
- Dennis, A.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Eichert, N.; Mouchti, S.; Pansini, M.; Roca-Fernandez, A.; Thomaides-Brears, H.; et al. Multi-organ impairment and long COVID: a 1-year prospective, longitudinal cohort study. J R Soc Med 2023, 116, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Sutton, R. Autonomic dysfunction and postural orthostatic tachycardia syndrome in post-acute COVID-19 syndrome. Nat Rev Cardiol 2023, 20, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Gonzalez, A.B.; Master, H.; Gall, N.; Halpin, S.; Rogers, N.; Greenhalgh, T. Orthostatic tachycardia after covid-19. Bmj 2023, 380, e073488. [Google Scholar] [CrossRef] [PubMed]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J 2018, 14, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhao, C.; He, M.Z.; Senter, C.; Zhou, Z.; Peng, J.; Li, S.; Fitzpatrick, A.L.; Lindström, S.; Stebbins, R.C.; et al. Long-term cardiac symptoms following COVID-19: a systematic review and meta-analysis. medRxiv 2023. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Oviedo, S.A.; Ali, M.K.; Ofotokun, I.; Gander, J.C.; Patel, S.A.; Magliano, D.J.; Patzer, R.E. The bidirectional association between diabetes and long-COVID-19 - A systematic review. Diabetes Res Clin Pract 2023, 195, 110202. [Google Scholar] [CrossRef]
- Nassar, M.; Daoud, A.; Nso, N.; Medina, L.; Ghernautan, V.; Bhangoo, H.; Nyein, A.; Mohamed, M.; Alqassieh, A.; Soliman, K.; et al. Diabetes Mellitus and COVID-19: Review Article. Diabetes Metab Syndr 2021, 15, 102268. [Google Scholar] [CrossRef]
- Karampela, I.; Vallianou, N.; Magkos, F.; Apovian, C.M.; Dalamaga, M. Obesity, Hypovitaminosis D, and COVID-19: the Bermuda Triangle in Public Health. Curr Obes Rep 2022, 11, 116–125. [Google Scholar] [CrossRef]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr Obes Rep 2021, 10, 214–243. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Kounatidis, D.; Stratigou, T.; Christodoulatos, G.S.; Karampela, I.; Dalamaga, M. Diabetes Mellitus and SARS-CoV-2 Infection: Pathophysiologic Mechanisms and Implications in Management. Curr Diabetes Rev 2021, 17, e123120189797. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z. Diabetes after SARS-CoV-2 infection. Lancet Diabetes Endocrinol 2023, 11, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.K.; Olaker, V.R.; Kaelber, D.C.; Xu, R.; Davis, P.B. Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw Open 2022, 5, e2233014. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Dalamaga, M.; Liu, J. SARS-CoV-2 adipose tissue infection and hyperglycemia: A further step towards the understanding of severe COVID-19. Metabol Open 2022, 13, 100163. [Google Scholar] [CrossRef] [PubMed]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.K.; Yu, M.W.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shin, H.S.; Park, H.Y.; Kim, J.L.; Lee, J.J.; Lee, H.; Won, S.D.; Han, W. Depression as a Mediator of Chronic Fatigue and Post-Traumatic Stress Symptoms in Middle East Respiratory Syndrome Survivors. Psychiatry Investig 2019, 16, 59–64. [Google Scholar] [CrossRef]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 2011, 11, 37. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994, 121, 953–959. [Google Scholar] [CrossRef]
- CDC. Myalgic encephalomyelitis/chronic fatigue syndrome: Symptoms. Available online: https://www.cdc.gov/me-cfs/symptoms-diagnosis/symptoms.html (accessed on 17 May 2023).
- Magnus, P.; Gunnes, N.; Tveito, K.; Bakken, I.J.; Ghaderi, S.; Stoltenberg, C.; Hornig, M.; Lipkin, W.I.; Trogstad, L.; Håberg, S.E. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 2015, 33, 6173–6177. [Google Scholar] [CrossRef]
- Wilson, H.W.; Amo-Addae, M.; Kenu, E.; Ilesanmi, O.S.; Ameme, D.K.; Sackey, S.O. Post-Ebola Syndrome among Ebola Virus Disease Survivors in Montserrado County, Liberia 2016. Biomed Res Int 2018, 2018, 1909410. [Google Scholar] [CrossRef]
- Garcia, M.N.; Hause, A.M.; Walker, C.M.; Orange, J.S.; Hasbun, R.; Murray, K.O. Evaluation of prolonged fatigue post-West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol 2014, 27, 327–333. [Google Scholar] [CrossRef]
- Shikova, E.; Reshkova, V.; Kumanova, А.; Raleva, S.; Alexandrova, D.; Capo, N.; Murovska, M. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J Med Virol 2020, 92, 3682–3688. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med 2020, 18, 100. [Google Scholar] [CrossRef]
- Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022, 10, 761–775. [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents 2020, 34, 9–14. [Google Scholar] [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab 2023, 34, 321–344. [Google Scholar] [CrossRef]
- Hu, F.; Chen, F.; Ou, Z.; Fan, Q.; Tan, X.; Wang, Y.; Pan, Y.; Ke, B.; Li, L.; Guan, Y.; et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol Immunol 2020, 17, 1119–1125. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Pinho, J.R.R.; Oliveira, K.G.; Sitnik, R.; Maluf, M.M.; Rodrigues, P.H.S.; Santana, R.A.F.; Welter, E.R.; Irony, O. Long term persistence of coronavirus SARS-CoV-2 infection. Einstein (Sao Paulo) 2021, 19, eRC6369. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Karn, V.; Ahmed, S.; Tsai, L.W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular Vesicle-Based Therapy for COVID-19: Promises, Challenges and Future Prospects. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- García-Abellán, J.; Padilla, S.; Fernández-González, M.; García, J.A.; Agulló, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutiérrez, F.; Masiá, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: a Longitudinal Study. J Clin Immunol 2021, 41, 1490–1501. [Google Scholar] [CrossRef]
- Spatola, M.; Nziza, N.; Jung, W.; Deng, Y.; Yuan, D.; Dinoto, A.; Bozzetti, S.; Chiodega, V.; Ferrari, S.; Lauffenburger, D.A.; et al. Neurologic sequelae of COVID-19 are determined by immunologic imprinting from previous coronaviruses. Brain 2023. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol 2021, 12, 698169. [Google Scholar] [CrossRef]
- Sausen, D.G.; Basith, A.; Muqeemuddin, S. EBV and Lymphomagenesis. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Lupo, J.; Truffot, A.; Andreani, J.; Habib, M.; Epaulard, O.; Morand, P.; Germi, R. Virological Markers in Epstein-Barr Virus-Associated Diseases. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Du Toit, A. EBV linked to multiple sclerosis. Nat Rev Microbiol 2022, 20, 189. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Tsilingiris, D.; Karampela, I.; Liu, J.; Dalamaga, M. Herpes zoster following COVID-19 vaccination in an immunocompetent and vaccinated for herpes zoster adult: A two-vaccine related event? Metabol Open 2022, 13, 100171. [Google Scholar] [CrossRef] [PubMed]
- Argyrakopoulou, G.; Dalamaga, M.; Spyrou, N.; Kokkinos, A. Gender Differences in Obesity-Related Cancers. Curr Obes Rep 2021, 10, 100–115. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K. Mounting evidence for EBV links to multiple sclerosis. Nat Med 2022, 28, 2450. [Google Scholar] [CrossRef] [PubMed]
- Nekoua, M.P.; Alidjinou, E.K.; Hober, D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 2022, 18, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Dai, X.C.; Liu, Z.J.; Zeng, Y.X.; Li, X.Y.; Wu, Q.J.; Lv, W.L. Helicobacter Pylori and Autoimmune Diseases: Involving Multiple Systems. Front Immunol 2022, 13, 833424. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadian, M.; Mirsaeidi, M.; Pooyafar, M.H.; Goudarzi, M.; Nasiri, M.J. Associations of Yersinia Enterocolitica Infection with Autoimmune Thyroid Diseases: A Systematic Review and Meta-Analysis. Endocr Metab Immune Disord Drug Targets 2021, 21, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Lake, C.M.; Breen, J.J. Sequence similarity between SARS-CoV-2 nucleocapsid and multiple sclerosis-associated proteins provides insight into viral neuropathogenesis following infection. Sci Rep 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story. Metabol Open 2021, 11, 100101. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.S.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Spyrou, N.; Vallianou, N.; Kadillari, J.; Dalamaga, M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin Cancer Biol 2021, 73, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Dalamaga, M.; Stratigou, T.; Karampela, I.; Tsigalou, C. Do Antibiotics Cause Obesity Through Long-term Alterations in the Gut Microbiome? A Review of Current Evidence. Curr Obes Rep 2021, 10, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Vallianou, N.; Dalamaga, M. Autoantibody Production in Obesity: Is There Evidence for a Link Between Obesity and Autoimmunity? Curr Obes Rep 2020, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. The Implication of Gut Hormones in the Regulation of Energy Homeostasis and Their Role in the Pathophysiology of Obesity. Curr Obes Rep 2020, 9, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Laurence, J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest 2022, 132. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 2020, 92, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Li, G.; Akmatbekov, A.; Harbert, J.L.; Lameira, F.S.; Brown, J.Q.; Vander Heide, R.S. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation 2020, 142, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Kassi, E.; Dalamaga, M.; Hroussalas, G.; Kazanis, K.; Merantzi, G.; Zachari, A.; Giamarellos-Bourboulis, E.J.; Dionyssiou-Asteriou, A. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas 2010, 67, 72–77. [Google Scholar] [CrossRef]
- Hroussalas, G.; Kassi, E.; Dalamaga, M.; Delimaris, I.; Zachari, A.; Dionyssiou-Asteriou, A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 2008, 59, 339–349. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Tzeravini, E.; Koliaki, C.; Dalamaga, M.; Kokkinos, A. The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: an Updated Overview. Curr Obes Rep 2021, 10, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Dalamaga, M.; Faviou, E.; Hroussalas, G.; Kazanis, K.; Nounopoulos, C.; Dionyssiou-Asteriou, A. Circulating oxidized LDL levels, current smoking and obesity in postmenopausal women. Atherosclerosis 2009, 205, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.M.; Kruger, A.; Proal, A.; Kell, D.B.; Pretorius, E. The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Wu, X.; Jing, H.; Novakovic, V.A.; Shi, J. The intersection of obesity and (long) COVID-19: Hypoxia, thrombotic inflammation, and vascular endothelial injury. Front Cardiovasc Med 2023, 10, 1062491. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J 2022, 479, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J Clin Transl Endocrinol 2022, 27, 100284. [Google Scholar] [CrossRef] [PubMed]
- Wheatland, R. Molecular mimicry of ACTH in SARS - implications for corticosteroid treatment and prophylaxis. Med Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased insulin resistance due to Long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Braz J Psychiatry 2023. [Google Scholar] [CrossRef]
- Dodd, S.; Sominsky, L.; Siskind, D.; Bortolasci, C.C.; Carvalho, A.F.; Maes, M.; Walker, A.J.; Walder, K.; Yung, A.R.; Williams, L.J.; et al. The role of metformin as a treatment for neuropsychiatric illness. Eur Neuropsychopharmacol 2022, 64, 32–43. [Google Scholar] [CrossRef]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. Elife 2022, 11. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. Mechanisms and clinical evidence to support melatonin’s use in severe COVID-19 patients to lower mortality. Life Sci 2022, 294, 120368. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Possible Application of Melatonin in Long COVID. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.M.; Martins, T.A.; Kulsvehagen, L.; Pössnecker, E.; Duchemin, W.; Hogan, S.; Sanabria-Diaz, G.; Müller, J.; Chiappini, A.; Rychen, J.; et al. Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: a prospective cross-sectional study. Nat Commun 2022, 13, 6777. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.R.; Bourne, K.M.; Stiles, L.E.; Miglis, M.G.; Cortez, M.M.; Miller, A.J.; Freeman, R.; Biaggioni, I.; Rowe, P.C.; Sheldon, R.S.; et al. Postural orthostatic tachycardia syndrome (POTS): Priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting - Part 2. Auton Neurosci 2021, 235, 102836. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.J.G.; Benitez-Trinidad, A.B.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Ortiz-Lazareno, P.C.; Girón-Pérez, D.A.; Bueno-Durán, A.Y.; Pérez-Díaz, D.A.; Barcelos-García, R.G.; Girón-Pérez, M.I. Loss of mitochondrial membrane potential (ΔΨ(m) ) in leucocytes as post-COVID-19 sequelae. J Leukoc Biol 2022, 112, 23–29. [Google Scholar] [CrossRef]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am J Hematol 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021, 42, 1866–1878. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating Chemerin and Its Kinetics May Be a Useful Diagnostic and Prognostic Biomarker in Critically Ill Patients with Sepsis: A Prospective Study. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Christodoulatos, G.S.; Dalamaga, M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr Obes Rep 2019, 8, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Karmaniolas, K.; Nikolaidou, A.; Papadavid, E. Hypocalcemia, hypomagnesemia, and hypokalemia following hydrofluoric acid chemical injury. J Burn Care Res 2008, 29, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Kandri, E.; Antonakos, G.; Vogiatzakis, E.; Christodoulatos, G.S.; Nikolaidou, A.; Dimopoulos, G.; Armaganidis, A.; Dalamaga, M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: A prospective study. J Crit Care 2017, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.H.; Stiles, L.E.; Bourne, K.; Green, E.A.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Diedrich, A.; Raj, V.; et al. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med 2019, 286, 438–448. [Google Scholar] [CrossRef]
- Roca-Fernandez, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Kelly, M.; Dennis, A.; Banerjee, R.; et al. Cardiac abnormalities in Long COVID 1-year post-SARS-CoV-2 infection. Open Heart 2023, 10. [Google Scholar] [CrossRef]
- Hughes, S.E.; Haroon, S.; Subramanian, A.; McMullan, C.; Aiyegbusi, O.L.; Turner, G.M.; Jackson, L.; Davies, E.H.; Frost, C.; McNamara, G.; et al. Development and validation of the symptom burden questionnaire for long covid (SBQ-LC): Rasch analysis. Bmj 2022, 377, e070230. [Google Scholar] [CrossRef]
- Jamal, S.M.; Landers, D.B.; Hollenberg, S.M.; Turi, Z.G.; Glotzer, T.V.; Tancredi, J.; Parrillo, J.E. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J Am Coll Cardiol 2022, 79, 2325–2330. [Google Scholar] [CrossRef]
- Stavileci, B.; Özdemir, E.; Özdemir, B.; Ereren, E.; Cengiz, M. De-novo development of fragmented QRS during a six-month follow-up period in patients with COVID-19 disease and its cardiac effects. J Electrocardiol 2022, 72, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Grist, J.T.; Collier, G.J.; Walters, H.; Kim, M.; Chen, M.; Abu Eid, G.; Laws, A.; Matthews, V.; Jacob, K.; Cross, S.; et al. Lung Abnormalities Detected with Hyperpolarized (129)Xe MRI in Patients with Long COVID. Radiology 2022, 305, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin Proc 2021, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Halim, A.; Halim, M.; Liu, S.; Aljeldah, M.; Al Shammari, B.R.; Alwarthan, S.; Alhajri, M.; Alawfi, A.; Alshengeti, A.; et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers. Rev Med Virol 2023, 33, e2424. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Agbana, Y.L.; Sun, Z.S.; Fei, S.W.; Zhao, H.Q.; Zhou, X.N.; Chen, J.H.; Kassegne, K. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty 2023, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Wood, J.; Jaycox, J.; Lu, P.; Dhodapkar, R.M.; Gehlhausen, J.R.; Tabachnikova, A.; Tabacof, L.; Malik, A.A.; Kamath, K.; et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv 2022. [Google Scholar] [CrossRef]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Krüger, K.; Baumgart, S.; Drube, S.; Lemhöfer, C.; Reuken, P.; Stallmach, A.; Müller, M.; et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients - indications for their potential involvement in the development of Long COVID? Front Immunol 2022, 13, 1070994. [Google Scholar] [CrossRef]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals With Long-COVID: Identification of Diagnostic Biomarkers. Front Immunol 2022, 13, 848886. [Google Scholar] [CrossRef]
- Utrero-Rico, A.; Ruiz-Ruigómez, M.; Laguna-Goya, R.; Arrieta-Ortubay, E.; Chivite-Lacaba, M.; González-Cuadrado, C.; Lalueza, A.; Almendro-Vazquez, P.; Serrano, A.; Aguado, J.M.; et al. A Short Corticosteroid Course Reduces Symptoms and Immunological Alterations Underlying Long-COVID. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Patterson, B.K.; Guevara-Coto, J.; Yogendra, R.; Francisco, E.B.; Long, E.; Pise, A.; Rodrigues, H.; Parikh, P.; Mora, J.; Mora-Rodríguez, R.A. Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning. Front Immunol 2021, 12, 700782. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin Infect Dis 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Gilboa, T.; Ogata, A.F.; Senussi, Y.; Lazarovits, R.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; Rivas, M.N.; Porritt, R.A.; et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Files, J.K.; Sarkar, S.; Fram, T.R.; Boppana, S.; Sterrett, S.; Qin, K.; Bansal, A.; Long, D.M.; Sabbaj, S.; Kobie, J.J.; et al. Duration of post-COVID-19 symptoms is associated with sustained SARS-CoV-2-specific immune responses. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021, 6, 100122. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 2021, 36, 109518. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Ryder, D.; Buck, A.; Beck-Engeser, G.; Chan, F.; Lu, S.; Goldberg, S.A.; Hoh, R.; et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest 2023, 133. [Google Scholar] [CrossRef]
- Zubchenko, S.; Kril, I.; Nadizhko, O.; Matsyura, O.; Chopyak, V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int 2022, 42, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Cecchinato, V.; Cavalli, A.; Shanbhag, A.A.; Matkovic, M.; Biggiogero, M.; Maida, P.A.; Moritz, J.; Toscano, C.; Ghovehoud, E.; et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nat Immunol 2023, 24, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Bodansky, A.; Wang, C.Y.; Saxena, A.; Mitchell, A.; Takahashi, S.; Anglin, K.; Huang, B.; Hoh, R.; Lu, S.; Goldberg, S.A.; et al. Autoantigen profiling reveals a shared post-COVID signature in fully recovered and Long COVID patients. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J 2023, 61. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Boesl, F.; Goereci, Y.; Gerhard, A.; Schweitzer, F.; Schroeder, M.; Foverskov-Rasmussen, H.; Heine, J.; Quitschau, A.; Kandil, F.I.; et al. Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav Immun 2023, 109, 139–143. [Google Scholar] [CrossRef]
- Peluso, M.J.; Mitchell, A.; Wang, C.Y.; Takahashi, S.; Hoh, R.; Tai, V.; Durstenfeld, M.S.; Hsue, P.Y.; Kelly, J.D.; Martin, J.N.; et al. Low Prevalence of Interferon α Autoantibodies in People Experiencing Symptoms of Post-Coronavirus Disease 2019 (COVID-19) Conditions, or Long COVID. J Infect Dis 2023, 227, 246–250. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med 2022, 20, 129. [Google Scholar] [CrossRef]
- Patel, M.A.; Knauer, M.J.; Nicholson, M.; Daley, M.; Van Nynatten, L.R.; Martin, C.; Patterson, E.K.; Cepinskas, G.; Seney, S.L.; Dobretzberger, V.; et al. Elevated vascular transformation blood biomarkers in Long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol Med 2022, 28, 122. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med 2022, 20, 138. [Google Scholar] [CrossRef]
- Tong, M.; Yan, X.; Jiang, Y.; Jin, Z.; Zhu, S.; Zou, L.; Liu, Y.; Zheng, Q.; Chen, G.; Gu, R.; et al. Endothelial Biomarkers in Patients Recovered from COVID-19 One Year after Hospital Discharge: A Cross-Sectional Study. Mediterr J Hematol Infect Dis 2022, 14, e2022033. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Bercu, A.; Kessler, A.; de Groot, R.; Dragunaite, B.; Heightman, M.; Hillman, T.; Price, L.C.; Brennan, E.; Sivera, R.; Vanhoorelbeke, K.; et al. Analysis of thrombogenicity under flow reveals new insights into the prothrombotic state of patients with post-COVID syndrome. J Thromb Haemost 2023, 21, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, L.; Valentini, P.; Sorrentino, S.; Ferretti, M.A.; De Candia, E.; Basso, M.; Lancellotti, S.; De Cristofaro, R.; De Rose, C.; Mariani, F.; et al. Extended coagulation profile of children with Long Covid: a prospective study. Sci Rep 2022, 12, 18392. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol 2022, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Sunada, N.; Honda, H.; Nakano, Y.; Yamamoto, K.; Tokumasu, K.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Otsuka, Y.; Obika, M.; et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr J 2022, 69, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Moon, J.C.; Topriceanu, C.C.; Joy, G.; Swadling, L.; Hallqvist, J.; Doykov, I.; Patel, N.; Spiewak, J.; Baldwin, T.; et al. Plasma proteomic signature predicts who will get persistent symptoms following SARS-CoV-2 infection. EBioMedicine 2022, 85, 104293. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; García Lopez, D.A.; Borrego, J.C.; Murgu, M.; Valdez, J.; López, J.A.; Monárrez-Espino, J. Untargeted analysis in post-COVID-19 patients reveals dysregulated lipid pathways two years after recovery. Front Mol Biosci 2023, 10, 1100486. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Aquino, J.M.; López, D.A.G.; Zheng, J.; Borrego, J.C.; Torres-Calzada, C.; Elizalde-Díaz, J.P.; Mandal, R.; Berjanskii, M.; Martínez-Martínez, E.; et al. The plasma metabolome of long COVID-19 patients two years after infection. 2023. [Google Scholar] [CrossRef]
- Cysique, L.A.; Jakabek, D.; Bracken, S.G.; Allen-Davidian, Y.; Heng, B.; Chow, S.; Dehhaghi, M.; Pires, A.S.; Darley, D.R.; Byrne, A.; et al. Post-acute COVID-19 cognitive impairment and decline uniquely associate with kynurenine pathway activation: a longitudinal observational study. 2022. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Ma, Y.; Chen, P.; Tang, J.; Yang, B.; Li, H.; Liang, M.; Xue, Y.; Liu, Y.; et al. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J Korean Med Sci 2023, 38, e120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Apple, A.C.; Oddi, A.; Peluso, M.J.; Asken, B.M.; Henrich, T.J.; Kelly, J.D.; Pleasure, S.J.; Deeks, S.G.; Allen, I.E.; Martin, J.N.; et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol 2022, 9, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Guasp, M.; Muñoz-Sánchez, G.; Martínez-Hernández, E.; Santana, D.; Carbayo, Á.; Naranjo, L.; Bolós, U.; Framil, M.; Saiz, A.; Balasa, M.; et al. CSF Biomarkers in COVID-19 Associated Encephalopathy and Encephalitis Predict Long-Term Outcome. Front Immunol 2022, 13, 866153. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, B.; Cosme, J.; Dupuis, C.; Coupez, E.; Adda, M.; Calvet, L.; Fabre, L.; Saint-Sardos, P.; Bereiziat, M.; Vidal, M.; et al. Severe COVID-19 is characterized by the co-occurrence of moderate cytokine inflammation and severe monocyte dysregulation. EBioMedicine 2021, 73, 103622. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med (Lausanne) 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis 2021, 224, 1839–1848. [Google Scholar] [CrossRef]
- Littlefield, K.M.; Watson, R.O.; Schneider, J.M.; Neff, C.P.; Yamada, E.; Zhang, M.; Campbell, T.B.; Falta, M.T.; Jolley, S.E.; Fontenot, A.P.; et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog 2022, 18, e1010359. [Google Scholar] [CrossRef]
- Cortellini, A.; Gennari, A.; Pommeret, F.; Patel, G.; Newsom-Davis, T.; Bertuzzi, A.; Viladot, M.; Aguilar-Company, J.; Mirallas, O.; Felip, E.; et al. COVID-19 Sequelae and the Host Proinflammatory Response: An Analysis From the OnCovid Registry. J Natl Cancer Inst 2022, 114, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Weng, Z.C. [Influence of stimulation of the skin receptive field on evoked discharges of the polymodal nociceptors in rats]. Sheng Li Xue Bao 1988, 40, 437–443. [Google Scholar] [PubMed]
- Queiroz, M.A.F.; Neves, P.; Lima, S.S.; Lopes, J.D.C.; Torres, M.; Vallinoto, I.; Bichara, C.D.A.; Dos Santos, E.F.; de Brito, M.; da Silva, A.L.S.; et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front Cell Infect Microbiol 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; Yalçın, B.; Taylor, K.R.; Dutton, S.; Acosta-Alvarez, L.; et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv 2022. [Google Scholar] [CrossRef]
- Venkataramani, V.; Winkler, F. Cognitive Deficits in Long Covid-19. N Engl J Med 2022, 387, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.; de Sarges, K.M.L.; Cantanhede, M.H.D.; da Costa, F.P.; Dos Santos, E.F.; Rodrigues, F.B.B.; de Nazaré do Socorro de Almeida Viana, M.; de Meira Leite, M.; da Silva, A.L.S.; de Brito, M.T.M.; et al. Thrombophilia and Immune-Related Genetic Markers in Long COVID. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Montoya, J.G.; Klimas, N.G.; Levine, S.; Felsenstein, D.; Bateman, L.; Peterson, D.L.; Gottschalk, C.G.; Schultz, A.F.; Che, X.; et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Sigal, G.B.; Novak, T.; Mathew, A.; Chou, J.; Zhang, Y.; Manjula, N.; Bathala, P.; Joe, J.; Padmanabhan, N.; Romero, D.; et al. Measurement of Severe Acute Respiratory Syndrome Coronavirus 2 Antigens in Plasma of Pediatric Patients With Acute Coronavirus Disease 2019 or Multisystem Inflammatory Syndrome in Children Using an Ultrasensitive and Quantitative Immunoassay. Clin Infect Dis 2022, 75, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, H.; Espín, E.; Tebbutt, S.J. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir Med 2023. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. Covid-19 Vaccines - Immunity, Variants, Boosters. N Engl J Med 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Banko, A.; Miljanovic, D.; Cirkovic, A. Systematic review with meta-analysis of active herpesvirus infections in patients with COVID-19: Old players on the new field. Int J Infect Dis 2023, 130, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Ying, L.Y. Epstein Barr Virus Reactivation during COVID-19 Hospitalization Significantly Increased Mortality/Death in SARS-CoV-2(+)/EBV(+) than SARS-CoV-2(+)/EBV(-) Patients: A Comparative Meta-Analysis. Int J Clin Pract 2023, 2023, 1068000. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pablos, M.; Paiva, B.; Montero-Mateo, R.; Garcia, N.; Zabaleta, A. Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Front Immunol 2021, 12, 656797. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, E.; Rizwan, M.; Moustardas, P.; Sjögren, P.; Bertilson, B.C.; Bragée, B.; Polo, O.; Rosén, A. Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome. Front Immunol 2022, 13, 949787. [Google Scholar] [CrossRef]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 2015, 125, 2194–2202. [Google Scholar] [CrossRef]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Manry, J.; Michailidis, E.; Hoffmann, H.H.; Eto, S.; Garcia-Prat, M.; et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Thomas, I.J.; Munter, S.E.; Deeks, S.G.; Henrich, T.J. Lack of Antinuclear Antibodies in Convalescent Coronavirus Disease 2019 Patients With Persistent Symptoms. Clin Infect Dis 2022, 74, 2083–2084. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS One 2021, 16, e0257016. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun 2021, 4, 100100. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 2021, 19, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”-A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol Res Perspect 2022, 10, e00911. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.E.; Wong, S.W.; Sum, C.L.L.; Lim, G.H.; Leung, B.P.; Tan, C.W.; Ramanathan, K.; Dalan, R.; Cheung, C.; Lim, X.R.; et al. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long-term outcomes in COVID-19 patients. Am J Hematol 2022, 97, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J 2022, 479, 1653–1708. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol 2022, 21, 148. [Google Scholar] [CrossRef]
- Du, W.N.; Zhang, Y.; Yu, Y.; Zhang, R.M. D-dimer levels is associated with severe COVID-19 infections: A meta-analysis. Int J Clin Pract 2021, 75, e14031. [Google Scholar] [CrossRef]
- Rushworth, R.L.; Torpy, D.J.; Falhammar, H. Adrenal Crisis. N Engl J Med 2019, 381, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Demitrack, M.A.; Dale, J.K.; Straus, S.E.; Laue, L.; Listwak, S.J.; Kruesi, M.J.; Chrousos, G.P.; Gold, P.W. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab 1991, 73, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Ko, Y.C.; Chow, L.H.; Hsiao, F.J.; Liu, H.Y.; Wang, P.N.; Chen, W.T. Salivary cortisol is associated with cognitive changes in patients with fibromyalgia. Sci Rep 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K.; Kwek, D.S.; Ng, A.W.; Ong, K.C.; Kaw, G.J.; Lee, L.S. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf) 2005, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W. Cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol 2020, 8, 808. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front Neurol 2023, 14, 1090747. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Christodoulatos, G.S. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Investig 2015, 23, 5–20. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Chamberland, J.; Nikolaidou, A.; Lekka, A.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Higher fetuin-A, lower adiponectin and free leptin levels mediate effects of excess body weight on insulin resistance and risk for myelodysplastic syndrome. Metabolism 2013, 62, 1830–1839. [Google Scholar] [CrossRef]
- Dalamaga, M.; Crotty, B.H.; Fargnoli, J.; Papadavid, E.; Lekka, A.; Triantafilli, M.; Karmaniolas, K.; Migdalis, I.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: a case-control study in Greece. Cancer Causes Control 2010, 21, 1451–1459. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Nikolaidou, A.; Chamberland, J.; Hsi, A.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Adiponectin and resistin are associated with risk for myelodysplastic syndrome, independently from the insulin-like growth factor-I (IGF-I) system. Eur J Cancer 2008, 44, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Nikolaidou, A.; Karmaniolas, K.; Hsi, A.; Chamberland, J.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: a case-control study. Oncology 2007, 73, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Marouga, A.; Dalamaga, M.; Kastania, A.N.; Antonakos, G.; Thrasyvoulides, A.; Kontelia, G.; Dimas, C.; Vlahakos, D.V. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab 2013, 59, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Gazi, S.; Dalamaga, M.; Stavrianeas, N.; Ntelis, V. Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: a case series of four patients and guidelines for management. J Eur Acad Dermatol Venereol 2008, 22, 380–382. [Google Scholar] [CrossRef]
- Papadavid, E.; Vlami, K.; Dalamaga, M.; Giatrakou, S.; Theodoropoulos, K.; Gyftopoulos, S.; Stavrianeas, N.; Papiris, S.; Rigopoulos, D. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol 2013, 27, 820–826. [Google Scholar] [CrossRef]
- Papadavid, E.; Dalamaga, M.; Vlami, K.; Koumaki, D.; Gyftopoulos, S.; Christodoulatos, G.S.; Papiris, S.; Rigopoulos, D. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: a large hospital-based case-control study. Sleep Breath 2017, 21, 949–958. [Google Scholar] [CrossRef]
- Pavlidou, A.; Dalamaga, M.; Kroupis, C.; Konstantoudakis, G.; Belimezi, M.; Athanasas, G.; Dimas, K. Survivin isoforms and clinicopathological characteristics in colorectal adenocarcinomas using real-time qPCR. World J Gastroenterol 2011, 17, 1614–1621. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Lekka, A.; Antonakos, G.; Thrasyvoulides, A.; Papadavid, E.; Spanos, N.; Dionyssiou-Asteriou, A. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Dis Markers 2010, 29, 55–61. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.; Nicklas, J.; Puskarich, M.A.; Cohen, K.; Belani, H.; Anderson, B.; Huling, J.D.; Tignanelli, C.; et al. Outpatient treatment of Covid-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up. medRxiv 2022. [Google Scholar] [CrossRef]
- Bruzzone, C.; Conde, R.; Embade, N.; Mato, J.M.; Millet, O. Metabolomics as a powerful tool for diagnostic, pronostic and drug intervention analysis in COVID-19. Front Mol Biosci 2023, 10, 1111482. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front Immunol 2021, 12, 628741. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin Cancer Biol 2023, 91, 70–98. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Zhang, F.; Lau, R.I.; Liu, Q.; Su, Q.; Chan, F.K.L.; Ng, S.C. Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat Rev Gastroenterol Hepatol 2023, 20, 323–337. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, V.M.; Engel, D.; Ricci, M.F.; Cruz, C.S.; Lopes, I.S.; Alves, D.A. et al. Mirna d’ Auriol5Gut microbiota from patients with mild COVID-19 cause alterations in mice that resemble post-COVID syndrome. 2022. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Arsenis, G.; Pantelaki, M.; Daskalopoulou, K.; Papadavid, E.; Migdalis, I. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns 2008, 34, 1205–1207. [Google Scholar] [CrossRef]
- Papadavid, E.; Dalamaga, M.; Kapniari, I.; Pantelidaki, E.; Papageorgiou, S.; Pappa, V.; Tsirigotis, P.; Dervenoulas, I.; Stavrianeas, N.; Rigopoulos, D. Lobomycosis: A case from Southeastern Europe and review of the literature. J Dermatol Case Rep 2012, 6, 65–69. [Google Scholar] [CrossRef]
- Bader-Larsen, K.S.; Larson, E.A.; Dalamaga, M.; Magkos, F. A Narrative Review of the Safety of Anti-COVID-19 Nutraceuticals for Patients with Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Giron, L.B.; Peluso, M.J.; Ding, J.; Kenny, G.; Zilberstein, N.F.; Koshy, J.; Hong, K.Y.; Rasmussen, H.; Miller, G.E.; Bishehsari, F.; et al. Markers of fungal translocation are elevated during post-acute sequelae of SARS-CoV-2 and induce NF-κB signaling. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Domingues, R.B.; Leite, F.; Senne, C. Cerebrospinal fluid analysis in patients with COVID-19-associated central nervous system manifestations: a systematic review. Arq Neuropsiquiatr 2022, 80, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Pache, F.; Körtvelyessy, P.; Jelčić, I.; Stettner, M.; Franciotta, D.; Keller, E.; Neumann, B.; Ringelstein, M.; Senel, M.; et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation 2022, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, H.; Kaczorowski, F.; San-Galli, A.; Michel, P.P.; Tressières, B.; Roze, E.; Quadrio, I.; Lannuzel, A. Cerebrospinal fluid biomarkers in SARS-CoV-2 patients with acute neurological syndromes. Rev Neurol (Paris) 2023, 179, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Guillot, F.; Garcia, A.; Salou, M.; Brouard, S.; Laplaud, D.A.; Nicot, A.B. Transcript analysis of laser capture microdissected white matter astrocytes and higher phenol sulfotransferase 1A1 expression during autoimmune neuroinflammation. J Neuroinflammation 2015, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Sans, H.M.; Forman, C.A.; Nylander, A.N.; Ho, H.E.; Lu, S.; Goldberg, S.A.; Hoh, R.; Tai, V.; Munter, S.E.; et al. Plasma Markers of Neurologic Injury and Inflammation in People With Self-Reported Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurol Neuroimmunol Neuroinflamm 2022, 9. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.-H.; Wood, J.; Yalçın, B.; Taylor, K.R.; Dutton, S.; Acosta-Alvarez, L.; et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. 2022. [Google Scholar] [CrossRef]
- Magdy, R.; Eid, R.A.; Fathy, W.; Abdel-Aziz, M.M.; Ibrahim, R.E.; Yehia, A.; Sheemy, M.S.; Hussein, M. Characteristics and Risk Factors of Persistent Neuropathic Pain in Recovered COVID-19 Patients. Pain Med 2022, 23, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G.; Mustapic, M.; Kapogiannis, D.; Henrich, T.J.; Lu, S.; Goldberg, S.A.; Hoh, R.; Chen, J.Y.; Martinez, E.O.; et al. SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19. Ann Neurol 2022, 91, 772–781. [Google Scholar] [CrossRef]
- Wu, J.; Tang, L.; Ma, Y.; Li, Y.; Zhang, D.; Li, Q.; Mei, H.; Hu, Y. Immunological Profiling of COVID-19 Patients with Pulmonary Sequelae. mBio 2021, 12, e0159921. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Apostolo, D.; D’Onghia, D.; Tonello, S.; Minisini, R.; Baricich, A.; Gramaglia, C.; Patrucco, F.; Zeppegno, P.; Acquaviva, A.; Balbo, P.E.; et al. Decreased Gas6 and sAxl Plasma Levels Are Associated with Hair Loss in COVID-19 Survivors. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Olivier, T.; Prasad, V. The definition of long COVID used in interventional studies. Eur J Clin Invest 2023, e13989. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, N.A.; Abou Shaar, B.; Taha, R.M.; Arabi, T.Z.; Sabbah, B.N.; Alkodaymi, M.S.; Omrani, O.A.; Makhzoum, T.; Almahfoudh, N.E.; Al-Hammad, Q.A.; et al. A systematic review of trials currently investigating therapeutic modalities for post-acute COVID-19 syndrome and registered on WHO International Clinical Trials Platform. Clin Microbiol Infect 2023, 29, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Sivan, M.; Delaney, B.; Evans, R.; Milne, R. Long covid-an update for primary care. Bmj 2022, 378, e072117. [Google Scholar] [CrossRef] [PubMed]
- Chadda, K.R.; Blakey, E.E.; Huang, C.L.; Jeevaratnam, K. Long COVID-19 and Postural Orthostatic Tachycardia Syndrome- Is Dysautonomia to Be Blamed? Front Cardiovasc Med 2022, 9, 860198. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Anglin, K.; Durstenfeld, M.S.; Martin, J.N.; Kelly, J.D.; Hsue, P.Y.; Henrich, T.J.; Deeks, S.G. Effect of Oral Nirmatrelvir on Long COVID Symptoms: 4 Cases and Rationale for Systematic Studies. Pathog Immun 2022, 7, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern Med 2023. [Google Scholar] [CrossRef]
- Bajema, K.L.; Berry, K.; Streja, E.; Rajeevan, N.; Li, Y.; Yan, L.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; Bohnert, A.; et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Lin, F.; Peyser, N.D.; Isasi, C.; Carton, T.W.; Henrich, T.J.; Deeks, S.G.; Olgin, J.E.; Pletcher, M.J.; et al. Association of Nirmatrelvir/Ritonavir Treatment with Long COVID Symptoms in an Online Cohort of Non-Hospitalized Individuals Experiencing Breakthrough SARS-CoV-2 Infection in the Omicron Era. 2023. [Google Scholar] [CrossRef]
- Parthasarathy, H.; Tandel, D.; Siddiqui, A.H.; Harshan, K.H. Metformin suppresses SARS-CoV-2 in cell culture. Virus Res 2022, 323, 199010. [Google Scholar] [CrossRef]
- Ventura-López, C.; Cervantes-Luevano, K.; Aguirre-Sánchez, J.S.; Flores-Caballero, J.C.; Alvarez-Delgado, C.; Bernaldez-Sarabia, J.; Sánchez-Campos, N.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Sander-Padilla, J.G.; et al. Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial. Biomed Pharmacother 2022, 152, 113223. [Google Scholar] [CrossRef] [PubMed]
- Daou, N.; Viader, A.; Cokol, M.; Nitzel, A.; Chakravarthy, M.V.; Afeyan, R.; Tramontin, T.; Marukian, S.; Hamill, M.J. A novel, multitargeted endogenous metabolic modulator composition impacts metabolism, inflammation, and fibrosis in nonalcoholic steatohepatitis-relevant primary human cell models. Sci Rep 2021, 11, 11861. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, L.E.M.; Cassar, M.P.; Koziel, M.J.; Pradines, J.; Lamlum, H.; Azer, K.; Kirby, D.; Montgomery, H.; Neubauer, S.; Valkovič, L.; Raman, B. Efficacy and tolerability of an endogenous metabolic modulator (AXA1125) in fatigue-predominant long COVID: a single-centre, double-blind, randomised controlled phase 2a pilot study. eClinicalMedicine. (In press). 2023, 101946. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ (accessed on 15 May 2023).
- Kritis, P.; Karampela, I.; Kokoris, S.; Dalamaga, M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol Open 2020, 8, 100066. [Google Scholar] [CrossRef]
| Main Characteristics | Acute severe COVID-19 | long COVID |
|---|---|---|
| Age | Older age | ↑ % of diagnoses between the ages 36-50 years |
| Gender | More frequent in males | More frequent in females |
| Predisposing factors & Comorbidities | -Older age, Obesity, T2DM, CVD, asthma or chronic lung disease, sickle cell disease, immunocompromised patients, hematologic malignancies, chronic kidney disease, patients under immunosuppressive treatments -Racial and ethnic minority groups -People with low income -Unvaccinated individuals |
-Obesity, T2DM, connective tissue disorders, allergic rhinitis, ADHD, -More frequent in Black and Hispanic Americans -People with low income -Poor rest in the early period after COVID-19 -↑ risk after severe COVID-19 -Most cases involve non-hospitalized patients with a mild acute COVID-19 |
| Laboratory findings & biomarkers | Lymphopenia, ↑CRP, ↑ Neutrophils, ↑ IL-6, ↑ IL-10, ↑ D-dimer, ↑ LDH, ↑ ferritin |
In certain patients: ↑ IL-6, ↑ CRP, ↑ D-dimer, detectable SARS-CoV-2 RNA in stool or gut mucosa, biomarkers of EBV reactivation, anti-IFN-α2 or anti-IFN-λAbs, ↑ ET-1 and ↓Ang-2, ↓ cortisol, metabolites of mitochondrial dysfunction, ↑% of CSF abnormalities and ↑ biomarkers of neuronal damage (For more details see Table 3) |
| CVD features | Pulmonary embolism, deep vein thrombosis, AMI, heart failure | Chest pain, palpitations, myocarditis, cardiac impairment, POTS |
| Neurologic features | Headache, Ischemic stroke, ataxia, seizures, anosmia, ageusia | Brain fog, fatigue, musculoskeletal pain, cognitive impairment, paresthesia, sleep disorder, dizziness, memory loss, dysautonomia, tinnitus |
| Pulmonary features | Cough, dyspnea, hypoxia, bilateral lung infiltrates | Cough, dyspnea, abnormal gas exchange, ground glass lung |
| Gastrointestinal features | Abdominal pain, nausea, vomiting, diarrhea, T1DM, ↑ transaminases | Abdominal pain, nausea |
| Endocrine abnormalities | ↑ T1DM, ↑ T2DM thyroiditis |
Diabetes, hypocortisolism |
| Dermatologic findings | Skin rashes (maculopapular, chilblain-like, urticarial, vesicular, livedoid, and petechial lesions), hair loss | Most common alopecia, various skin rashes |
| Renal manifestations | Acute Kidney Injury Acute Tubular Necrosis |
↓eGFR, |
| Manifestations from the reproductive system | Menstrual irregularities ↑ urinary frequency and nocturia |
Menstrual irregularities ↑ premenstrual symptoms Erectile dysfunction ↓ sperm count |
| General laboratory work-up |
| Complete blood count |
| Erythrocyte sedimentation rate |
| General biochemical tests: Glucose, HbA1c, urea, creatinine, electrolytes (sodium, potassium, calcium, phosphate) |
| Indices of hepatic function: AST, ALT, γ-GT, ALP |
| CPK, ferritin, LDH |
| Indices of cardiac function: troponin, BNP or NT-proBNP |
| Indices of thyroid function: TSH, fT4 |
| Indices of coagulation and fibrinolysis: D-dimer, fibrinogen, PT, aPTT |
| Indices of inflammation: CRP, IL-6 |
| Vitamins: 25-OHD, B12 |
| Autoantibodies and Complement: RF, anti-CCP, ANA, ACA, C3, C4 |
| Imaging and other function tests |
| High resolution Chest Computed Tomography |
| Computed Tomography Pulmonary Angiogram (CTPA) |
| Pulmonary function tests (spirometry, diffusion capacity, lung volumes) |
| Pulse Oxymetry |
| Six minute walk test (6MWT) |
| Electrocardiogram |
| Cardiac ultrasound |
| Cardiac magnetic resonance |
| Cardiopulmonary exercise testing (CPET) |
| Brain magnetic resonance |
| Questionnaires and clinical tests |
| Tilt table test for POTS or 10-Minute NASA Lean Test for orthostatic intolerance |
| Dyspnea scales (mMRC, NYHA) |
| Questionnaire SBQ-LC |
| Questionnaires for anxiety: BAI, HAM-A, GAD-7 |
| Questionnaires for depression: BDI, HAM-D, PHQ-2,-9 |
| Some Promising tests |
| Cortisol/ four-point salivary cortisol test |
| IgG, IgA, IgM |
| Natural Killer cell function tests |
| Panels for reactivated herpesviruses (EBV, CMV, VZV, HHV-6) |
| SARS-CoV-2 RNA in stool or gut mucosa |
| Endothelin-1 and Angiopoietin-2 |
| Hyperpolarized gas magnetic resonance of the lungs |
| Study, year | Study design/population | Main Findings | Utility |
|---|---|---|---|
| Biomarkers of systemic inflammation | |||
| Yong SJ et al., 2023 [150] | Meta-analysis of 23 studies (14 prospective and 9 retrospective case control) with 18 meta-analyzed biomarkers | -↑ CRP, D-dimer, LDH and leukocytes in LC patients than those without LC -↑ lymphocytes and IL-6 in LC patients than those without LC -↑ IL-8 in LC than healthy controls |
-Diagnostic utility in LC; however small effects -↑ D-dimer, LDH, and lymphocytes in patients with organ abnormalities -↑ IL-6 in patients with symptom persistence - ↑ LDH, leucocytes, NT-pro-BNP in patients with duration of symptoms <6 months -↑ D-dimer in patients with duration of symptoms ≥6 months |
| Yin JX et al., 2022 [151] | Meta-analysis of 22 case-control observational studies | -↑ IL-6 levels in LC patients than controls -↑ IL-6 levels in LC patients than those with non-postacute sequelae of severe COVID-19 -↑ IL-6 levels in patients with acute COVID-19 than patients with LC |
-IL-6 may predict LC -IL-6 may characterize early stage of LC. |
| Lopez-Leon S et al., 2021 [15] | 21 meta-analyses on the prevalence of long-term effects in LC, 15 studies, 47,910 patients (age: 17–87 years). | -↑ CRP in 8% of patients (95% CI: 5–12) -↑ ferritin in 8% of patients (95% CI: 4–14) -↑ procalcitonin in 4% of patients (95% CI: 2–9) -↑ IL-6 in 3% of patients (95% CI: 1–7) |
Limited evidence for systemic inflammation after 4 months approximately post-viral infection. |
| Immune profiling in long COVID | |||
| Klein J et al., 2022 [152] | 220 participants (101 LC, 41 CC, 41 HC, and 37 HCW) at >400 days post infection Cross-sectional study |
LC is characterized by: ↑ non-classical monocytes, ↑ activated B cells, ↑ double-negative B cells, ↑ exhausted T cells, ↑ IL-4/IL-6 secreting CD4 T cells, ↓ conventional DC1 ↓ central memory CD4 T cells |
Integration of immune phenotyping data into machine learning models may identify distinguishing features in the classification of LC |
| Haunhorst S et al., 2022 [153] | Scoping review of 3 studies examining Tregs in LC | -Galán M et al. [154] 2.5X ↑Tregs in LC compared to subjects that recovered completely -Utrero-Rico et al. [155] ↓ Tregs in LC compared to subjects that recovered completely - ↓ Tregs in LC compared to seronegative controls [156] |
No firm conclusions can be drawn about Treg alterations in LC |
| Phetsouphanh C et al., 2022 [85] | LC group (n= 31) asymptomatic matched controls/MC (n = 31) compared to individuals infected with common cold coronavirus (HCoV) (n= 25) and unexposed healthy donors (n= 13) |
↑ activated innate immune cells, ↓ naive T and B cells ↑ expression of type I IFN (IFN-β) and type III IFN (IFN-λ1) that remained persistently high at 8 months after infection. |
|
| Patterson BK et al., 2021 [156] | Case-control & longitudinal study 121 cases with LC 29 healthy controls 26 cases with mild-moderate COVID-19 48 cases with severe COVID-19 |
-↑ B-cells in LC compared to HC -↑ inflammatory monocytes CD14+, CD16+, CCR5+ compared to HC -↓CD4+ and CD8+ T-cells expressing PD-1 (exhausted lymphocytes), and T-reg in LC compared to HC |
-↑ Inflammatory monocytes and ↓ exhausted lymphocytes detected in patients who later developed LC (predictive biomarkers). - ↑ Inflammatory monocytes and ↑ exhausted lymphocytes in the convalescence period of LC |
| Biomarkers reflecting SARS-CoV-2 persistence | |||
| Swank Z et al., 2022 [157] | 63 individuals previously infected with SARS-CoV-2, 37 of whom were diagnosed with LC |
↑ circulating Spike protein in LC cases, but not in convalescent controls up to 12 months | SARS-CoV-2 viral reservoirs may persist in the body |
| Natarajan A et al., 2022 [158] | 113 individuals with mild to moderate COVID-19 Longitudinal study |
- SARS-CoV-2 RNA in the feces at 4 months after diagnosis (12.7%) - SARS-CoV-2 RNA in the feces at 7 months after diagnosis (3.8%) |
GI symptoms (abdominal pain, nausea, vomiting) are related with fecal shedding of SARS-CoV-2 RNA |
| Stein SR et al., 2022 [87] | Autopsies on 44 patients who died with COVID-19 | - Persistent SARS-CoV-2 RNA in multiple anatomic sites, including throughout the brain, as late as 230 day - ≥50% of late cases had persistent RNA in the myocardium, lymph nodes from the head and neck and from the thorax, sciatic nerve, ocular tissue, and in all sampled regions of the CNS, except the dura mater. |
SARS-CoV-2 can cause systemic infection and persist in the body for months in certain patients |
| Tejerina F et al., 2022 [159] | Cohort of 29 patients who reported persistent symptoms at least 4w after COVID-19 | - 45% had positive plasma RT-PCR results -51% were positive in at least one RT-PCR sample (plasma, urine, or stool). |
Potential systemic viral persistence associated with persistent symptoms |
| Zollner A et al., 2022 [160] | Cohort of 46 patients with IBD 219 days (range, 94-257) after confirmed COVID-19 | - SARS-CoV-2 RNA in the gut mucosa ∼7 months after mild acute COVID-19 in 69.5% - Nucleocapsid protein persisted in 52.2% patients in gut epithelium and CD8+ T cells. -Not detectable viral antigens in stool |
-Viral persistence markers associated with symptoms of LC |
| Yonker LM et al., 2021 [161] | 19 children with MIS-C, 26 with acute COVID-19, and 55 controls. | -89% of cases with MIS-C had GI symptoms versus 27% of children with acute COVID-19 - Detectable viral loads in the stool (1.5 × 102 to 2.5 × 107 RNA copies/mL) in the majority of cases -↑ plasma zonulin and LBP compared to controls - ↑ SARS-CoV-2 spike, S1, and nucleocapsid antigens compared to controls |
- Prolonged exposure to SARS-CoV-2 in the GI tract of children with MIS -Loss of mucosal barrier integrity -SARS-CoV-2 antigenemia may trigger the hyperinflammatory responses defining MIS-C |
| Humoral and cellular response against SARS-CoV-2 in long COVID | |||
| Klein J et al., 2022 [152] | 220 participants (101 LC, 41 CC, 41 HC, and 37 HCW) at >400 days post infection Cross-sectional study |
↑ IgG against Spike and S1 in LC compared to vaccination-matched controls | -Potential predictive biomarker of LC |
| Files JK et al., 2021 [162] | A longitudinal study of 50 cases with COVID-19: -20 with persistent symptoms (30-208 days) -30 with symptom resolution within 20 days. |
In patients with persistent symptoms: -↑ IgG avidity to SARS-CoV-2 spike protein - sustained T cell response magnitudes - ↑ antigen-specific CD4+ T cell responses against Spike (late phase) - ↑ antigen-specific CD8+ T cell populations |
↑ T cell response magnitude in individuals with prolonged symptoms |
| García-Abellán J et al., 2021 [89] | Prospective, longitudinal study of 146 hospitalized COVID-19 patients | ↓ peak IgG against Spike associated with LC symptoms at 6 months | -Potential predictive biomarker of LC |
| Augustin M et al., 2021 [163] | A longitudinal prospective cohort study of 958 non- hospitalized patients with confirmed COVID-19 | ↓ baseline levels of IgG against S1 domain of the spike associated with long-term symptoms | -Potential predictive biomarker of LC |
| Blomberg B et al., 2021 [164] | A prospective cohort study of 312 patients (247 home-isolated and 65 hospitalized) | -↑ IgG against spike protein and -↑ microneutralizing antibody titers detected after 2 months independently associated with both persistent fatigue and total number of symptoms at 6 months | -Increased antibody titers predictive biomarker of LC symptoms |
| Peluso MJ et al., 2021 [165] | A prospective cohort study of 70 individuals with PCR-confirmed COVID-19 | - No significant differences in early and late (1 and 4 months) antibody levels (IgG against SARS-CoV-2 Spike, RBD, and two preparations of the N protein) between cases with and without persistent symptoms. - No differences in T cell responses at initial time point between cases with and without persistent symptoms -↓ CD8+ T cells expressing CD107a against N peptide in cases with LC -↓ N-specific interferon-γ-producing CD8+ T cells in cases with LC |
|
| Biomarkers reflecting reactivation of latent viruses | |||
| Peluso MJ et al., 2023 [166] | A cohort of 280 adults with prior COVID-19 | -↑ early antigen-diffuse EBV IgG positivity associated with fatigue at 4m -↑ IgG against EBNA associated with fatigue and neurocognitive dysfunction, at 4 m after infection |
Reactivation of EBV is associated with fatigue and cognitive dysfunction in LC |
| Klein J et al., 2022 [152] | 220 participants (101 LC, 41 CC, 41 HC, and 37 HCW) at >400 days post infection Cross-sectional study |
↑ titers of anti-EBV antibodies, even though overall seroprevalence is not different from HC or CC | Altered humoral responses to distinct herpesviruses in LC as predictive biomarker |
| Zubchenko S et al., 2022 [167] | 88 patients with LC | - EBV reactivation in 42.6% -HHV6 reactivation in 25.0% -EBV plus HHV6 reactivation in 32.4% |
Reactivation was associated with ↑ slight fever temperature, headache, psycho-neurological disorders, pulmonary abnormalities and myalgia, liver enzymes, CRP and D-dimer, and ↓ cellular immune response |
| Su Y et al., 2022 [168] | A cohort of 309 COVID-19 patients from initial diagnosis to convalescence (2-3 months later) 457 HC |
-EBV viremia at T1 was associated with LC (fatigue and respiratory symptoms) at 3m post-infection | -EBV viremia may be a predictive biomarker of LC |
| Gold JE et al., 2021 [169] | A cohort of randomly 185 surveyed COVID-19 patients -56 developed LC |
-↑ EBV early antigen-diffuse IgG or ↑EBV viral capsid antigen (VCA) IgM in 66.7% of LC versus 10% of controls | -EBV reactivation may be a predictive biomarker of LC |
| Biomarkers reflecting autoimmunity | |||
| Muri J et al., 2023 [170] | A cohort of 71 COVID-19 convalescent patients at month 6 (on average) post disease onset, 23 HC | -IgG antibodies against chemokines CCL21, CXCL13 and CXCL16 at month 6 distinguished LC from no LC groups - Levels of CCL21, CXCL13 and CXCL16 predicted the absence of persistent symptoms with 77.8% accuracy |
-Ab against specific chemokines were associated with favorable disease outcome and negatively correlated with the development of LC at 1 year post-infection. |
| Bodansky A et al., 2023 [171] | A cohort of 121 individuals with LC, 64 with prior COVID-19 and full recovery, and 57 pre-COVID controls. | - Significant differences in autoreactivity between COVID-19 patients and pre-COVID controls. -No patterns of autoreactivity that separate individuals with LC to individuals fully recovered from COVID-19. |
Absence of LC specific autoreactivities |
| Son K et al., 2023 [172] | A cohort of 106 convalescent COVID-19 patients with varying acute phase severities at 3, 6 and 12 months post-recovery, 22 HCs and 34 with other respiratory infections | -Abs to U1-snRNP and anti-SS-B/La were both positively associated with persistent symptoms of fatigue and dyspnea - Pro-inflammatory cytokines such as TNF-α and CRP predicted ↑ ANAs at 12 months. |
Persistently positive ANAs at 12 months post-COVID are associated with persisting symptoms and inflammation in a subset of COVID-19 survivors |
| Franke C et al., 2023 [173] | A prospective study of 50 patients with reported cognitive problems | -92% had normal routine CSF parameters -52% had anti-neuronal Abs (n = 9 in serum only, n = 3 in CSF only and n = 14 in both, including those against myelin, Yo, Ma2/Ta, GAD65 and NMDA receptor, but also a variety of undetermined epitopes on brain sections). |
Abnormal cognitive status is associated with anti-neuronal Abs in CSF |
| Peluso MJ et al., 2022 [174] |
A prospective study of 215 participants with convalescent COVID-19 tested over 394 time points, including 121 people with LC | -2 out of 215 had IFN-α2-specific autoantibodies across all sample timepoints | No detectable anti-IFN-α2 antibodies in LC |
| Klein J et al., 2022 [152] | 220 participants (101 LC, 41 CC, 41 HC, and 37 HCW) Cross-sectional study |
No significant differences in the total number of autoantibody reactivities per participant across groups using REAP, a high throughput method for the measurement of Ab reactivity against >6,000 extracellular and secreted human proteins |
No specific autoAbs that could differentiate participants with LC from controls |
| Su Y et al., 2022 [168] | A cohort of 309 COVID-19 patients from initial diagnosis to convalescence (2-3 months later). 457 HC Determination of an auto-Ab panel: anti-IFN-α2, and 5 anti-nuclear auto-Abs (Ro/SS-A, La/SS-B, U1-snRNP, Jo-1, and P1) at clinical diagnosis and convalescence |
- Patients with autoAbs at convalescence (44%) already exhibited mature (class-switched) auto-Abs as early as at diagnosis (56%). -Inverse correlations between SARS-CoV-2 IgGs (class-switched) and autoAbs. - |
- IFN-α2 autoAbs uniquely associated with respiratory symptoms of LC - ↑ multiple autoAbs at convalescence are associated with GI symptoms and sputum production of LC |
| Rojas M et al., 2022 [175] | Case-control study 100 patients with LC with a median post-COVID time: 219 (IQR: 143 to 258) days. 30 pre-pandemic HC |
-Latent autoimmunity in 83% of patients -Polyautoimmunity in 62% of patients -anti-IFN autoAbs in 5-10% of patients -Anti-SARS-CoV-2 IgG correlated with autoAbs |
-IgG anti-IFN-λ Abs were associated with the persistence of respiratory symptoms. -Latent autoimmunity correlates with Ab response against SARS-CoV-2 |
| Endothelial or vascular biomarkers | |||
| Patel MA et al., 2022 [176] | A case-control study of 23 LC patients, one to six months post-infection, and 23 ward COVID patients, 23 ICU COVID patients and 23 HCs | -Angiogenesis markers (ANG-1 and P-SEL) had excellent sensitivity and specificity for LC status (AUC = 1.00) among 16 blood biomarkers of vascular transformation. -ANG-1 levels were associated with female sex and a lack of disease interventions at follow-up |
-Diagnostic utility and classification of LC status (accuracy 96%) |
| Haffke M et al., 2022 [177] | A case-control study of 30 LC patients with persistent fatigue and exertion intolerance (14 with 14 post-COVID ME/CFS) and 15 age- and sex matched seronegative HCs |
-↑ ET-1 concentration in both ME/CFS and LC patients compared to HCs and post-convalescent controls -↓ Ang-2 in both LC patients and post-convalescent controls compared to HCs |
-LC patients with fatigue present ↑ ET-1 and ↓ Ang-2 - Ang-2 levels exclusively in LC could be a differentiation biomarker between PCS and ME/CFS |
| Tong M et al., 2022 [178] | A cross-sectional study of 345 COVID-19 (39% had LC symptoms) and 119 age and gender-matched HCWs | -No significant differences in vascular biomarkers (serum levels of VCAM-1, ICAM-1, P-selectin) between COVID-19 survivors and controls | |
| Biomarkers of coagulation and fibrinolysis | |||
| Yong SJ et al., 2023 [150] | Meta-analysis of 23 studies (14 prospective and 9 retrospective case control) with 18 meta-analyzed biomarkers | -↑ D-dimer, CRP, LDH and leukocytes in LC patients than those without LC -↑ lymphocytes and IL-6 in LC patients than those without LC -↑ IL-8 in LC than healthy controls |
-Diagnostic utility in LC; however small effects -↑ D-dimer, LDH, and lymphocytes in patients with organ abnormalities -↑ D-dimer in patients with duration of symptoms ≥6 months |
| Constantinescu-Bercu A et al., 2023 [179] | A cohort of 21 patients with LC with a median time of 23 months of follow up and controls | -↑ platelet binding on both collagen and anti-VWF A3 in patients with LC compared with controls, which positively correlated with VWF, VWF(Ag):ADAMTS13 ratio, and inversely correlated with ADAMTS13 activity | -LC is a prothrombotic state |
| Di Gennaro L et al., 2022 [180] | A case-control study of 75 children with previously confirmed COVID-19 in a pediatric post-covid unit ≥ 8 weeks after the initial infection | -↑ D-Dimer levels in LC children compared to children that had fully recovered at the 8-12 weeks (p = 0.04), and 12 week follow up or more (p = 0.05), and in children with three or more symptoms at 12 weeks (p = 0.002). -No significant differences in other coagulation factors between LC children and controls |
Abnormal D-dimer levels in children with LC and more symptoms |
| Kruger et al., 2022 [181] | A case-control study of 99 LC patients and 29 HCs. | -↑ platelet factor 4 (PF4), VWF, and α-2 antiplasmin (α-2-AP) in LC - ↓ plasma kallikrein in LC - Significant platelet hyperactivation was noted in LC |
Failed fibrinolytic system in LC |
| Pretorius E et al., 2021 [182] | A cross sectional study of 11 patients with LC, 13 HCs, 15 with COVID-19 (before treatment), 10 with T2DM | - Microclots in both acute COVID-19 and LC plasma samples are resistant to fibrinolysis (compared to plasma from controls and T2DM) -↑ SAA in LC than controls - Platelets from LC patients are hyperactivated -in the digested clots ↑ α2 antiplasmin, plasminogen, coagulation factor XIII A chain, vWF, fibrinogen alpha chain, C7, CRP in LC than controls |
Abnormal fibrinolysis and coagulopathy in LC |
| Hormonal and metabolic biomarkers | |||
| Al-Hakeim HK et al., 2023 [124] | Retrospective cohort study with controls 86 patients with LC 39 HC |
-↑ HOMA2-IR, fasting blood glucose, and insulin levels in LC -33.7% of patients vs 0% of controls had HOMA-IR >1.8 |
- LC is associated with new-onset IR -↑ IR was significantly associated with depressive symptoms |
| Su Y et al., 2022 [168] | A cohort of 309 COVID-19 patients from initial diagnosis to convalescence (2-3 months later) 457 HC |
-↓ cortisol and cortisone in patients with LC at convalescence - ↑ proteins associated with the negative regulation of the circadian sleep/wake cycle in patients with neurological symptoms |
- Patients with respiratory symptoms at convalescence exhibited ↓ cortisol and cortisone |
| Klein J et al., 2022 [152] | 220 participants (101 LC, 41 CC, 41 HC, and 37 HCW) Cross-sectional study |
↓ serum cortisol in LC ↑ in healthy (uninfected) controls, ↓ in convalescent controls, and ↓↓ in cases with LC |
-Serum cortisol was the most significant individual predictor of LC |
| Sunada N et al., 2022 [183] | 186 patients with LC Retrospective analysis |
-↓ serum GH and ↑ serum FT4 in patients with general fatigue -↓cortisol in patients with dysosmia/dysgeusia -↑ serum TSH and ↓ ratio of FT4/TSH in initial severe LC cases |
|
| Other proteins as biomarkers | |||
| The PHOSP-COVID Collaborative Group, 2022 [81] | A prospective, longitudinal cohort study 2,320 participants discharged from hospital; 807 (32·7%) participants completed 5-month and 1-year visits. |
Plasma proteome data for 296 protein features: -↑ of 13 proteins in the severe group of LC |
-↑ IL-6 and CD70 in cognitive impairment cluster compared with the mild cluster |
| Captur G et al., 2022 [184] | A nested longitudinal proteomic case-control study of 156 healthcare workers | Differentially abundant proteins in HCW with persistent symptoms (>6w): proteins with lipid, atherosclerosis and cholesterol metabolism pathways, complement and coagulation cascades, autophagy, and lysosomal function | Potential predictive value for LC at the time of seroconversion |
| Metabolites as biomarkers | |||
| López-Hernández Y et al., 2023 [185] | A longitudinal retrospective analysis of COVID-19 patients (n=22), LC patients (n=25) and controls (n=15) | - Fatigue (59%) and musculoskeletal (50%) were most relevant and persistent symptoms. - Sterols, bile acids, isoprenoids, and fatty esters were affected in both COVID-19 and postCOVID-19 patients -↑ species of phosphatidylcholines and sphingomyelins in LC compared to controls |
- Dysregulation in sphingolipid metabolism could be associated with fatigue and muscular pain |
| López-Hernández Y et al., 2023 [186] | A retrospective longitudinal analysis of 108 participants (HC, COVID-19 and LC patients) | -↑ Lactic acid, lactate/pyruvate ratio, ornithine/citrulline ratio, sarcosine, and arginine in LC -↑ IL-17 in LC |
- Mitochondrial dysfunction, redox state imbalance, impaired energy metabolism, and chronic immune dysregulation in LC |
| Guntur VP et al., 2022 [134] | Case-control study of 29 patients with LC, 16 CC and 30 HC | -↑ free- and carnitine-conjugated mono-, poly-, and highly unsaturated fatty acids, ↓ levels of mono-,di- and tricarboxylates, polyamines (spermine) and taurine in LC -milder disturbances in fatty acid metabolism and ↑ spermine and taurine in recovered patients -tryptophan depletion not normalized in LC |
-Altered fatty acid metabolism and dysfunctional mitochondria-dependent lipid catabolism associated with mitochondrial dysfunction during exercise |
| Cysique LA et al., 2022 [187] | A prospective study of 128 SARS-CoV-2 positive patients | - ↑ quinolinic acid, 3-hydroxyanthranilic acid, and kynurenine were significantly associated with cognitive decline. | The kynurenine pathway metabolites are potential therapeutic targets for COVID-19-related cognitive impairment. |
| Microbiota alterations in long COVID | |||
| Zhang D et al., 2023 [188] | A prospective follow-up study of 187 recovered subjects; 84 reported LC symptoms at one-year after discharge; 32 HCs 16S rRNA sequencing of stool samples |
- Gut microbiota dysbiosis in symptomatic recovered patients -↓ bacterial diversities in LC -↓ relative abundance of SCFAs-producing salutary symbionts, such as Eubacterium_hallii group, Subdoligranulum, Ruminococcus, Dorea, Coprococcus, and Eubacterium_ventriosum group in LC |
-Gut microbiota dysbiosis in recovered patients at one-year after discharge -Gut microbiota dysbiosis in LC |
| Liu Q et al., 2022 [189] | A prospective study of 106 patients with a spectrum of COVID-19 severity from admission to 6 months and 68 non-COVID-19 controls | -↑ Ruminococcus gnavus, Bacteroides vulgatus in LC -↓ Faecalibacterium prausnitzii in LC -↓ Butyrate-producing bacteria in LC |
- Gut microbiota composition at admission was predictive of LC occurrence. |
| Cerebrospinal fluid biomarkers | |||
| Etter MM et al., 2022 [129] | A cohort study of 40 neuro-COVID patients; 25 HCs and 25 non-MS inflammatory neurological disease controls | - CSF levels: ↑ pro-inflammatory proteins (TNFRSF9, IFN-γ) and ↓ anti-inflammatory mediators (TRANCE(RANKL), TRAIL) are predictive for LC - plasma CLM-6, MCP-3 and ST1A1 may predict LC |
-Prediction of LC |
| Apple AC et al., 2022 [190] | A case-control study of 22 participants with cognitive LC and 10 cognitive controls within the prospective study LIINC | - CSF abnormality in 77% of LC patients versus 0% of cognitive controls - Normal values for CSF white blood cells, glucose, calculated CSF/serum albumin ratio, IgG index, CSF IgG level, and serum IgG level in all participants - Abnormal oligoclonal banding in 69% of LC patients versus 0% of cognitive controls |
- ↑% of CSF abnormalities in patients with LC and cognitive impairment after mild COVID-19 |
| Guasp M et al., 2022 [191] | A prospective study of 60 hospitalized neuro-COVID patients, 25 of them with encephalopathy and 14 with encephalitis. 46 serum samples from HCs and 24 CSF samples from subjects with mild subjective cognitive complaints Follow-up: 18 months | - ↑ IL-18, IL-6, and IL-8 in both serum and CSF in neuro-COVID patients compared to HCs -↑ 14-3-3, NfL and IL-18, IL-1RA and IL-8 are associated with acute COVID-19 severity -↑ CSF 14-3-3 and NfL correlate with the degree of neurologic disability in the daily activities at 18 months |
- ↑ CSF levels of neuronal damage biomarkers during the acute phase of COVID-19 are prognostic biomarkers of worse long-term clinical outcome of patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





