Introduction
Armand Trousseau’s observation dating back nearly a century and a half from the association of thrombo-embolic disease and cancer has been widely validated in clinical practice. The relationship between thrombosis and cancer is in fact reciprocal: cancer predisposes to the onset of thrombosis and the development of the tumor process is linked to this state of hypercoagulability (1).
The risk of venous thromboembolic disease is increased in cancer patients (Relative Risk (RR) * 3 to 6) and is doubled in the case of associated chemotherapy (2). The unexpected occurrence of thrombosis may reveal occult neoplasia in 10 to 25% of cases. There is a close relationship between idiopathic thrombosis and cancer. The development of neoplasia in patients with idiopathic bilateral venous thrombosis is more frequent than in unilateral episodes. Also, the risk of discovery of occult cancer is 2 to 3 times higher in the first year with an incidence ranging from 7 to 20%. Cancer doubles the risk of postoperative thrombosis and triples the risk of fatal pulmonary embolism.
Cancer is therefore associated with a state of hypercoagulability manifested by clinical thrombotic episodes or biological abnormalities of coagulation which are responsible for the adverse evolution and the greatest morbimortality (3). It appears that survival is significantly shortened in the case of associated VTE. This earlier death may be related to massive pulmonary embolism or major haemorrhage induced by anticoagulants reflecting greater comorbidity or even metastatic evolution. Venous thrombosis is the second leading cause of death in cancer patients.
In this landscape, thrombosis appears to be a major issue. For a long time, generations of doctors have learned that thrombosis can reveal cancer. Today, the link between haemostasis / thrombosis and tumor development is established, both from information derived from basic research and from clinical and epidemiological data.
For a long time, several studies and research have been carried out in this area, some highlighting the strong link between venous thromboembolic disease and cancer, while others highlight the high prevalence of cancer patients who have died from pulmonary embolism, and thus confirming the major role of these events in cancer mortality. Nevertheless, the predominance of pulmonary embolism in a cancerous environment is still underestimated, as the incidence of asymptomatic pulmonary embolism is not well known. It is increasingly common to find cases of asymptomatic pulmonary embolism on thoracic CT scans performed during the overall management of cancer patients.
The aim of our study is to determine the local prevalence of asymptomatic pulmonary embolism in cancer patients, the characteristics of patients developing this type of event, which is most often underestimated, and the fate of these patients during the evolution of their cancer pathology.
Patients and Methods
This is a prospective study carried out on patients attending the Radiology department, exploring a thorax scannographic examination over a period of 12 months from March 1, 2022 to February 28, 2023. This study concerned two types of patients, on the one hand hospitalized patients for diagnostic and therapeutic treatment of a cancerous disease, and on the other hand cancer patients, followed on an outpatient basis to monitor the post-therapeutic evolution. These patients are explored by thoracic computed tomography (CAT) scans for a number of reasons, mainly as part of the extension of cancer disease, or search for a recurrence treatment. The search for asymptomatic pulmonary embolism cases consisted of triage of the thoracic tomodensitometry from the Radioloy department on a weekly basis, and then analyzing.
In the case of a positive diagnosis of asymptomatic pulmonary embolism, accurate analysis of the patient’s entire medical record is used to verify the indication of the CT scan and to seek clinical details that may reclassify pulmonary embolism as symptomatic, including clinical symptoms that have not been noticed or attributed to other causes, such as dyspnea, cough, hemoptysis, chest pain, or symptomatology in favor of deep venous thrombosis Unnoticed. Also, all of the venous thromboembolic disease (diagnosis and treatment) has been documented. Details on the subsequent management of these patients were also recorded, mainly, anticoagulant therapy and its hemorrhagic complications, asymptomatic deep vein thrombosis by venous Doppler Ultrasound examination of the lower limbs, as well as the search for complications of a possible asymptomatic pulmonary embolism passed unnoticed (post embolic PAH ...).
At the same time, the analysis of the clinical files covered the clinical data of each patient, ie age, sex, cancer pathology and its evolutionary profile: Type of tumor, stage, therapeutics Chemotherapy (active principle and therapeutic protocol), radiotherapy, as well as surgery. Our analysis also focused on the search for a history of venous thromboembolic disease and its risk factors for each patient.
Patients were considered to have had recent chemotherapy if they had received treatment within 30 days prior to their CT scan. Also, details regarding recent surgery, radiation therapy or hormone therapy as well as the state of coagulation were recorded.
In the oncology department, a standard protocol is used to organize the pathway of the patient with a cancerous pathology, in particular with regard to diagnosis and spread of the disease, post-therapeutic surveillance as well as the restoration of malignant tumors and finally the search for a possible recurrence. This protocol is based on a battery of biological and radiological examinations based mainly on the realization of a thoraco-abdomino-pelvic computed tomography with contrast enhancement. Only patients undergoing this imaging protocol were recruited during the course of this study.
Patients with clinical suspicion of pulmonary embolism, patients not receiving thoracic CT scan, and patients with contraindications to iodinated contrast media (iodine allergy, renal failure, and pregnancy) were excluded of asymptomatic pulmonary embolism.
In addition, patients requiring different imaging protocols (eg, devoted to the liver, pancreas, or kidneys) were also excluded. The ethics approval was obtained from the heads of the diagnostic imaging department and the medical oncology department of the Saint André hospital, in order to be able to exploit the data of the patients’ medical records.
All examinations that have detected asymptomatic pulmonary embolism are reviewed by two radiologists working in this unit.
Results
From March 1, 2022 to February 28 th, 2023, of the 389 thoracic CT scans meeting the inclusion criteria from the Radiology Department, 12 asymptomatic pulmonary embolisms were detected, ie 3.1% of all the patients analyzed.
In our series, 10 patients are male, against 2 female patients. The mean age of the entire group was 67 years (the ages ranged from 46 years to 84 years).
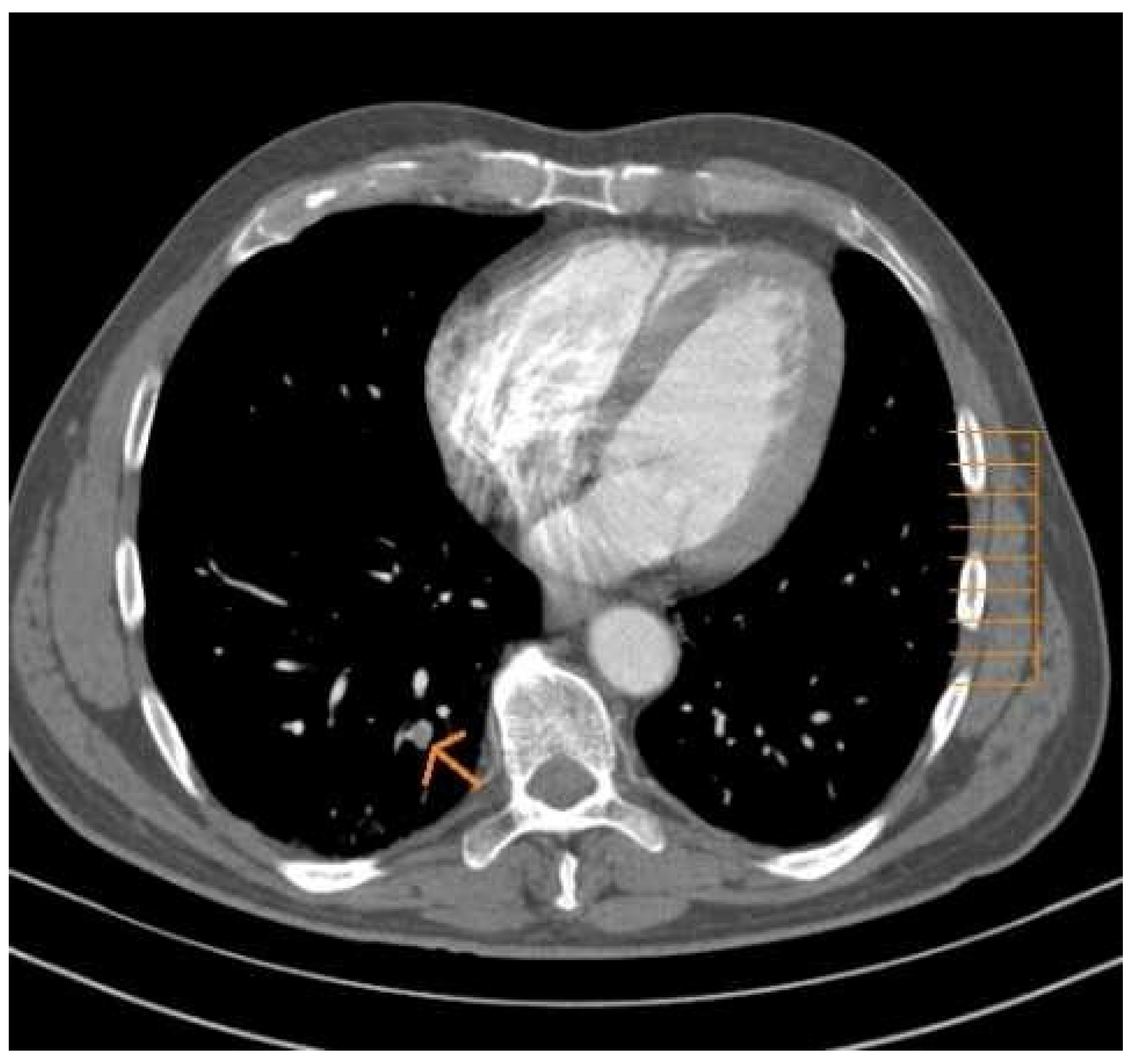
Asymptomatic pulmonary embolism was proximal localization in 4 patients, whereas it was considered peripheral, involving a segmental or sub-segmental arterial branch in 8 patients.
All patients in our series were carriers of a metastatic cancer. Also, 10 patients were undergoing anti-cancer therapeutic protocol based on Chemotherapy, either isolated or in combination with radiotherapy and / or surgery or with hormone therapy.
3 patients in our series were hospitalized at diagnosis, while the remaining patients (9 patients) were followed on an outpatient basis for post-therapeutic monitoring of their neoplastic pathology.
10 patients in our series are carriers of a carcinoma type neoplasm. 1 patient has a melanoma. Finally, 1 patient carries a lymphoma.
Regarding therapeutic management, 10 patients underwent chemotherapy, 8/10 of which were mono-chemotherapy-based, while 2/10 of these patients underwent multidrug therapy.
3 patients also underwent radiotherapy sessions according to the therapeutic requirements of the tumor stage. On the other hand, the association of radiotherapy and chemotherapy was objectified in 2 cases.
Surgery was in fact the cornerstone at the beginning of the oncological therapeutic protocol in 7 cases of our series, mainly with respect to kidney and colon cancer.
A patient with prostatic adenocarcinoma is put on chemotherapy in combination with anti-androgenic hormone therapy.
Finally, therapeutic abstention was observed in a single patient monitored semi-annually for splenic lymphoma in the marginal zone (Stage I of the Ann ARBOR classification of malignant lymphomas).
Concerning venous thromboembolic disease, 2 patients with asymptomatic pulmonary embolism have a history of deep venous thrombosis, the first patient presented a table of proximal pulmonary embolism associated with a thrombosis in the vena cava, which led to the discovery of its cancerous pathology, while the second patient, had splenomesenteric trunk thrombosis as antecedent. The rest of the patients have no history of thromboembolism.
Following the discovery of asymptomatic embolism, all patients in our series were treated with low-molecular-weight heparin (LMWH) curative-dose anticoagulant therapy in 10 patients, fondaparinux 7.5 mg in one Patient and finally based on ORGARAN with relay by long-term K antivitamins in a patient who presented an allergy to heparin during an old thromboembolic event. Also, 4 patients were examined by an Doppler Ultrasounds examination for deep vein thrombosis of the lower limbs. All Doppler Ultrasounds reported the existence of DVT, active in 1/4 patients with superior pole thrombus at the superficial femoral level, sequellar in 2 patients, whereas Doppler Ultrasound was normal in a single patient.
The follow-up of patients on anticoagulant therapy did not reveal any particular haemorrhagic complications. Also, all patients are still alive with stabilization of their carcinological status.
Discussion
Asymptomatic pulmonary embolisms are not uncommon in daily routine practice, especially in patients with high thromboembolic risk, particularly in oncological settings. The incidence of asymptomatic pulmonary embolism varies Depending on the population studied, the diagnostic criteria, and the diagnostic techniques used, in particular, in thoracic imaging, some studies have reported an incidence ranging from 0.5% to 5% (20, 21).
In a recent study, the medical records of 1921 cancer patients, having already started chemotherapy-based therapeutic protocols were examined. Overall, there were 101 (5.3%) venous thromboembolic events, of which 62 (3.2%) were asymptomatic, and more than one third (24/62) were pulmonary embolisms (22).
In another retrospective study involving 435 thoraco-abdomino-pelvic scans performed in a group of patients with cancer, pulmonary embolism was detected in 13 computed tomography (3.3%), While 6.8% of patients had asymptomatic iliofemoral DVT and 1.2% had common Iliac DVT (
Figure 1). The association DVT / PE was more common in inpatients (p = 0.002, 0.004, relative risk [RR] = 1.6 / 2.1 respectively) and this was observed mainly in patients with a metastatic stage of their cancer pathology (23).
The moderate variation in the incidence rate of asymptomatic pulmonary embolism could be attributed to the heterogeneity of the patient population studied. However, the technique of computed tomography, including the thickness of the cut, also plays a very important role. The scan symptomatology of PE has been well established (24,25). The analysis of the segmental and sub-segmental branches is made more and more possible by means of thinner collimation (26), which significantly improves the detection capabilities of peripheral, segmental and sub-segmental pulmonary embolisms. By decreasing the collimation thickness by 3 to 1 mm, there is an improvement of about 40% in the detection potential of sub- segmental PEs (27).
Furthermore, accidental detection of asymptomatic pulmonary embolisms has been reported in several series of patients using cutting thicknesses of 5 to 10 mm. Winston found PEs in 0.96% of the 1879 patients who had Thoracic CT scan for several indications, including assessment of tumor pathology, and trauma (28). Gosselin objectivized asymptomatic pulmonary embolism in 1.5% of 785 patients with thoracic CT with a cut thickness of 5 to 8 mm (29). The majority of patients in this study were carriers of neoplasia. Asymptomatic pulmonary embolism was observed in 2.5% of the 364 thoracic CT performed with cutting thickness between 5 and 8 mm.
Also, 2.1% of asymptomatic pulmonary embolism was observed by Boswell, in a group of 2085 cancer patients with a collimation of the order of 2mm, only 0.4% (9 patients) had a significant pulmonary embolism or A pulmonary embolism at the right or left pulmonary artery (15).
Thus, cancer patients are often subjected to multiple thoracic scannographic investigations for several reasons, but mainly for the restaging of the neoplastic pathology, as well as surveillance under specific treatment (Surgery, radiotherapy or chemotherapy), and which will reveal asymptomatic pulmonary embolism in a considerable percentage of patients. The best method of detection is to have a fenestration specifically adapted to the pulmonary arterial tree, since the usual parenchymal window does not allow visualizing them well.
CT diagnosis of defective filling or pulmonary arterial endoluminal defects using thick cutting edges may be difficult due to the considerable risk of false positives in relation to partial filling, movement artifacts and then the presence of adjacent lymph nodes. Advice on the interpretation of thoracic scans should be extended for future studies. The diagnosis of asymptomatic pulmonary embolism requires certain criteria including:
- The necessity to visualize the appearance of a “Mint polo” or rail appearance, or pulmonary embolism is surrounded by blood;
- The requirement that the filling defect should be displayed on 2 consecutive shots.
Patients with asymptomatic pulmonary embolism can be treated with curative anticoagulant therapy. Proof of this attitude can probably only be based on extrapolation from symptomatic PE management.
A recent meta-analysis of 12 published studies of asymptomatic pulmonary embolism that included more than 10,000 patients found a prevalence of 2.0% when using a CT scan with 5 mm of slice thickness comparatively to a prevalence of 3.0% when using test protocols with 3 mm slice thickness (30). This issue was also addressed in another study, in which asymptomatic pulmonary embolisms were more frequent with sweeping using a slice thickness of about 1 mm (6%) compared to 2 or 3 mm as the cutting thickness (4.7%), although this difference was not statistically significant (31).
Also, another study documents the prevalence of accessory pulmonary embolism in hospitalized patients undergoing CT-scan. All patients were scanned by a 16-slice CT-scan machine, with a routine interpretation report.
Subsequently, CTs were reviewed by an experienced thoracic radiologist who was unaware of the results of the first interpretation. In 28 scanners judged positive by the expert, 9 were considered as free from asymptomatic embolism during the first interpretation. These embolisms were localized at the segmental (n = 6) and sub-segmental (n = 3) levels.
On the whole, 28 cases of asymptomatic pulmonary embolism were detected among 487 thoracic scans (5.7%) (48).
Several studies also show that the evolution of asymptomatic pulmonary embolism occurring during an episode of DVT is not the same as that of asymptomatic embolism without DVT. Patients with DVT may develop asymptomatic pulmonary embolism detectable with imaging, which may have a more pejorative evolution compared to pulmonary embolisms without deep venous thrombosis. In a literature review, asymptomatic PEs were diagnosed in 1665 (32%) of 5233 patients with a DVT episode. Also, the incidence of asymptomatic PE was higher with proximal DVT than with distal DVT (32). This raises the question about the possibility of identifying clinical indices, or eventually paraclinical, which may reflect the thromboembolic risk in the patient with cancer pathology.
Several studies have been conducted on this segment of patients with symptomatic venous thromboembolic disease. They were able to bring out certain clinical variables to predict patients at high risk of developing a thromboembolic event.
First, the risk of VTE is higher in certain types of cancer such as the brain, ovaries, stomach, and tumors of the pancreas (33,34). This risk was lower in other sites such as skin, breast and thyroid (35,36).
Secondly, the risk of VTE is higher during the first half of the year following the diagnosis of the cancer pathology (37).
Third, the risk varies according to the stage of the neoplasm, being much higher in the advanced stage and metastatic than in the early stages (37).
Fourth, the risk is also higher in patients with cancer pathology and following a therapeutic protocol, mainly based on chemotherapy with or without combination with radiotherapy (38).
In addition to what has been reported for VTE in cancer patients, most of our asymptomatic pulmonary embolisms have been diagnosed in patients undergoing an active therapeutic protocol of chemotherapy, radiotherapy, and surgery or hormone therapy during the early stages of their cancer. Thereby, it can be concluded that there are other important characteristics for predicting the risk of asymptomatic pulmonary embolism, including the evolutionary stage of neoplasia, as well as chemotherapy treatment. However, given the relatively small number of cases of pulmonary embolism reported in our series, the strength of the selection bias associated with a higher rate of scannographic examination for certain types of tumors should not be overlooked, by virtue of the disease itself or in relation to a particular therapeutic and surveillance protocol.
In addition, it should be taken into account that in our study, the search for pulmonary embolism was carried out within the database of the radiology department of the Saint André hospital, which raises the question about a likely referral towards skin cancers, the abdominal digestive and renal sphere. Thus, it would be difficult to assert that asymptomatic pulmonary embolism is more frequent in certain types of tumors or to suggest establishing a protocol for the diagnosis or screening of this form of venous thromboembolic disease for certain cancers by in relation to others.
The management of asymptomatic pulmonary embolisms is not yet very clear in the minds of the majority of clinicians. Current recommendations published by the American College of Chest encourage practitioners to treat asymptomatic pulmonary embolism in a similar way to symptomatic PE. (39)
Nonetheless, other studies have highlighted the need to evaluate the benefit / risk ratio of introducing anticoagulant therapy for asymptomatic pulmonary embolism (segmental / sub-segmental), argue that a similar outcome can be achieved without anticoagulant (40). Also, it should be noted that these studies were performed in non-cancer patients, where the indication of anticoagulant therapy with its risks should be well evaluated. Also, a a related issue is the in the management of asymptomatic pulmonary embolisms, is there a selection bias towards cancer pathology? Cancer patients are more likely than others to undergo thoracic CT examinations for a variety of reasons, including monitoring and restaging neoplasms, or even screening for recurrence.
So it is perhaps not surprising that cancer patients have a higher proportion of asymptomatic pulmonary embolism on CT exams.
The experience of oncology centers is similar: The prevalence of asymptomatic pulmonary embolism was only 4% (16/403 patients) in a recent study (47). The results presented by Ritchie and his team show, for the first time, that there is no significant difference in the prevalence of segmental asymptomatic PE between cancer patients and other patients: (18/343 (5.2%) vs 10/144 6.9%)) (48). Thus, Ritchie and his team conclude that:
- Despite the strong link between cancer pathology and venous thromboembolic disease, the reverse scenario (that cancer patients are more likely to have an asymptomatic PE) is not necessarily true.
- The search for accessory pulmonary embolism should not, in the absence of clinical suspicion, trigger a battery of paraclinical examinations for underlying cancer.
In view of the foregoing, the conduct to be held before asymptomatic pulmonary embolism in cancer patients remains blurred. It is up to the clinician to make the therapeutic decision that is rarely easy. Pragmatically, anticoagulation is justified whenever pulmonary embolism is established, whether symptomatic or not. The deviation from this practice is not easy to accept because, until now, the state of knowledge is not dense enough to be able to guess the natural evolution of untreated asymptomatic pulmonary embolism. Asymptomatic character of pulmonary embolism.
In the absence of convincing evidence from large randomized studies, therapeutic indications, as well as the prognosis of asymptomatic pulmonary embolisms, are not yet clear.
However, there are some data that can guide the management of this type of condition. First, it is known that pulmonary embolisms are not all fatal. Studies indicate that between 9% and 63% of pulmonary embolisms are sub-segmental and are unlikely to contribute to life-threatening outcomes.
In a retrospective study, Engelke and Al reported the results of treatment of asymptomatic PE in a group of 96 patients, the majority of whom were carriers of cancerous pathology. 49 patients (51%) received curative anticoagulant therapy, while 21 patients (22%) received prophylactic dose anticoagulation therapy, finally, 21 patients (22%) received no treatment. PEs who received anticoagulant therapy were more severe than those who were not on anticoagulants. Hemorrhagic complications were more frequent with therapeutic anticoagulation (2 deaths, 5 major haemorrhages and one case of minor bleeding, P = 0.037). The study also reported 8 premature deaths, 7 in the group of patients under curative anticoagulant therapy and 1 in the control group (P = 0.037). The authors concluded that peripheral asymptomatic pulmonary embolism could progress favorably without anticoagulant therapy, which, if initiated, could be the source of significant hemorrhagic complications (41). Nevertheless, caution should be exercised when applying such findings in everyday routine practice.
Although defined as asymptomatic, our study showed that with careful examination of each patient’s medical record, symptoms or signs suggestive of pulmonary embolism, found to be present, such as brief chest pain, minimal haemoptysis, or some ECG repolarization disorders, but which have been attributed to other diagnoses (3/12). This has been observed in 3 cases of embolism, and is all proximal PEs.
Our study also helps to confirm the obvious association of venous thromboembolic disease and cancer pathology. 9 patients in our series had their embolism without a history of recent hospitalization, 4 patients undergoing outpatient chemotherapy.
Khorana et Al. have attempted to establish a model for assessing the risk of VTE in ambulatory cancer patients after initiation of chemotherapy. The primary site of the tumor, platelet count, leukocyte count and hemoglobin, use of erythropoiesis-stimulating agents, and body mass index were classified as predictive factors for the occurrence of VTE Chemotherapy for outpatient cancer patients (41).
In another recent study by the Vienna group on thrombosis and cancer, researchers reported that a serum with a high P-selectin concentration would predict the risk of VTE in 687 newly diagnosed cancer patients. The cumulative probability of VTE after 6 months of follow-up was 11.9% in patients with high serum P-selectin, compared to 3.7% in those with a low rate (P = 0.002) (42). In our study, the series of patients detected, as well as the series of other similar studies, is too small to be able to draw definitive conclusions about the characters of asymptomatic pulmonary embolism in the patient with a cancerous pathology, As well as its risk factors.
More work would probably be needed to establish a clearer picture of the management of asymptomatic pulmonary embolism, which would seem to be underestimated by the majority of clinicians.
The prospective nature of our study and the active anticoagulation of the majority of our patients in the study do not allow us to trace the clinical relevance of the risk associated with asymptomatic and especially peripheral pulmonary embolisms. Previous studies have shown that patients with cancer have a 4 to 5-fold higher risk of dying from an acute episode of venous thromboembolic disease (VTE) than non-cancer patients, Under estimation of VTE in the overall management of cancer pathology, and in particular asymptomatic episodes (43,44).
In another study, Sorensen et al. found that the 1-year survival rate for cancer patients with a thromboembolic event of thrombosis was 12% versus 36% in cancer patients without VTE (45). O’connell et al. confirmed this finding by proving that cancer patients with asymptomatic pulmonary embolism had a much higher mortality rate than other cancer patients and that this embolism appears to be correlated with poorer survival (46).
Although our study cannot confirm a negative evolutionary impact of asymptomatic pulmonary embolisms on the overall evolution of our patients, the results of the similar studies carried out suggest some indications that asymptomatic pulmonary embolism could be a sign of poor prognosis of the asymptomatic pulmonary embolism, could be a sign of poor prognosis regarding the evolution of the cancer disease.
Few studies have attempted to describe the natural evolution of asymptomatic embolisms, and these studies have been performed primarily in non-cancer patient groups. This encourages investing in other broader studies of interest to the patient population with cancer pathology, in order to be able to draw a clear line of conduct regarding the asymptomatic form of the VTE and to specify and to specify its evolutionary potential, especially the risk of recurrence in a silent form or even in the form of an acute event involving the immediate prognosis of the cancer patient (47).
Conclusions
In conclusion, asymptomatic pulmonary embolism in the patient with a cancerous pathology is more and more frequent. In our series, the prevalence is of the order of 3%. A large number of cases have been diagnosed in patients with advanced neoplasia and especially during chemotherapy. The clinical consequences of proximal asymptomatic pulmonary embolism, ie, the risk of recurrence, pos-embolic PAH and the risk of sudden death appears to be comparable to symptomatic PE data.
Several parameters appear to be involved in the occurrence of this form of venous thromboembolic disease in the cancer patient, mainly the type of cancer pathology (carcinomas?) as well as its stage, and the initiation of multidrug therapy.
The detection of asymptomatic pulmonary embolism depends largely on the quality of the CT scan, the radiologist’s awareness and especially his experience in the interpretation of thoracic imaging.
Management of this condition is still imprecise, and several questions require a clear answer, particularly with regard to therapeutic management, significance and prognostic value of asymptomatic pulmonary embolism.
Should we consider the occurrence of asymptomatic PE during cancer, as a factor of poor prognosis for the overall course of the patient?
Is the discovery of asymptomatic PE during post-treatment surveillance an implicit index of recurrence of neoplasia? And, therefore, should an investigation be undertaken to look for neoplastic relapse?
Are there differences in incidence related to cancer histology? If this is true, should we strengthen the management of venous thromboembolic disease for cancers more than others?
We do not have an answer to these questions. To be able to answer, it is necessary to carry out large randomized studies.
Patients and methods
The work was based on the Analysis of the CT-examinations performed on patients of the Radiology department, for reasons other than the search for pulmonary embolism. The examinations are interpreted by 2 radiologist doctors.
Results
In 389 patients, 12 cases of asymptomatic pulmonary embolism were detected, representing a prevalence of the order of 3%. The mean age of onset is 67 years. 11 patients had metastatic cancer at the time of diagnosis of pulmonary embolism. 4 PE patients are proximal, while 8 others had segmental and / or sub-segmental PE. 10 patients are treated for carcinomas, the rest for melanoma and splenic lymphoma. After analysis of clinical records, 3 of the 4 proximal pulmonary embolisms were manifested by a discrete clinical symptom attributed to diagnoses other than pulmonary embolism. 10 patients are undergoing chemotherapy alone or combined with radiotherapy, surgery or hormone therapy. At the time of the diagnosis of pulmonary embolism, 3 patients were hospitalized, while 9 were followed up on an outpatient basis.
Conclusions
Asymptomatic pulmonary embolism in cancer patients is becoming more frequent. Asymptomatic pulmonary embolisms are mainly peripheral, segmental or sub-segmental.
As for symptomatic VTE, asymptomatic pulmonary embolism is diagnosed in patients with metastatic cancer, especially carcinoma type, and treated with chemotherapy.
References
- Zwicker Jl, Furie B. Cancer-associated thrombosis.Crit Rev Oncol hematol 2007; 62: 126-36.
- Heit JA, Silverstein MD, Lohr DN et al. Risk factors for deep vein thrombosis ans pulmonary embolism: A population based casecontrol study. Arch Intern Med 2000; 160: 809–15. [CrossRef]
- Elalamy I, Gerotziafas G, Verdy E et al. Physio pathogénie de la maladie thrombo-embolique veineuse au cours du cancer. Pathol Biol sous presse 2008.
- Decicco, M. The prothrombotic state in cancer: pathogenic mechanisms. Crit. Rev. Oncol. 2004. [Google Scholar] [CrossRef]
- Tsai, A.W.; Cushman, M.; Rosamond, W.D.; Heckbert, S.R.; Tracy, R.P.; Aleksic, N.; Folsom, A.R. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am. J. Med. 2002, 113, 636–642. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Karimi, M.; Mosalaei, A.; Canciani, M.T.; Peyvandi, F. Patients with localized and disseminated tumors have reduced but measurable levels of ADAMTS-13 (von Willebrand factor cleaving protease). . 2003, 88, 454–8. [Google Scholar]
- Belting, M.; Ahamed, J.; Ruf, W.; H, Z.; A, S.; Z, L.; Z, H.; S, Z.; X, M.; Y, Z.; et al. Signaling of the Tissue Factor Coagulation Pathway in Angiogenesis and Cancer. Arter. Thromb. Vasc. Biol. 2005, 25, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Yu JL, May L, Lhotak V et al. Oncologic events regulate tissue factor expression in colorectal cancer cells: implications for tumors progression and angiogenesis. Blood 2005; 105: 1734-41. [CrossRef]
- Hallquist Viale, P. Abnormal clottiing in cancer: An overview of pathophysiology and etiology. Sem Oncol Nurs 2005:21 (suppl 1): 12–20. [CrossRef]
- Wahrenbrock, M.; Borsig, L.; Le, D.; Varki, N.; Varki, A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Investig. 2003, 112, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Durand MK, Bodker JS, Christensen A, et al. Plasminogen-Activator Inhibitor-I ans Tumor growth, invasion, and metastasis. Thromb haemost 2004; 91 :438-49. [CrossRef]
- Ma, L.; Perini, R.; McKnight, W.; Dicay, M.; Klein, A.; Hollenberg, M.D.; Wallace, J.L. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc. Natl. Acad. Sci. USA 2005, 102, 216–220. [Google Scholar] [CrossRef]
- Drouet, L. Risque thrombo-embolique de la thalidomide et des thérapeutiques anti-angiogéniques utilisées dans les cancers. STV 2008 ; 20 : 227–38. [CrossRef]
- El Accaoui RN, Shamseddeen WA, Taher AT. Thalidomide and thrombosis. A meta-analysis. Thromb haemost 2007; 97: 1031-6. [CrossRef]
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thrombo-embolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458-64. [CrossRef]
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715-22. [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Verso M, Agnelli G. Venous thrombo-embolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003; 21:3665-75.
- Staton, C.A.; Lewis, C.E. Angiogenesis inhibitors found within the haemostasis pathway. J. Cell. Mol. Med. 2005, 9, 286–302. [Google Scholar] [CrossRef]
- Engelke, C.; Manstein, P.; Rummeny, E.; Marten, K. Suspected and incidental pulmonary embolism on multidetector-row CT: Analysis of technical and morphological factors influencing the diagnosis in a cross-sectional cancer centre patient cohort. Clin. Radiol. 2006, 61, 71–80. [Google Scholar] [CrossRef]
- Sebastian, A.; Paddon, A. Clinically unsuspected pulmonary embolism—an important secondary finding in oncology CT. Clin. Radiol. 2006, 61, 81–85. [Google Scholar] [CrossRef]
- Ferrante, N.; De Tursi, M.; Iacobelli, S.; Cuccurullo, F.; Büller, H.R.; Feragalli, B.; Porreca, E.; Di Nisio, M. Incidental venous thromboembolism in ambulatory cancer patients receiving chemotherapy. 104, 1049. [Google Scholar] [CrossRef]
- Browne, A.M.; Cronin, C.G.; English, C.; NiMhuircheartaigh, J.; Murphy, J.M.; Bruzzi, J.F. Unsuspected Pulmonary Emboli in Oncology Patients Undergoing Routine Computed Tomography Imaging. J. Thorac. Oncol. 2010, 5, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Monreal, M.; Salvador, R.; Soriano, V.; Sabria, M. Cancer and Deep Venous Thrombosis. Arch. Intern. Med. 1988, 148, 485–485. [Google Scholar] [CrossRef] [PubMed]
- Van Doormaal FF, Terpstra W, Van Der Griend R, Prins MH, Nijziel MR, Van De Ree MA, et al. Is extensive screening for cancer in idiopathic venous thrombo-embolism warranted? J Thromb Haemost 2011;9:79-84. [CrossRef]
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thrombo-embolism and its effect on survival among patients with common cancers. Arch Intern Med 2006; 166:458-64.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293: 715-22. [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Verso M, Agnelli G. Venous thrombo-embolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003;21:3665-75.
- Dentali, F.; Ageno, W.; Becattini, C.; Galli, L.; Gianni, M.; Riva, N.; Imberti, D.; Squizzato, A.; Venco, A.; Agnelli, G. Prevalence and Clinical History of Incidental, Asymptomatic Pulmonary Embolism: A Meta-Analysis. Thromb. Res. 2010, 125, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.; McGurk, S.; McCreath, C.; Graham, C.; Murchison, J.T. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007, 62, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Matta, F.; Musani, M.H.; Diaczok, B. Silent Pulmonary Embolism in Patients with Deep Venous Thrombosis: A Systematic Review. Am. J. Med. 2010, 123, 426–431. [Google Scholar] [CrossRef]
- Wan, J.Y.; Nguyen, N.P.; Sallah, S. Venous Thrombosis in Patients with Solid Tumors: Determination of Frequency and Characteristics. Arthritis Res. Ther. 2002, 87, 575–579. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and Its Effect on Survival Among Patients With Common Cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Babiera, G.; Singletary, S.E.; Hunt, K.K.; Meric-Bernstam, F.; Feig, B.W.; Ames, F.C.; Ross, M.I.; Dejesus, Y.; Kuerer, H.M. Incidence and Prevention of Venous Thromboembolism in Patients Undergoing Breast Cancer Surgery and Treated According to Clinical Pathways. Ann. Surg. 2006, 243, 96–101. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and the Impact on Survival in Breast Cancer Patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef]
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and risk of venous thrombosis. AMA.2005;293: 715–722. [CrossRef]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest ;6002.133:454–545. [CrossRef]
- Engelke, C.; Rummeny, E.J.; Marten, K. Pulmonary Embolism at Multi–Detector Row CT of Chest: One-year Survival of Treated and Untreated Patients. Radiology 2006, 239, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Simanek, R.; Vormittag, R.; Dunkler, D.; Alguel, G.; Koder, S.; Kornek, G.; Marosi, C.; Wagner, O.; Zielinski, C.; et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112, 2703–2708. [Google Scholar] [CrossRef]
- Carson, J.L.; Kelley, M.A.; Duff, A.; Weg, J.G.; Fulkerson, W.J.; Palevsky, H.I.; Schwartz, J.S.; Thompson, B.T.; Popovich, J.; Hobbins, T.E.; et al. The Clinical Course of Pulmonary Embolism. New Engl. J. Med. 1992, 326, 1240–1245. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The Long-Term Clinical Course of Acute Deep Venous Thrombosis. 125, 7. [CrossRef]
- S ّrensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000; 343: 1846–1850. [CrossRef]
- O’Connell C, Razavi P, Ghalichi M, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging MDCT scanning. J Thromb Haemost. 2011;9: 305 311. [CrossRef]
- Gladish, G.W.; Choe, D.H.; Marom, E.M.; Sabloff, B.S.; Broemeling, L.D.; Munden, R.F. Incidental Pulmonary Emboli in Oncology Patients: Prevalence, CT Evaluation, and Natural History. 240. [CrossRef]
- Ritchie, G.; McGurk, S.; McCreath, C.; Graham, C.; Murchison, J.T. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007, 62, 536–540. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).