Submitted:
17 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.1. Methods
2.2. Cells
2.3. Materials
| PROs | CONs | References | |
|---|---|---|---|
| Inkjet | Low cost; high resolution; fast printing speed; chemical and photocrosslinking gelation method; thermal-, or piezoelectric-driven. | Narrow ranges of printable biomaterial viscosities; high probability of cell damage, and cell lysis; non-uniform droplet size; nozzle clogging risk. |
[17,26,27,28,29,30] |
| Laser-assisted | >95% cell viability; low risk of contamination; laser pulse-driven; nozzle-free; high viscosity and resolution. | High printer cost; cell damage due to high laser energy; difficulty of use. | [29,31,32,33] |
| Extrusion | Good quality of vertical structure; chemical, photocrosslinking; shear thinning and temperature gelation method; microscale resolution; high cell density; piston-, pneumatic- or screw-driven. | Slow print speed; poor cell viability (40-80%) due to shear damage; low resolution. | [17,18,34,35,36,37] |
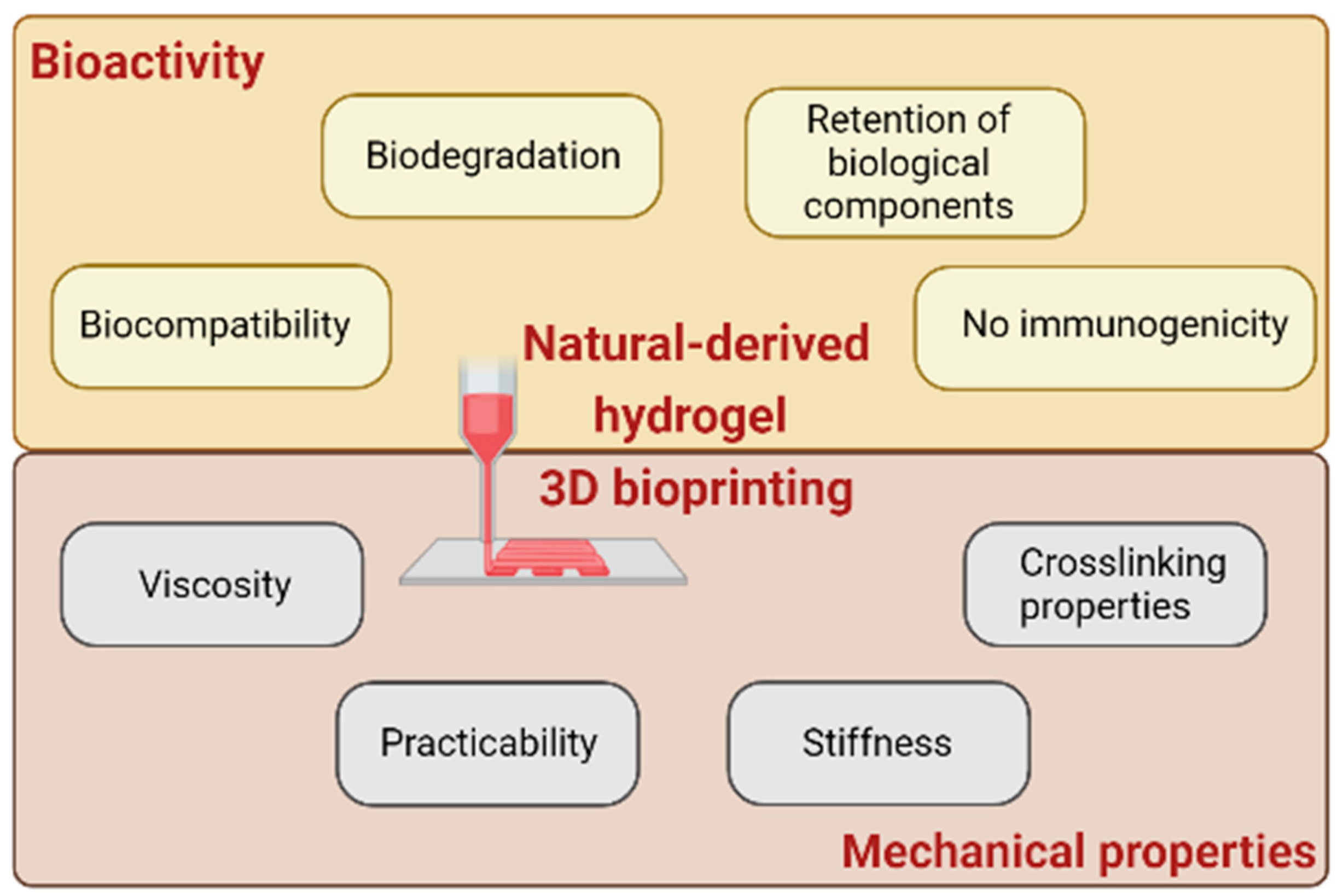
3. Natural-Derived Bioink Advantages: Bioactivity and Biocompatibility
- Ability to maintain the same biological activity of the natural matrix [96]. dECM hydrogels retain numerous structural and soluble components found in native tissue such as cell adhesion proteins, growth factors, and glycosaminoglycans. The presence of bioactive factors (e.g., cytokines, growth and differentiation factors, transforming growth factor, etc.) within a bioink can enhance cell viability and proliferation. Indeed, it was shown that after incorporation of bioactive factors into bioinks where cells are cultured, cell proliferation and ECM protein production increased compared to hydrogels without bioactive factors [97]. The characteristic of including a variety of structural proteins together with soluble factors and cytokines makes these types of hydrogels much more complete than other bioinks of natural origin. In addition, they support a constructive, site-appropriate remodeling response when implanted in a wide variety of anatomic sites [98,99,100].
- No immunogenic cell material due to decellularization. This prevents infection transmission and avoids an immune reaction, allowing the use of allogeneic or xenogeneic dECM [101].
- Injectability. The dECM pre-gel fluid can be extruded or injected directly into targeted areas or tissues using minimally invasive techniques [102,103] and can be induced to polymerize at physiological temperature to form a hydrogel that perfectly fits the targeted organ, stimulating regeneration and ultimately serving as carrier of factors or molecules [104].
4. Natural-Derived Bioink Challenges: Mechanical Properties
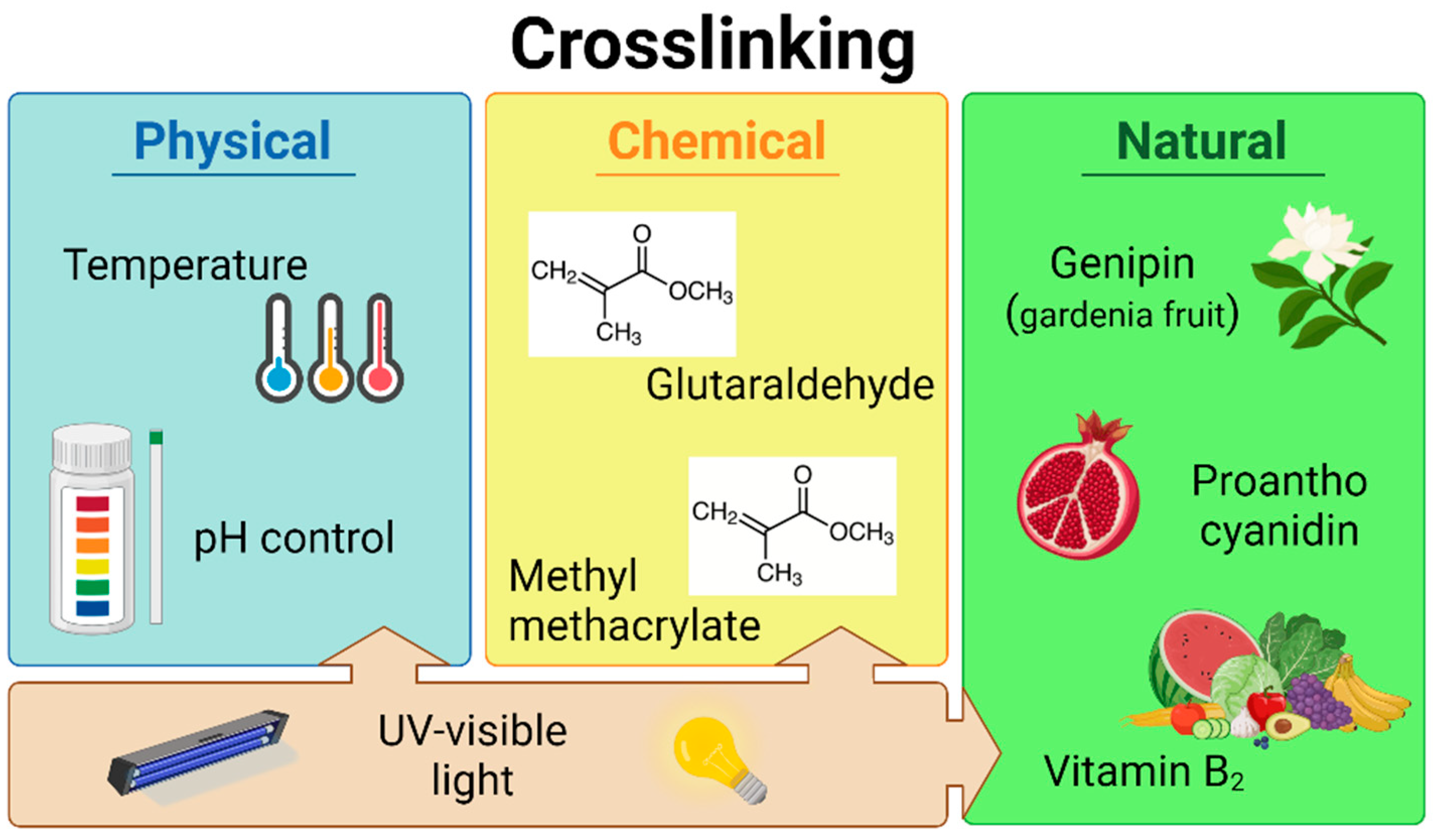
5. Bioink Reinforcement and Crosslinking
5.1. Physical Crosslinking
5.1.1. Temperature-Triggered Hydrogels
5.1.3. Ion-Responsive Hydrogels
5.1.4. Light-Responsive Hydrogels
- Photosensitive hydrogels can absorb and emit light as energy. Light can be converted into heat through photosensitive moieties to trigger the polymer phase transition temperature and the consequent polymerization. This approach occurs in a similar way to temperature-sensitive hydrogels [175].
- Photosensitive molecules can be ionized through light irradiation to produce ion-sensitive hydrogels or crosslinking induced by variation in ionic concentration.
- Chromophoric groups can be incorporated into the hydrogel matrix to alter physical properties (geometry, dipole moments) under light irradiation. This method can facilitate the formation of hydrogels after in vivo injection, which is attractive for drug delivery and tissue engineering [176].
5.2. Chemical Crosslinking
5.2.1. Small Molecule Crosslinking Agents
5.2.2. Free Radical Polymerization Crosslinking
5.2.3. Enzymatically Crosslinked Hydrogels
5.3. Natural crosslinkers
5.3.1. Genipin
5.3.2. Proanthocyanidin
5.3.3. Vitamin B2
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine 2010, 16, 927–933. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nature medicine 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A. , et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Science translational medicine 2014, 6, 234ra258. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D. , et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Goodbye, flat biology? Nature 2003, 424, 861. [CrossRef]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philosophy, ethics, and humanities in medicine : PEHM 2009, 4, 2. [Google Scholar] [CrossRef]
- Rezaei, N.T.; Kumar, H.; Liu, H.; Lee, S.S.; Park, S.S.; Kim, K. Recent advances in organ-on-chips integrated with bioprinting technologies for drug screening. Advanced healthcare materials 2023, e2203172. [Google Scholar] [CrossRef]
- Landers, R.; Hubner, U.; Schmelzeisen, R.; Mulhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3d bioprinting in organ manufacturing. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Potyondy, T.; Uquillas, J.A.; Tebon, P.J.; Byambaa, B.; Hasan, A.; Tavafoghi, M.; Mary, H.; Aninwene, G.E., 2nd; Pountos, I.; Khademhosseini, A. , et al. Recent advances in 3d bioprinting of musculoskeletal tissues. Biofabrication 2021, 13. [Google Scholar] [CrossRef]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3d bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. Journal of materials chemistry. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef]
- Chae, S.; Cho, D.W. Biomaterial-based 3d bioprinting strategy for orthopedic tissue engineering. Acta biomaterialia 2023, 156, 4–20. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3d bioprinting: From printing methods to biomedical applications. Asian journal of pharmaceutical sciences 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Kocak, E.; Yildiz, A.; Acarturk, F. Three dimensional bioprinting technology: Applications in pharmaceutical and biomedical area. Colloids and surfaces. B, Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3d bioprinting for engineering complex tissues. Biotechnology advances 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3d bioprinting system to produce human-scale tissue constructs with structural integrity. Nature biotechnology 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kulkarni, P.; Dutta, D. An accurate slicing procedure for layered manufacturing. Compufer-Aided Design 1996, 28, 683–697. [Google Scholar] [CrossRef]
- Pandey, P.M.; Reddy, N.V.; Dhande, S.G. Slicing procedures in layered manufacturing: A review. Emerald Insight 2003, 9, 274–288. [Google Scholar]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta biomaterialia 2017, 61, 41–53. [Google Scholar] [CrossRef]
- Wust, S.; Godla, M.E.; Muller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta biomaterialia 2014, 10, 630–640. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3d bioprinting in tissue engineering and regenerative medicine. Journal of clinical medicine 2021, 10. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-d bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes & diseases 2017, 4, 185–195. [Google Scholar]
- Boso, D.; Maghin, E.; Carraro, E.; Giagante, M.; Pavan, P.; Piccoli, M. Extracellular matrix-derived hydrogels as biomaterial for different skeletal muscle tissue replacements. Materials 2020, 13. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE transactions on bio-medical engineering 2013, 60, 691–699. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Advanced materials 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3d bioprinting of tissues and organs. Nature biotechnology 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed chinese hamster ovary cells. Biotechnology and bioengineering 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3d printable hydrogels and constructs. Materials and Design 2018, 20–38. [Google Scholar] [CrossRef]
- I. , D.; J.C.M., v.H.; N.R., C. Bio-inks for 3d bioprinting: Recent advances and future prospects. Polymer Chemistry 2017, 4451–4471. [Google Scholar]
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.Z.; Lee, J.; Lee, D. Current advances in 3d bioprinting technology and its applications for tissue engineering. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnology journal 2017, 12. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3d bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Applied materials today 2020, 18. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3d printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3d bioprinting is a key factor to balance printing resolution and stem cell integrity. Advanced healthcare materials 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Polak, J.M.; Mantalaris, S. Stem cells bioprocessing: An important milestone to move regenerative medicine research into the clinical arena. Pediatric research 2008, 63, 461–466. [Google Scholar] [CrossRef]
- Knowlton, S.; Cho, Y.; Li, X.J.; Khademhosseini, A.; Tasoglu, S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomaterials science 2016, 4, 768–784. [Google Scholar] [CrossRef]
- Ramalho-Santos, M.; Willenbring, H. On the origin of the term "stem cell". Cell stem cell 2007, 1, 35–38. [Google Scholar] [CrossRef]
- Dobos, A.; Gantner, F.; Markovic, M.; Van Hoorick, J.; Tytgat, L.; Van Vlierberghe, S.; Ovsianikov, A. On-chip high-definition bioprinting of microvascular structures. Biofabrication 2021, 13, 015016. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M. , et al. 3d printed stem-cell derived neural progenitors generate spinal cord scaffolds. Advanced functional materials 2018, 28. [Google Scholar]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3d printing of personalized thick and perfusable cardiac patches and hearts. Advanced science 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-dimensional bioprinting of mesenchymal stem cells using an osteoinductive bioink containing alginate and bmp-2-loaded plga nanoparticles for bone tissue engineering. Biomaterials advances 2022, 136, 212789. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3d printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomaterials science 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J. , et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Scientific reports 2019, 9, 1856. [Google Scholar] [CrossRef]
- Zidaric, T.; Milojevic, M.; Gradisnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-based bioink formulation for 3d bioprinting of an in vitro model of the human dermis. Nanomaterials 2020, 10. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jimenez, G.; Chocarro, C.; Carrillo, E.; Montanez, E.; Galvez-Martin, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3d bioprinting of articular cartilage engineering constructs. Acta biomaterialia 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3d bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Science advances 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Rashid, A.A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A review of processes, materials and applications. Bioprinting 2021, 23, e00148. [Google Scholar] [CrossRef]
- Stanton, M.M.; Samitier, J.; Sanchez, S. Bioprinting of 3d hydrogels. Lab on a chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, P.; Toit, L.C.d.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-based bioinks for 3d bioprinting in tissue regeneration. Frontiers in Materials 2020, 7. [Google Scholar] [CrossRef]
- Almoshari, Y. Novel hydrogels for topical applications: An updated comprehensive review based on source. Gels 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Materials science & engineering. C, Materials for biological applications 2015, 57, 414–433. [Google Scholar]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering bioinks for 3d bioprinting. Biofabrication 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3d printing. Biomaterials research 2018, 22, 11. [Google Scholar] [CrossRef]
- Peng, B.; Du, L.; Zhang, T.; Chen, J.; Xu, B. Research progress in decellularized extracellular matrix hydrogels for intervertebral disc degeneration. Biomaterials science 2023, 11, 1981–1993. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3d bioprinting: An overview. Biomaterials science 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Moon, S.H.; Choi, H.N.; Yang, Y.J. Natural/synthetic polymer materials for bioink development. Biotechnology and Bioprocess Engineering 2022, 27, 482–493. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. Journal of tissue engineering 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Deries, M.; Cachaco, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Developmental biology 2011, 354, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural biomaterials and their use as bioinks for printing tissues. Bioengineering 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regenerative therapy 2021, 18, 88–96. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue engineering. Part B, Reviews 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. Journal of controlled release : official journal of the Controlled Release Society 2012, 161, 680–692. [Google Scholar] [CrossRef]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and injectable hydrogel for drug delivery in soft tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Chen, G.; Li, Z.; Liu, Y.; Wang, G.; Wu, X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and plga microspheres for management of non-healing infected wounds. Materials science & engineering. C, Materials for biological applications 2018, 89, 213–222. [Google Scholar]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Seminars in cell & developmental biology 2009, 20, 646–655. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Hubbell, J.A. Biomaterials in tissue engineering. Bio/technology 1995, 13, 565–576. [Google Scholar] [CrossRef] [PubMed]
- W. E., H.; C.F., v.N. Novel crosslinking methods to design hydrogels. Advanced Drug Delivery Reviews 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Callegari, A.; Bollini, S.; Iop, L.; Chiavegato, A.; Torregrossa, G.; Pozzobon, M.; Gerosa, G.; De Coppi, P.; Elvassore, N.; Sartore, S. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 2007, 28, 5449–5461. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, Y.; Ma, Z.; Lin, H.; Zhu, X.; Xiao, Y.; Zhang, X. Collagen hydrogel viscoelasticity regulates msc chondrogenesis in a rock-dependent manner. Science advances 2023, 9, eade9497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.Y.; Cabral, A.T.; Rossello-Martinez, A.; Zulli, A.; Gong, X.; Zhang, Q.; Yan, J.; Mak, M. Tunable mesoscopic collagen island architectures modulate stem cell behavior. Advanced materials 2023, e2207882. [Google Scholar] [CrossRef]
- Cameron, A.P.; Gao, S.; Liu, Y.; Zhao, C.X. Impact of hydrogel biophysical properties on tumor spheroid growth and drug response. Biomaterials advances 2023, 149, 213421. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Kolesnik-Gray, M.; Angeloni, M.; Schruefer, S.; Fiedler, M.; Schubert, D.W.; Ferrazzi, F.; Krstic, V.; Engel, F.B. Collagen hydrogel containing polyethylenimine-gold nanoparticles for drug release and enhanced beating properties of engineered cardiac tissues. Advanced healthcare materials 2023, e2202408. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnology advances 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Rossi, C.A.; Flaibani, M.; Blaauw, B.; Pozzobon, M.; Figallo, E.; Reggiani, C.; Vitiello, L.; Elvassore, N.; De Coppi, P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011, 25, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type i-hyaluronan hybrid bioink for 3d bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.M.; Tuttle, T.; Guo, Y.; Miles, D.; Buganza-Tepole, A.; Calve, S. Multiscale mechanical characterization and computational modeling of fibrin gels. Acta biomaterialia 2023. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nature communications 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Singh, N.K.; Kim, J.J.; Kim, H.; Kim, B.S.; Park, J.Y.; Jang, J.; Cho, D.W. Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials 2019, 224, 119496. [Google Scholar] [CrossRef]
- Zhu, L.; Yuhan, J.; Yu, H.; Zhang, B.; Huang, K.; Zhu, L. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment. Small 2023, e2207752. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L. , et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nature communications 2019, 10, 5658. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.H. 3d bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta biomaterialia 2021, 119, 75–88. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3d cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ullah, I.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 035023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, G.; Kim, C.; Lee, J.S.; Lee, I.G.; An, S.H.; Yun, W.S.; Kim, S.Y.; Shim, J.H. Synergistic effects of beta tri-calcium phosphate and porcine-derived decellularized bone extracellular matrix in 3d-printed polycaprolactone scaffold on bone regeneration. Macromolecular bioscience 2018, 18, e1800025. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, T.G.; Jeong, J.; Yi, H.G.; Park, J.W.; Hwang, W.; Cho, D.W. 3d cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Advanced healthcare materials 2016, 5, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Scientific reports 2019, 9, 14933. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, R.A.; Young, B.M.; Link, P.A.; Park, H.E.; Kahn, A.R.; Shankar, K.; Schneck, M.B.; Weiss, D.J.; Heise, R.L. Porcine lung-derived extracellular matrix hydrogel properties are dependent on pepsin digestion time. Tissue engineering. Part C, Methods 2020, 26, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D'Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Yoo, Y.; Nam, S.E.; Hyun, H.; Lee, D.W.; Um, S.; Kim, S.Y.; Hong, S.O.; Yang, D.H.; Chun, H.J. The cocktail effect of bmp-2 and tgf-beta1 loaded in visible light-cured glycol chitosan hydrogels for the enhancement of bone formation in a rat tibial defect model. Marine drugs 2018, 16. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef]
- Quarti, A.; Nardone, S.; Colaneri, M.; Santoro, G.; Pozzi, M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interactive cardiovascular and thoracic surgery 2011, 13, 569–572. [Google Scholar] [CrossRef]
- Nagao, R.J.; Lundy, S.; Khaing, Z.Z.; Schmidt, C.E. Functional characterization of optimized acellular peripheral nerve graft in a rat sciatic nerve injury model. Neurological research 2011, 33, 600–608. [Google Scholar] [CrossRef]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3d bioprinting. Progress in Natural Science: Materials International 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, L.; Liu, C.; Wen, H.; Wang, S.; Guan, P.; Chen, D.; Ning, C.; Zhou, L.; Tan, G. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioactive materials 2022, 7, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of nanocomposite injectable hydrogels for minimally invasive surgery. Accounts of chemical research 2019, 52, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, L.; Xiong, H.; Li, Q.; Wu, C.; Huang, Y.; Xie, H.; Shen, B. Injectable decellularized cartilage matrix hydrogel encapsulating urine-derived stem cells for immunomodulatory and cartilage defect regeneration. NPJ Regenerative medicine 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Parak, A.; Pradeep, P.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Pillay, V. Functionalizing bioinks for 3d bioprinting applications. Drug discovery today 2019, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. 'Printability' of candidate biomaterials for extrusion based 3d printing: State-of-the-art. Advanced healthcare materials 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, Y.; Yin, J.; Xu, C.; Xiong, R.; Christensen, K.; Ringeisen, B.R.; Chrisey, D.B.; Huang, Y. Evaluation of bioink printability for bioprinting applications. Applied Physics Reviews 2018, 5. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3d bioprinting. Scientific reports 2016, 6, 29977. [Google Scholar] [CrossRef]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue engineering. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef]
- Tirella, A.; Orsini, A.; Vozzi, G.; Ahluwalia, A. A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication 2009, 1, 045002. [Google Scholar] [CrossRef]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3d bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chemical reviews 2020, 120, 10834–10886. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; An, J.; Chua, C.K.; Tan, L.P. Bioprinting of 3d in vitro skeletal muscle models: A review. Materials & Design 2020, 193. [Google Scholar]
- Sabzi, M.; Samadi, N.; Abbasi, F.; Mahdavinia, G.R.; Babaahmadi, M. Bioinspired fully physically cross-linked double network hydrogels with a robust, tough and self-healing structure. Materials science & engineering. C, Materials for biological applications 2017, 74, 374–381. [Google Scholar] [PubMed]
- Zhang, Y.; Chen, M.; Tian, J.; Gu, P.; Cao, H.; Fan, X.; Zhang, W. In situ bone regeneration enabled by a biodegradable hybrid double-network hydrogel. Biomaterials science 2019, 7, 3266–3276. [Google Scholar] [CrossRef]
- Ye, L.; Lv, Q.; Sun, X.; Liang, Y.; Fang, P.; Yuan, X.; Li, M.; Zhang, X.; Shang, X.; Liang, H. Fully physically cross-linked double network hydrogels with strong mechanical properties, good recovery and self-healing properties. Soft matter 2020, 16, 1840–1849. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Hammink, R.; Nelissen, F.H.T.; Rowan, A.E.; Heus, H.A. Biomimetic stress sensitive hydrogel controlled by DNA nanoswitches. Biomacromolecules 2017, 18, 3310–3317. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Rossetti, F.F.; Kaufmann, S.; Kaindl, T.; Madsen, J.; Engel, U.; Lewis, A.L.; Armes, S.P.; Tanaka, M. Quantitative evaluation of mechanosensing of cells on dynamically tunable hydrogels. Journal of the American Chemical Society 2011, 133, 1367–1374. [Google Scholar] [CrossRef]
- Nguyen, M.; Liu, J.C.; Panitch, A. Physical and bioactive properties of glycosaminoglycan hydrogels modulated by polymer design parameters and polymer ratio. Biomacromolecules 2021, 22, 4316–4326. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, K.; Deng, R.; Ren, X.; Wu, C.; Li, J. Tunable stiffness of graphene oxide/polyacrylamide composite scaffolds regulates cytoskeleton assembly. Chemical science 2018, 9, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta biomaterialia 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Sun, J.; Xiang, Z. Induction of m2-type macrophage differentiation for bone defect repair via an interpenetration network hydrogel with a go-based controlled release system. Advanced healthcare materials 2021, 10, e2001502. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A review on the design of hydrogels with different stiffness and their effects on tissue repair. Frontiers in bioengineering and biotechnology 2022, 10, 817391. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Gupta, S.; Mitra, K.; Rizvanov, A.A.; Solovyeva, V.V.; Antmen, E.; Salehi, M.; Ehterami, A.; Pourchet, L.; Barthes, J. , et al. From 3d printing to 3d bioprinting: The material properties of polymeric material and its derived bioink for achieving tissue specific architectures. Cell and tissue banking 2022, 23, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Mitchell, K.; Raymond, L.; Godina, B.; Zhao, D.; Zhou, W.; Jin, Y. Fluid bath-assisted 3d printing for biomedical applications: From pre- to postprinting stages. ACS biomaterials science & engineering 2021, 7, 4736–4756. [Google Scholar]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel bioink reinforcement for additive manufacturing: A focused review of emerging strategies. Advanced materials 2020, 32, e1902026. [Google Scholar] [CrossRef]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired materials for controlling stem cell fate. Accounts of chemical research 2010, 43, 419–428. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Advanced materials 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Brunel, L.G.; Hull, S.M.; Heilshorn, S.C. Engineered assistive materials for 3d bioprinting: Support baths and sacrificial inks. Biofabrication 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science advances 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Song, K.; Gellermann, N.; Huang, Y. Printing of hydrophobic materials in fumed silica nanoparticle suspension. ACS applied materials & interfaces 2019, 11, 29207–29217. [Google Scholar]
- Jin, Y.; Compaan, A.; Chai, W.; Huang, Y. Functional nanoclay suspension for printing-then-solidification of liquid materials. ACS applied materials & interfaces 2017, 9, 20057–20066. [Google Scholar]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of fresh 3d printing as a platform for advanced tissue biofabrication. APL bioengineering 2021, 5, 010904. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3d bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. Journal of controlled release : official journal of the Controlled Release Society 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Wu, C.A.; Zhu, Y.; Venkatesh, A.; Stark, C.J.; Lee, S.H.; Woo, Y.J. Optimization of freeform reversible embedding of suspended hydrogel microspheres for substantially improved three-dimensional bioprinting capabilities. Tissue engineering. Part C, Methods 2023, 29, 85–94. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue engineering. Part B, Reviews 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Zhou, K.; Dey, M.; Ayan, B.; Zhang, Z.; Ozbolat, V.; Kim, M.H.; Khristov, V.; Ozbolat, I.T. Fabrication of pdms microfluidic devices using nanoclay-reinforced pluronic f-127 as a sacrificial ink. Biomedical materials 2021. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. Journal of cell science 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert review of medical devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Coyle, A. Application of uva-riboflavin crosslinking to enhance the mechanical properties of extracellular matrix derived hydrogels. Journal of the mechanical behavior of biomedical materials 2016, 54, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Gao, G.; Cho, D.W. Tissue-specific decellularized extracellular matrix bioinks for musculoskeletal tissue regeneration and modeling using 3d bioprinting technology. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in extrusion 3d bioprinting: A focus on multicomponent hydrogel-based bioinks. Advanced healthcare materials 2020, 9, e1901648. [Google Scholar] [CrossRef]
- Kort-Mascort, J.; Flores-Torres, S.; Peza-Chavez, O.; Jang, J.H.; Pardo, L.A.; Tran, S.D.; Kinsella, J. Decellularized ecm hydrogels: Prior use considerations, applications, and opportunities in tissue engineering and biofabrication. Biomaterials science 2023, 11, 400–431. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3d printing: A materials science perspective. Annals of biomedical engineering 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Advanced materials 2014, 26, 85–123. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomaterials science 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Wim, E. Hennink; Nostrum, C.F.v. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 2002, 54, 13–36. [Google Scholar]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3d bioprinting and photocrosslinking: Emerging strategies & future perspectives. Biomaterials advances 2022, 134, 112576. [Google Scholar]
- Holzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3d bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chemical Society reviews 2016, 45, 4013–4031. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Frontiers in chemistry 2018, 6, 499. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, Y.; Park, T.G. Thermo-sensitive and biodegradable hydrogels based on stereocomplexed pluronic multi-block copolymers for controlled protein delivery. Journal of controlled release : official journal of the Controlled Release Society 2008, 127, 22–30. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3d bioprinting of polymeric hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. Journal of the Royal Society, Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive hydrogels and advances in their application in disease therapy. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tsao, C.T.; Kyomoto, M.; Zhang, M. Injectable natural polymer hydrogels for treatment of knee osteoarthritis. Advanced healthcare materials 2022, 11, e2101479. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Umair Hassan, M.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare applications of ph-sensitive hydrogel-based devices: A review. International journal of nanomedicine 2020, 15, 3887–3901. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Hsiao, M.H.; Zhang, M.Y.; Levengood, S.L.; Zhang, M. Chitosan-peg hydrogel with sol-gel transition triggerable by multiple external stimuli. Macromolecular rapid communications 2015, 36, 332–338. [Google Scholar] [CrossRef]
- Du, H.; Liu, M.; Yang, X.; Zhai, G. The design of ph-sensitive chitosan-based formulations for gastrointestinal delivery. Drug discovery today 2015, 20, 1004–1011. [Google Scholar] [CrossRef]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nuske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Muller, P.; Taubenberger, A.; Maharana, S. , et al. A ph-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 2016, 5. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Materials science & engineering. R, Reports : a review journal 2015, 93, 1–49. [Google Scholar]
- Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. Journal of biomedical materials research. Part A 2018, 106, 2762–2776. [Google Scholar] [CrossRef]
- Lin, H.; Li, Q.; Du, Q.; Wang, O.; Wang, Z.; Akert, L.; Carlson, M.A.; Zhang, C.; Subramanian, A.; Zhang, C. , et al. Integrated generation of induced pluripotent stem cells in a low-cost device. Biomaterials 2019, 189, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ramey-Ward, A.N.; Salaita, K. Programmable mechanically active hydrogel-based materials. Advanced materials 2021, 33, e2006600. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Alsberg, E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Progress in polymer science 2014, 39, 1236–1265. [Google Scholar] [CrossRef] [PubMed]
- X. , F.; L., H.-R.; R., C.; J., C. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chemical reviews 2018, 4, 2084–2107. [Google Scholar]
- Meng, W.; Gao, L.; Venkatesan, J.K.; Wang, G.; Madry, H.; Cucchiarini, M. Translational applications of photopolymerizable hydrogels for cartilage repair. Journal of experimental orthopaedics 2019, 6, 47. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioactive materials 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. International journal of biological macromolecules 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regenerative biomaterials 2014, 1, 81–89. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. The Korean journal of thoracic and cardiovascular surgery 2011, 44, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. International journal of biological macromolecules 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue engineering. Part C, Methods 2011, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fukazawa, K.; Ishihara, K. Photoinduced inhibition of DNA unwinding in vitro with water-soluble polymers containing both phosphorylcholine and photoreactive groups. Acta biomaterialia 2016, 40, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xu, J.; Yu, Q.; Zhang, J.; Hu, X.; Sun, C.; Zhang, H. Photo-crosslinkable hydrogels for 3d bioprinting in the repair of osteochondral defects: A review of present applications and future perspectives. Micromachines 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3d bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3d bioprinting of low-concentration cell-laden gelatin methacrylate (gelma) bioinks with a two-step cross-linking strategy. ACS applied materials & interfaces 2018, 10, 6849–6857. [Google Scholar]
- Ying, G.L.; Jiang, N.; Maharjan, S.; Yin, Y.X.; Chai, R.R.; Cao, X.; Yang, J.Z.; Miri, A.K.; Hassan, S.; Zhang, Y.S. Aqueous two-phase emulsion bioink-enabled 3d bioprinting of porous hydrogels. Advanced materials 2018, 30, e1805460. [Google Scholar] [CrossRef]
- Komez, A.; Baran, E.T.; Erdem, U.; Hasirci, N.; Hasirci, V. Construction of a patterned hydrogel-fibrous mat bilayer structure to mimic choroid and bruch's membrane layers of retina. Journal of biomedical materials research. Part A 2016, 104, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G.H. Efficient myotube formation in 3d bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2020, 230, 119632. [Google Scholar] [CrossRef] [PubMed]
- Toledano, S.; Williams, R.J.; Jayawarna, V.; Ulijn, R.V. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. Journal of the American Chemical Society 2006, 128, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. Journal of controlled release : official journal of the Controlled Release Society 2011, 152, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. Journal of bioscience and bioengineering 2007, 104, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. European Polymer Journal 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An interpenetrating alginate/gelatin network for three-dimensional (3d) cell cultures and organ bioprinting. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. Journal of materials chemistry. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Montemurro, F.; De Maria, C.; Orsi, G.; Ghezzi, L.; Tine, M.R.; Vozzi, G. Genipin diffusion and reaction into a gelatin matrix for tissue engineering applications. Journal of biomedical materials research. Part B, Applied biomaterials 2017, 105, 473–480. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomaterials science 2021, 9, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Chang, W.H.; Ma, C.Y.; Lee, M.H. Crosslinking of biological tissues using genipin and/or carbodiimide. Journal of biomedical materials research. Part A 2003, 64, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Q.; Zhu, J.; Xiao, J.; Wan, P.; Zhou, C.; Huang, Z.; Qiang, N.; Zhang, W.; Wu, Z. , et al. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials 2012, 33, 7336–7346. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. Journal of biomaterials science. Polymer edition 1999, 10, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Boso, D.; Carraro, E.; Maghin, E.; Todros, S.; Dedja, A.; Giomo, M.; Elvassore, N.; De Coppi, P.; Pavan, P.G.; Piccoli, M. Porcine decellularized diaphragm hydrogel: A new option for skeletal muscle malformations. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. Journal of biomedical materials research. Part A 2003, 65, 118–124. [Google Scholar] [CrossRef]
- Teixeira, S. Bioflavonoids: Proanthocyanidins and quercetin and their potential roles in treating musculoskeletal conditions. The Journal of orthopaedic and sports physical therapy 2002, 32, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/gelatin-based films crosslinked by proanthocyanidin. Journal of biomedical materials research. Part B, Applied biomaterials 2005, 75, 442–450. [Google Scholar] [CrossRef]
- Liu, B.S. Fabrication and evaluation of a biodegradable proanthocyanidin-crosslinked gelatin conduit in peripheral nerve repair. Journal of biomedical materials research. Part A 2008, 87, 1092–1102. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. American journal of ophthalmology 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Tirella, A.; Liberto, T.; Ahluwalia, A.D. Riboflavin and collagen: New crosslinking methods to tailor the stiffness of hydrogels. Materials Letters 2012, 74, 58–61. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-a-induced cross-linking. Journal of cataract and refractive surgery 2003, 29, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin b2-induced photo-crosslinking. Acta biomaterialia 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





