1. Introduction
Alginate is a bioactive polymer extracted from brown algae (Phaeophyceae) such as
Sargassum, Laminaria, Tubinaria, Macrocystis, and
Ascophyllum grown in tropical and subtropical coasts [
1,
2,
3]. Alginates exist in seaweed cell walls and play a remarkable role in other fields. For example, food, functional food, pharmaceutical and different industries also in the economy [
4,
5]. Alginate is composed of basic units of (1→4) α-L-guluronic acid (G) and (1→4) β-D-mannuronic acid (M) [
6,
7,
8]. The arrangement of M and G in alginate leads to the different structures and activities of alginate, and this depends on the species of seaweed, the season, and the geographical location of the algae. Alginate possesses different bioactivities, for example, antioxidant [
9,
10], anti-tumour, anti-fungal, neuroprotective, anticancer, and immuno-stimulation [
11,
12,
13]. In brown algae,
Sargassum in Vietnam, alginate content is about 16 to 36 (%, wt) of dry algae, and the yield of sargassum seaweed gets about 10,000 kg dry per year.
Lignins belong to the polyphenol group in trees, crops, and plants [
14]. They are diverse in biological activities and non-toxic. Different plants have different lignin in terms of structure, composition and bioactivity [
15]. Lignins show great potential for numerous fields such as food, functional food, pharmaceutical, fertilizer, fodder, and fuel. Lignins are found in a mixture of cellulose, hemicellulose, lignin, and extractives of plants [
15,
16,
17]. Softwood, herbaceous plants, and hardwood species include 27–32%, 0–40%, and 21–31% lignin, respectively [
18]. Lignins have structure types sulfur lignin (kraft and lignosulfonates lignin) and sulfur-free lignin (alkaline and organosolv lignin) [
16,
17]. Phenolic hydroxyl and sulfur (SO
32− and HSO
3−) groups are found in a large amount in kraft and lignosulfonates lignin, respectively. Kraft and lignosulfonates lignin is dissolved in water [
18,
19,
20]. Organosolv is soluble in organic solvents and insoluble in water. Nitrogen and silicate content in alkaline lignin is more than in other lignins [
21,
22,
23]. In Vietnam, corn stalks are about 120 – 135 tons/ha/year, and the corn planting area is about 1.1 million hectares.
Alginate and lignin possess diverse biological activities, such as anticancer, antibacterial, and antioxidant. Physico-chemistry properties of alginate and lignin are interesting. Hence, the applications of alginate and lignin in different fields are very diverse, for example, carriers of biologically active ingredients in pharmaceuticals, pharmaceutical materials, food packaging films, drug packaging films, and state-forming agents [
2,
4,
15,
16,
17]. The combination of alginate and lignin for forming different biomaterials is of great interest, such as alginate/lignin films. However, the notices on alginate and lignin particles and their properties have not appeared. Today, aging, cancer, lack of medicinal herbs, food, drugs, functional foods, and environmental pollution are existential dangers to humans.
Therefore, the study focused on alginate/lignin characteristics composed of antioxidant activity, anticancer activity, physico-chemistry properties, and acute oral toxicity. Lignin and alginate were extracted from by-products of maize harvest (stalks and leaves) and brown algae processing.
2. Results
2.1. Antioxidant Activities
The total antioxidant activity and reducing power activity of alginate/lignin was 218.73 ± 10.45 mg ascorbic acid equivalent/g DW and 479.62 ± 23.18 mg FeSO
4 equivalent/g DW. DPPH free radical scavenging capacity of alginate/lignin got the highest value of 19.75 ± 4.07 (%) and the lowest value of 0.53 ± 0.78 (%), corresponding to the concentration of 2000 (µg alginate/lignin/mL) and 31.3 (µg alginate/lignin/mL), respectively. A positive control (Trolox) possessed the highest value (81.48 ± 3.30, %) and the lowest value (5.20 ± 0.75, %) of DPPH free radical scavenging activity, corresponding to 180 (µg Trolox/mL) and 11.25 (µg Trolox/mL), respectively (
Table 1). Alginate/lignin and Trolox got the average value of alginate/lignin which corresponded to 8.39 ± 1.56 (%) and 37.98 ± 1.40 (%), respectively.
α – glucosidase inhibition activity of alginate/lignin was in the range of 27.33 ± 3.62 to 87.62 ± 1.13 (%) with IC50 of 50.56 ± 0.8 (µg/mL). The lowest value and highest value of α – glucosidase inhibition activity corresponded to 27.33 ± 3.62 and 87.62 ± 1.13 (%), respectively, occurring as alginate/lignin content got 15.63 and 250 (µg alginate/lignin /mL), respectively. A positive control of acarbose got α – glucosidase inhibition activity of 65.95 ± 0.72 (%), which corresponded to 1000 (µg acarbose/mL) (
Table 2).
2.2. Anticancer Activities
Alginate/lignin did not exhibit anticancer activity on NCI H460 line. Camptothecin possessed anticancer activity on four cell lines composed of NCl-H460, fibroblast, hepG2, and MCF-7, corresponding to 64.93 ± 1.58, 47.89 ± 2.58, 57.62 ± 2.06, and 53.89 ± 3.28 (%), respectively, at camptothecin concentration of 0.007, 2.5, 0.07, and 0.05 (µg camptothecin/mL), respectively. Alginate/lignin showed anticancer activity on two cell lines of fibroblast (14.80 ± 2.00, %) and HepG2 (3.71 ± 4.35, %) at the concentration of 1000 (µg alginate/lignin /mL) (
Table 3).
2.3. Physico-chemistry Characteristics
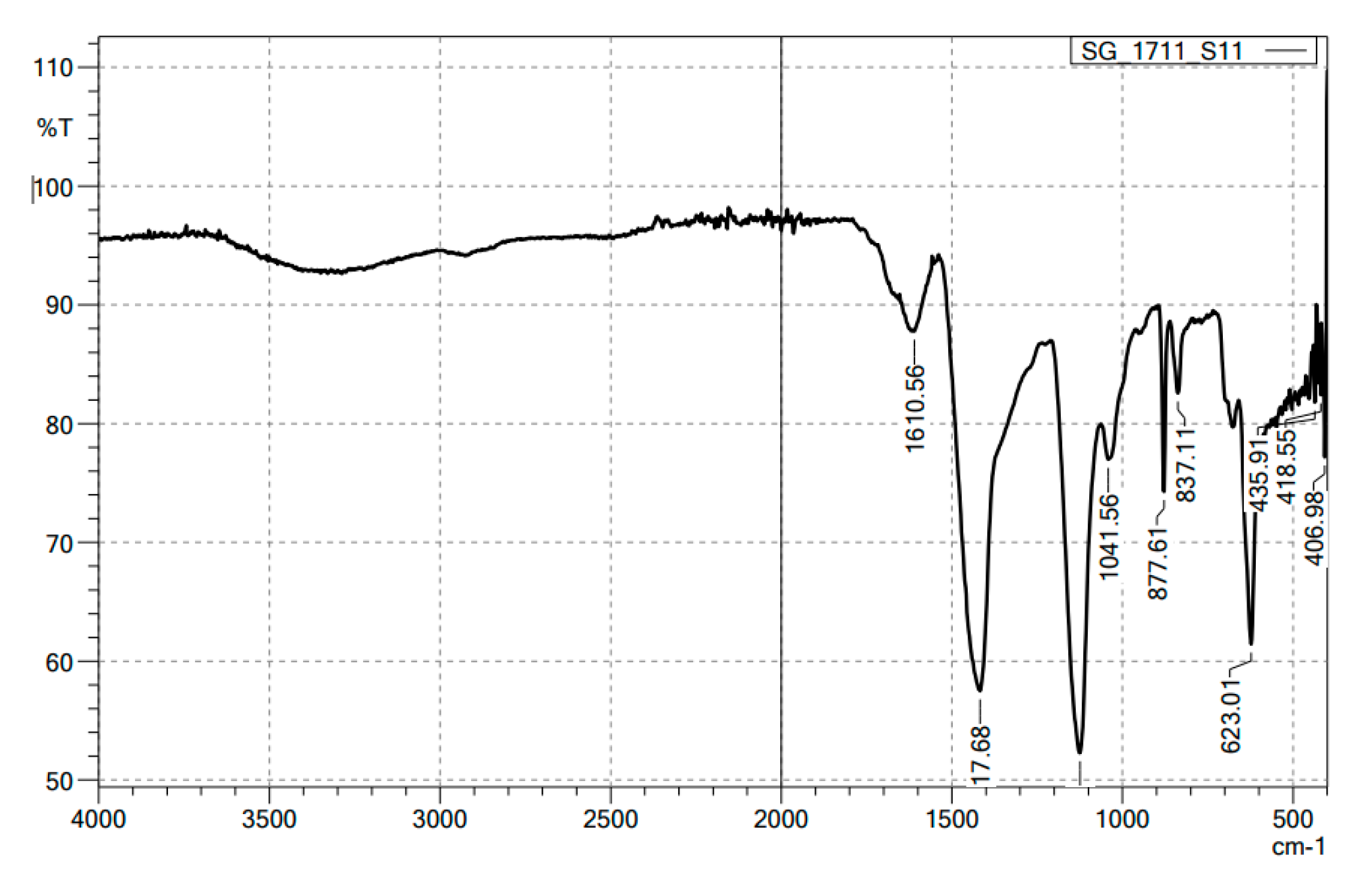
FTIR spectrum of alginate/lignin exhibited ten peaks composed of 1610.56, 1417.68, 1041.56, 877.61, 837.11, 623.01, 435.91, 418.55, 406.98 cm
-1, corresponding to the functional groups of C=C, O-H, S=O, C-H, C-S, and oxide groups (
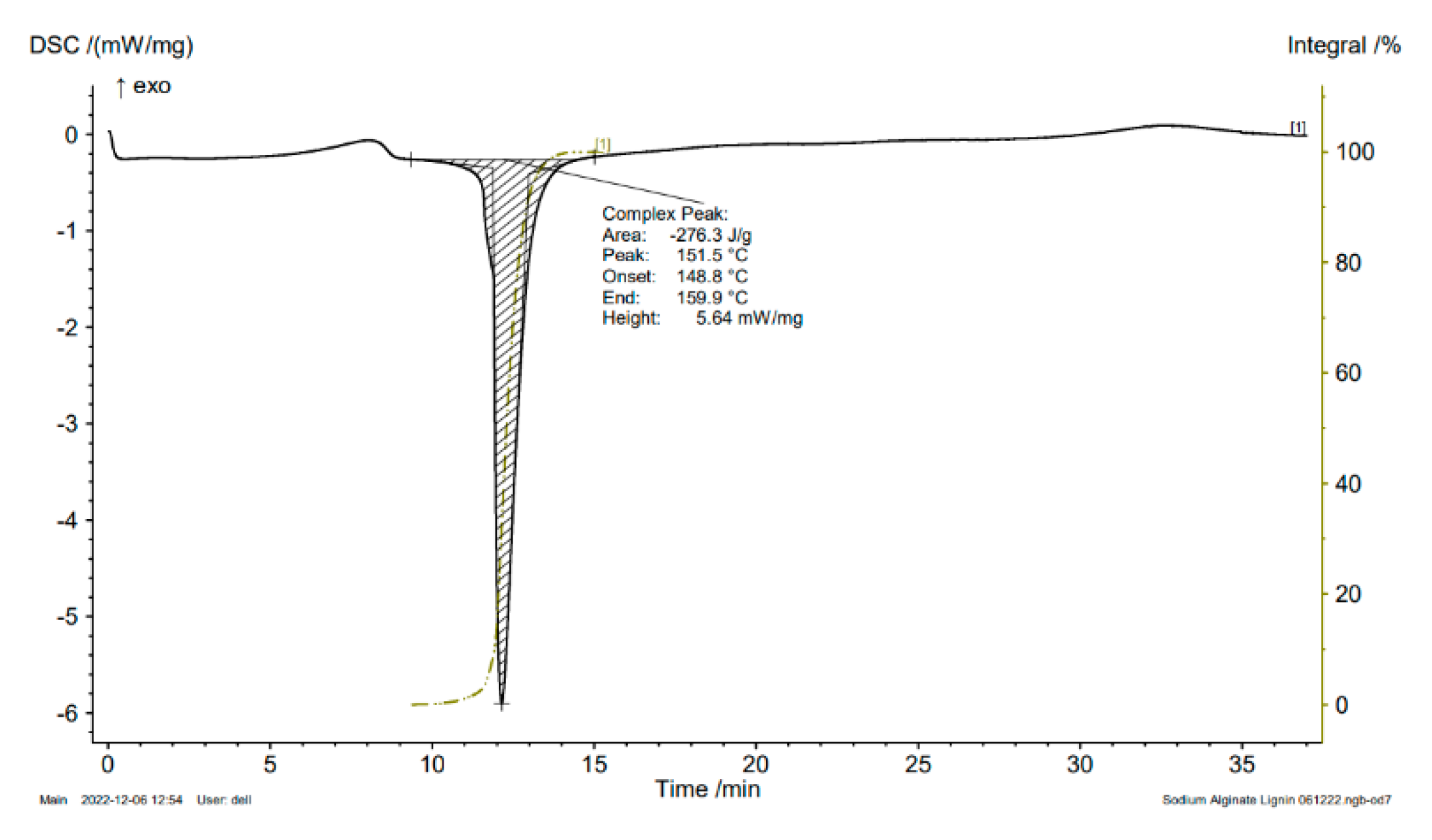
Figure 1). Alginate/lignin was crystalized at about 100
oC and melted for continuous degradation at 151.5
oC. The region of melting temperature (T
m) of alginate/lignin was in the range of 148.8 to 159.9
oC with an area of – 276.3 J/g (
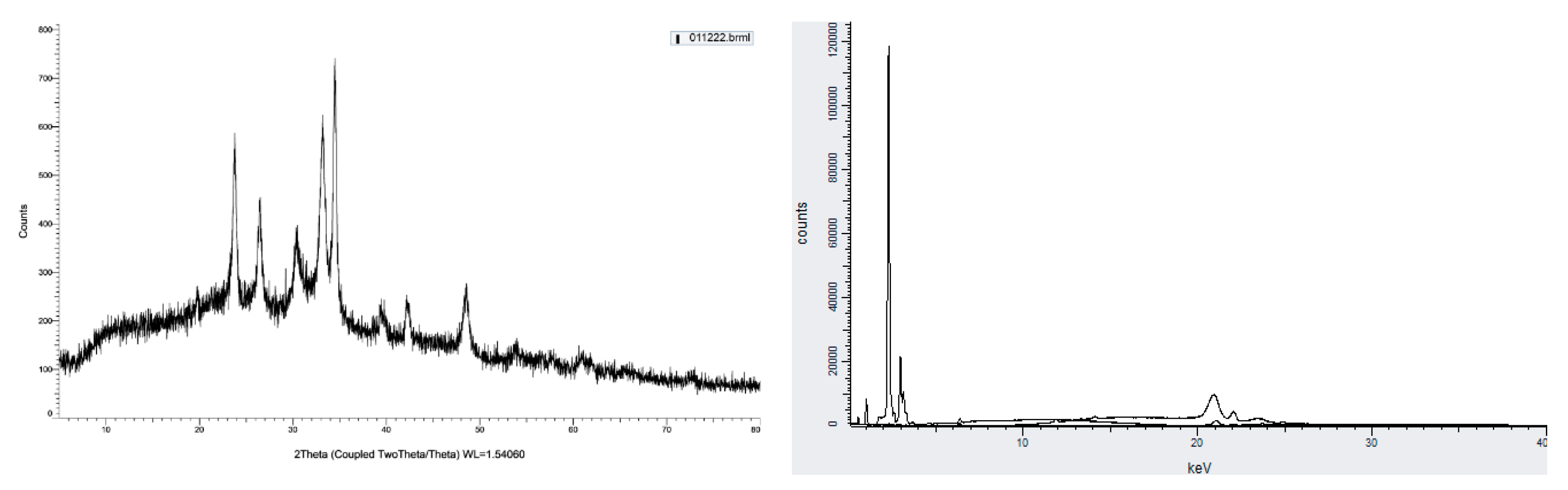
Figure 2). The structure of alginate/lignin did not exist in a linear or branched chain but crystallographic characteristics (
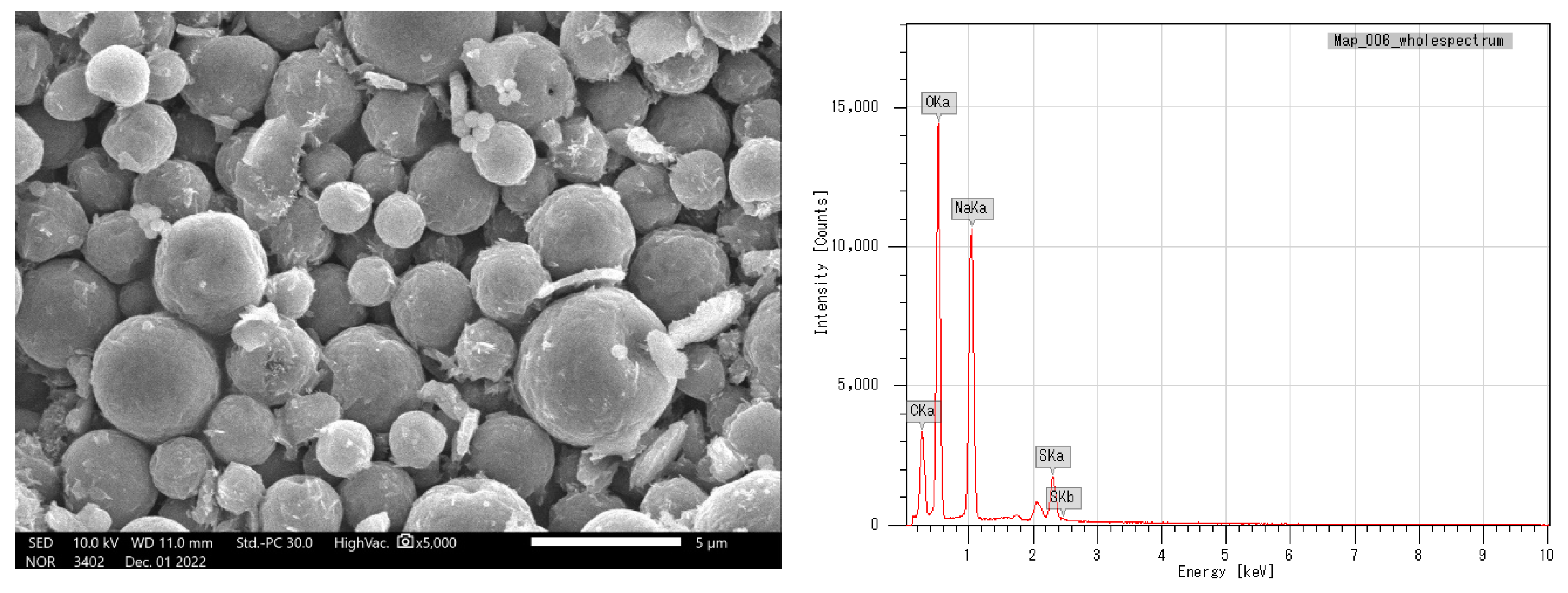
Figure 3a). Alginate/lignin was found in the shape of spherical particles and not broken (
Figure 4a). The chemical composition of alginate/lignin focused on the range of 1 to 3 and 21 – 22 keV (
Figure 3b) when analysis of them was by XRF spectrum. CK
a, OK
a, NaK
a, SK
a, and SK
b of alginate/lignin occurred in the energy range under 03 keV (
Figure 4b). The results of SEM_EDS mapping presented the mass percentage of elements C, O, Na, and S of alginate/lignin corresponding to 22.16 ± 0.08, 41.55 ± 0.13, 27.62 ± 0.12, 8.66 ± 0.08 (%), respectively. The atom percentage of elements C, O, Na, and S of alginate/lignin got 31.2 ± 0.11, 43.91 ± 0.13, 20.32 ± 0.09, 4.57 ± 0.04 (%), respectively (
Table S1).
Eight metals were detected in alginate/lignin composed of Si, Ta, Fe, Mg, Na, Na, K, R/R0, and P. Content of eight metals got a value from 0.1 to 54.9%, corresponding to Ta and Na, respectively. Nineteen oxides of alginate/lignin were shown including to SiO2 P
2O
5, SO
3, K
2O, Fe
2O
3, CoO, NiO, ZnO, As
2O
3, SrO, Sb
2O
3, CdO, SnO
2, HfO
2, Ta
2O
5, WO
3, and PbO. The oxide of SO
3 reached the highest value in the detected oxide of alginate/lignin, in fact, 43.74 (%). CoO, NiO, ZnO, As
2O
3, Sb
2O
3, and SnO
2 got the lowest value (0.01%) in the oxide that was determined in alginate/lignin (
Table 4).
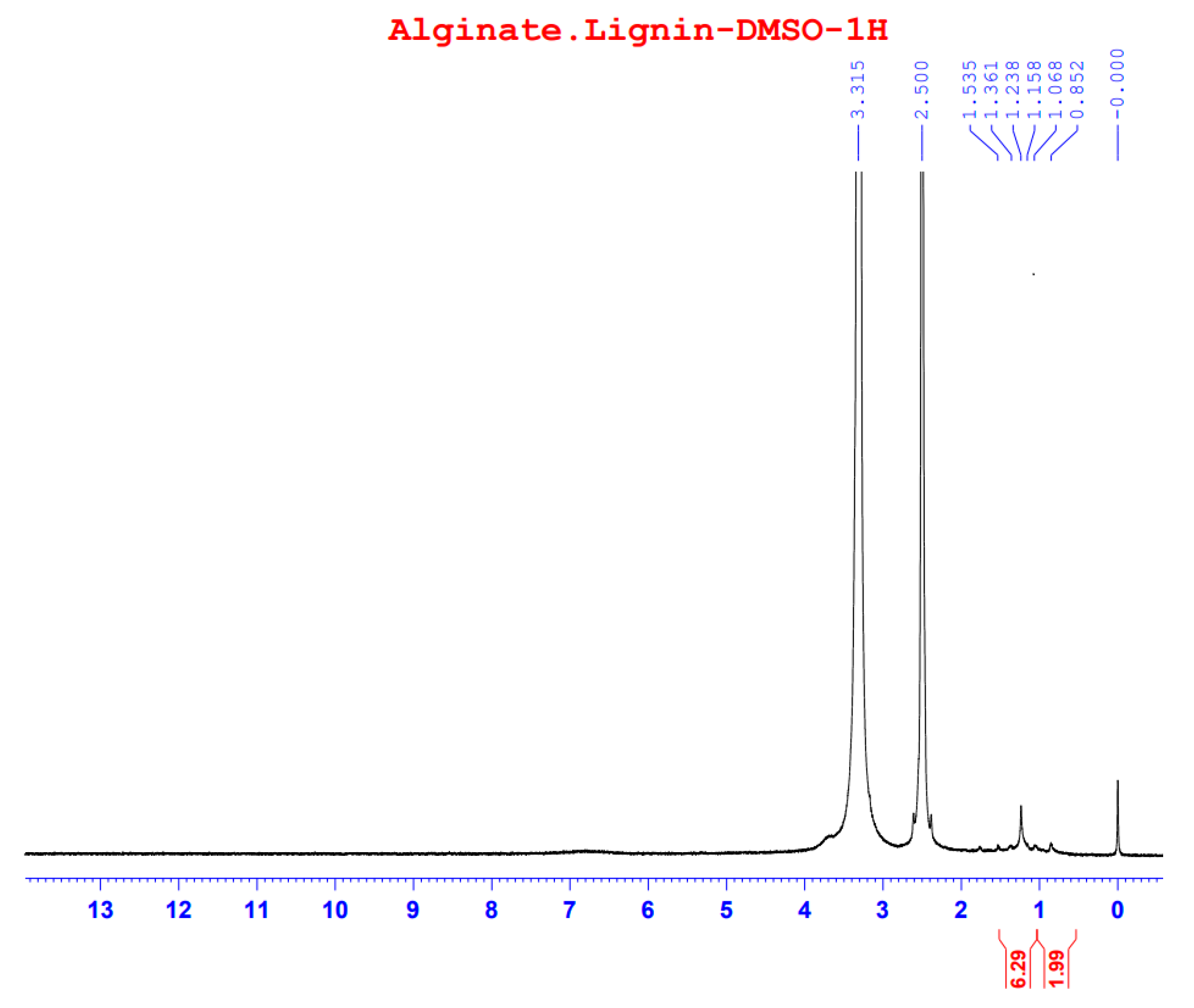
The
1H-NMR spectrum of alginate/lignin gave signals at 0.852, 1.068, 1.158, 1.238, 1.361, and 1.535 ppm (
Figure 5) with DMSO solvent for running spectrum. The solvent peak presented in the signal at 2.5 ppm.
2.4. Acute Toxicity
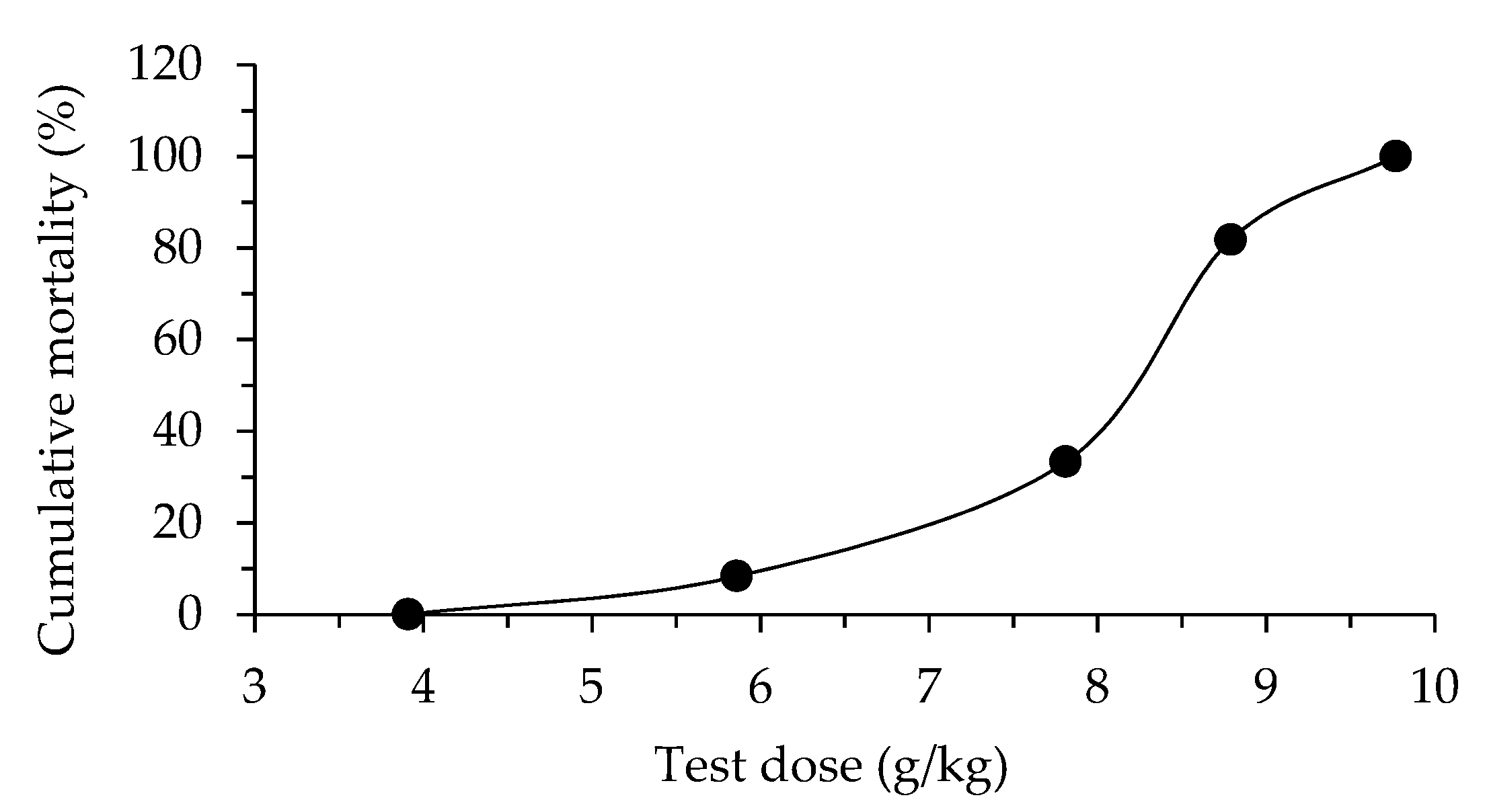
Acute oral toxicity of alginate/lignin material in mice showed that LD
0 and LD
100 were 3.91 g/kg and 9.77 g/kg, respectively.
From
Figure 6 and Equation 1, LD
16 and LD
84 were determined 6.67 g/kg and 8.91 g/kg, respectively.
The standard error of LD
50 was calculated by the formula:
In there: k = 0.66: constant Behrens; S: Normal distribution; d: Dose jump between 2 doses LD50, d = D2 – D1 = 8.79 – 7.81 = 0.98 g/kg; n: Average number of tested mice in 2 batches near the dose LD50, n = = 7 mice.
Hence, LD50 of material alginate/lignin = 8.15 ± 0.32 (g/kg).
The cumulative mortality percentage (%) changed according to the non-linear equation of level 2 (Eq. 1) (
Figure 6).
3. Discussion
The total antioxidant activity of alginate/lignin was higher than one of alginate in the previous notice [
9,
10] and polyphenol of sweet rowanberry cultivars [
24]. Reducing power activity of alginate/lignin exhibited higher than that of alginate of brow algae
Cystoseira schiffneri [
25]. The total antioxidant activity of alginate/lignin exhibited alginate/lignin possessing antioxidants, and the assay is a pioneer test for further antioxidant assay. Alginates play a role in the absorbent substance for metal, so their reducing power activity is usually high. Alginate/lignin showed the capacity of methylene blue movement [
26] and metal in the industry [
27]. Reducing power activity is necessary for numerous applications of alginate/lignin. DPPH free radical scavenging activity of alginate/lignin in the current study was higher than one in the notice [
28]. The highest DPPH free radical scavenging activity of alginate/lignin only corresponded to Trolox at 22.5 µg/mL. DPPH free radical scavenging efficiency of alginate/lignin is only 1% of that of Trolox. α – glucosidase inhibition activity of alginate/lignin was ten times more than acarbose. The results were potential in the application of diabetes medicine, functional food, or pharmaceutical raw materials. The results of α – glucosidase inhibition activity in the current study showed higher than previous notices on benzoylphloroglucinols from
Garcinia schomburgakiana [
29] and flavonoids [
30]. Antioxidant activities in the current study were not found in previous publications.
The results on the anticancer activity of alginate/lignin showed that alginate/lignin was non-selective on tested cancer cells because the inhibition percentage of cancer cells is lower than that of fibroblast cells. Alginate/lignin did not have potential in the research and development of K treatment products.
Functional groups of alginate and lignin did not found in the structure of alginate/lignin. Alginate/lignin only contained functional groups belonging to peaks of 1610.56 to 406.98 cm
-1. Peaks of 435.91, 418.55, and 406.98 cm
-1 showed the presentation of oxide groups in alginate/lignin. Peak 1417.68 cm
-1 exhibited the bending of carboxylic acid. The strong stretching of the C-O group got a peak of 1041.56 cm
-1. The strong bending of the C=C group presented at a peak of 837.11 cm
-1 [
31,
32]. The peak at 1610.56 and 877.61 cm
-1 represented the tensile vibration of the group - COO- and the oscillation of the glycoside ring in the alginate structure, respectively. The peak at 837.11 cm
-1 was typical for the aromatic ring vibration of lignin. The disappearance of the characteristic peaks of each starting material was also found, such as the peak of the -COOH group in alginate at about 1738 cm
-1 lost. Some typical peaks of lignin disappeared, such as the typical band for the symmetric and asymmetrical vibrations of the CH
3 group at 2920 and 2850 cm
-1, respectively, and the characterized peak for the C=C oscillation in the aromatic ring at about 1510 and 1460 cm
-1 [
33]. The band disappearance is exhibited by the interaction of two materials to form a stable composite system. DSC spectrum of alginate/lignin showed the melting temperature was similar to lignin in the notice [
34,
35]. SEM_EDS, SEM_EDS mapping, XRD, and XRF have shown the morphology, elemental distribution, and content of metal and oxide. Some flattened flakes are observed, which may be the beginning materials that were decomposed/torn and not attached to the composite system. Synthetic Alginate/lignin particles have a perfect spherical structure. The spheres are unequal in size, intertwined, and stick together. The XRD spectrum of the Alginate/lignin system shows the formation of a crystalline phase with sharp and sharp peaks on the amorphous background. In previous studies, the XRD spectra of alginate and lignin often show broad peaks of the carbon system, which are typical for the amorphous or poor crystalline structure of the materials [
36,
37]. The combination of alginate and lignin forms a composite system with the enhancement of the crystalline phase on an amorphous substrate. Based on these results, can completely guide the application of alginate/lignin as pharmaceutical materials and functional foods. The structural properties of alginate/lignin are necessary continuously studied by other solvent systems.
In, LD
0 and LD
100 is the highest doses that do not cause death in test mice, and the lowest dose causes 100% death in experimental mice of alginate/lignin material. The lethal dose of 50% of the test mice (LD
50) is shown in
Table 5 and the mice were tested over 3 dose levels with 6 to 8 mice (50% male, 50% female) per dose. After drinking alginate/lignin for 30-45 minutes, the mice decreased their activity, lay still, and had diarrhea. Some mice had severe diarrhea, weak breathing and died within 2-4 hours. The proportion of mice with diarrhea was proportional to the oral dose (
Table 5). The surviving mice recovered, and diarrhea stopped after 8-24 hours of drinking the sample. These mice eat bran pellets and drink water normally. Their stool, urine, and weight were not unusual after recovery. Abnormalities in circulatory function, digestive, sensory reflexes, excretory status, and hair of mice were absent. Mice lived within the first 72 h of observation and for 14 days of follow-up. The recovery time was proportional to the test dose level. Died mice during the observation period were operated on for further studies. For example, macroscopic examination showed that the internal organs and organs (heart, lungs, liver, stomach, intestines) were not abnormal. The alginate/lignin material exhibited acute oral toxicity in mice with an LD
50 value of 8.15 ± 0.32 g/kg, corresponding to an oral dose of 55 kg average adult of 36.443 ± 1.43 g /day (the dose conversion factor between adults and mice is 12.3). After taking alginate/lignin material, some mice showed diarrhea and decreased activity after 30-45 minutes; no abnormalities in circulation and sensory reflexes; then the mouse died within 2-4 hours.
4. Materials and Methods
4.1. Sample preparation
4.1.1. Extraction of Sodium Alginate
Brown seaweed Sargassum polycystum was selected, cleaned, and stored below 10 oC during transportation to the laboratory. Salt and impurities of seaweed were removed with fresh water in the laboratory and dried to a moisture content of 19±1%. After that, the seaweed is ground into a fine powder and stored at 10 oC for further studies. Phlorotannin was separated out of brown algae by 96% ethanol according to the ratio of 1/10 (w/v). The extraction of fucoidan was by H2SO4 solution (pH 2) for 2 hours at 80 oC. After fucoidan extraction, the residue was pressed to separate the liquid for extracting alginate. Alginate was extracted from the residue for 4 hours at 60 oC with the ratio Na2CO3 (pH 9)/dried seaweed (40/1, v/w). After that, the mixture was filtered hot to collect the filtrate and added to 10% CaCl2 solution (the ratio of CaCl2 to alginate was 2.0 / 1.0) to form a calcium alginate precipitate. Calcium alginate was washed with distilled water and decolorized with 20-30 mL of chlorine solution (1% chlorine solution/100 g calcium alginate antioxidant) for 30 min, and the movement of residual chlorine was with fresh water. Bleached calcium alginate was converted to alginic acid by solution (pH 2.0). Next, the alginic acid is converted to sodium alginate by dissolving the alginic acid in a Na2CO3 solution with a Na2CO3-alginic acid ratio of 0.35/1 (w/w). The mixture was filtered to remove the insoluble fraction of Na2CO3 solution and precipitated sodium alginate in 40% ethanol. The sodium alginate precipitate was filtered and dried at 50 oC under a vacuum to obtain the sodium alginate dry powder.
4.1.2. Extraction of alkaline lignin
Corn by-products (corn stalks and leaves) were extracted polyphenols and chlorophyll by 96% ethanol and were dried in the aerated shade until the moisture was below 20±1% for ground and storage in breathable solid bags for further studies. Samples were soaked in 4N NaOH at 80 oC for 150 minutes and filtered. The filtrate was adjusted to pH 5 to form a cellulose precipitate, and then collect the filtrate. The filtrate was adjusted to 70% ethanol concentration using 96% ethanol to obtain a hemicellulose precipitate. After the removed hemicellulose, the filtrate was adjusted to pH 2 to collect precipitated lignin. After centrifugation, the lignin precipitate was washed with clean water and dried at 50 oC under vacuum conditions to constant weight.
4.1.3. Preparation of alginate/lignin particles
Alginate/lignin powder was prepared by a spray-drying method. Sodium alginate in section 4.1.2. (5 % w/v) was dissolved in de-ionized water at 80 °C for 30 min with stirring at 500 rpm, followed by lignin addition in section 4.1.3 (2 % w/v, in 0.1 N NaOH) according to the alginate-to-lignin ratio of 90:10 and assimilated at 500 rpm. Tween 80 and tripolyphosphate were, in turn, added to the ultrasound-assisted mixture at the rate of 0.1%, compared with the total content of alginate and lignin. The mixture was continuously homogenized during spray drying. The spray drying was at the condition as follows: a pump speed of 1,500 mL/hour, pressure in the chamber of 0.2 atm, air heating temperature of 180 oC, and outlet temperature of 90 oC. Alginate/lignin powder was sifted and preserved in a sealed aluminum bag for further evaluation, for example, antioxidant activity, anticancer activity, physico-chemistry characteristics, and acute toxicity.
4.2. Determination of Antioxidant Activity
4.2.1. Total Antioxidant Activity
100 µL sample was diluted ten times with distilled water and mixed into solution A (0.6 M H
2SO
4, 28 mM sodium phosphate, and 04 mM ammonium molybdate) for 5 minutes. Then, the mixture incubation was at 95 °C for 90 min, and measured the mixture absorbance at the wavelength of 695 nm. Ascorbic acid was the standard substance [
38].
4.2.2. Reducing Power Activity
The determination of reducing power activity was according to the description of Zhu et al. (2002) [
39]. 500 μL sample was mixed, in turn, 0.5 mL of phosphate buffer (pH 7.2), 0.2 mL of 1% K
3[Fe(CN)
6], and kept for 20 min at 50 °C. Then, the mixture was, in turn, added to 500 μL of 10% CCl
3COOH, 300 μL distilled water, and 80 μL of 0.1% FeCl
3 for vortexing for 5 minutes. The absorbance measurement of the compound was at 655 nm with the FeSO
4 standard.
4.2.3. DPPH Free Radical Scavenging Activity
Dilute DPPH in 80% methanol to form 150 µM of DPPH solution and use immediately. Each well on a 96-well plate was, in turn, added to 200 µL of 150 µM DPPH and 25 µL of the sample at different concentrations. Measurement of the optical density (OD) of the compound was at 517 nm for 30 minutes with a jump of 5 minutes/ time. The positive control was Trolox [
40].
The percentage calculation of DPPH free radical scavenging activity was by the formula:
ODt and ODc were the optical density of the test sample and the control, respectively, and these OD value have been subtracted the OD value of solution wells of non-contain DPPH. SC50 value (test concentration of 50% DPPH free radical scavenger) determined based on a standard curve of optical density values of samples at different concentrations (Using Prism software with multi-parameter nonlinear regression and R2 > 0.9).
4.2.4. α-glucosidase Inhibition Activity
Add 120 µL of sample and 20 µL of α-glucosidase (1 unit/mL) to each well on a 96-well plate to incubate the mixture at 37 °C for 15 min. Then, add 20 µL of 5 mM
p-nitrophenyl-α-D glucopyranoside solution /well and incubate for 15 minutes at 37
oC. Finish the reaction by adding 80 µL of 0.2 M Na
2CO
3 solution /well. The absorbance measurement of the mixture was at the wavelength of 405 nm, and the positive control was acarbose [
41].
The percentage of α-glucosidase inhibition was calculated using the formula:
ODt and ODc were the optical density of the test and control samples, respectively. The OD value of these samples did not include blank OD value (without α-glucosidase). IC50 value (concentration of 50% α-glucosidase inhibitor test substance) was determined based on the standard curve of optical density values of samples at different concentrations (Using Prism software with R2 > 0.9).
4.3. Determination of Anticancer Activity
4.3.1. Cell Culture
The cell line of breast cancer (MCF-7), lung cancer (NCI H460), liver cancer (Hep G2), and fibroblast provided by ATCC (USA), raised cultured in E'MEM medium (MCF-7, NCI H460, fibroblast, and Hep G2) supplemented with L-glutamine (2 mM), HEPES (20 mM), amphotericin B (0.025 μg/mL), penicillin G (100 UI/mL), streptomycin (100 μg/mL), 10% (v/v) serum FBS bovine fetus and incubated at 37 oC, 5% CO2.
4.3.2. Investigation of Cancer Cytotoxic Activity by SRB Method
Inoculation of single cells was on 96-well plates at a density of 104 cells/well (for HeLa, Hep G2, fibroblast, and MCF-7 cell lines), 7.5x103 cells/well (for the NCI H460 line), and 5x104 cells/well for the Jurkat cell line. After 24 h of culture, the cell population was incubated with the probe at different concentrations for 48 h. Then, the total protein fixing of test cells was with a cold solution of 50% trichloroacetic acid (Sigma) (Jurkat alone was 70%) and stained with 0.2% sulforhodamine B solution (Sigma). The results read was with an ELISA reader at two wavelengths of 492 nm and 620 nm. The experiments were triplicated and presented the results on mean ± standard deviation.
After obtaining the optical density values at 492 nm and 620 nm (denoted OD
492 and OD
620):
Calculate the percentage (%) of cytotoxicity according to the formula:
With:
ODav: average OD value of the cells well
ODblank: OD value of blank well (no cells)
ODTS: the OD value of the test sample calculated from formulas (1) and (2)
ODC: OD value of the control specimen calculated from formulas (1) and (2)
IC
50 was determined using Prism software with regression multi-parameter non-linearity and R
2 > 0.9 [
42,
43].
4.4. Determination of Physico-Chemistry Characteristics
4.4.1. Functional Groups
The sample was measured at a wavelength range from 4000 to 500 cm−1 on an IRAffinity-1S of Shimadzu.
4.4.2. Surface Morphology and Elemental Composition
Alginate/lignin was determined on surface morphology and elemental composition by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) (SEM_EDS) method.
The method SEM_EDS (Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) mapping method) was used for analyzing the surface morphology and the distribution of elemental components of alginate/lignin.
4.4.3. Crystallographic characteristics
Crystallographic characteristics of alginate/lignin were determined according to the X-ray diffraction (XRD) method on a Brucker D2 Phaser instrument. Sample measurement parameters: Voltage: 30 KV; Current: 10 mA; Tube: Cu tube with 1.54184 [Å]; Detector: Lynxeye (1D mode); 2ϕ angle: 5-80 degrees; Stepsize (step measurement): 0.02 degrees.
The elemental compositions of alginate/lignin were analyzed by X-ray fluorescence (XRF) method on a Brucker S2 PUMA instrument. Sample measurement parameters: Voltage: 20 kV; Current: 100 µA, 2000 mW; Detector: X-Flash Energy; Energy: -958 eV to 40138 eV.
4.4.4. Thermal analysis
Thermal analysis of alginate/lignin was according to the differential scanning calorimetry (DSC) method on a NETZSCH DSC 204F1 Phoenix. The surveyed temperature was 30 oC/10.0(K/min)/400 oC and atmosphere of N2, 40.0 mL/min / N2, 60.0 mL/min.
4.4.5. NMR spectra
NMR spectra were measured on a Bruker AVANCE Neo 600MHz instrument at 70 °C, using DMSO as solvent and DSS as an internal standard with a water-reduced measurement technique.
4.5. Determination of Acute Toxicity
4.5.1. Test mice
ICR strain white mice (Swiss albino) composed of male and female at six weeks old with a weight of 20 to 26 g, provided by Nha Trang Institute of Vaccines and Medical Biologicals. Mice were healthy without abnormal expression raised in the standard experimental condition for five days. Mice were supplied adequate food and water in the size cages of 25x35x15 cm.
4.5.2. Investigation of Acute Oral Toxicity
Principle: Give the test rats the same dose of the test sample under the same steady-state conditions, and observe the reactions occurring within 72 hours and 14 days.
Procedure: Ten mice were fasted for at least 12 hours before giving them the maximum possible oral dose of the test sample with a volume of 50 ml/kg [
44]. Recording the general movement, behavior, hair state, eating, urination, and death of mice was done within 72 hours. If the mouse shows no abnormality or dies after 72 hours, continue monitoring for 14 days. There are three possible cases:
- Case 1: After the mice drank the test sample, the mice did not die, continuously determining the highest possible dose of the test sample through the needle without causing the mouse to die (Dmax).
- Case 2: After giving the test sample to mice, the mortality rate is 100%, then try with a dose of ½ of the first dose until a minimum amount is lethal to 100% of mice (LD100) and a maximum amount that is not lethal to rats (LD0). Conduct testing to determine LD50:
Dividing mice into four lots, each batch of 6 mice. Divide the four doses by an exponential interval from LD0 - LD100. At doses close to LD50, the number of mice was increased for more accurate measurements and monitoring for 72 hours to record the movements of mice, and the number of dead mice in each batch, and calculate the mortality fraction to find LD50.
- Case 3: After giving the test sample, the mouse death rate is lower than 100%, the dose of LD100 cannot be determined, and the LD50 cannot be determined. In this case, it is only possible the determination of the maximum dose which is not lethal to mice, called sub-lethal dose (LD0).
The LD
50 value determined by the Behrens method based on two doses close to the LD
50 lethal dose:
In which:
D1 is the lethal dose a% of test animals (dose close to 50%).
D2 is the deadly dose b% of test animals (the upper dose is close to 50%).
d = D2 – D1 is the dose step between 2 doses near LD50.
4.6. Data Analysis
Each experiment was triplicated (n=3) and presented the results under mean ± SD. Analysis of ANOVA and statistics were on the software MS. Excel 2010. IC50 determination of cell toxicity activity, DPPH free radical scavenging activity, and α-glucosidase inhibition activity were analyzed using Prism software.
5. Conclusions
Alginate/lignin is a potential antioxidant material for pharmaceutical materials, functional foods, and supporting diabetes treatment. Total antioxidant activity and reducing power activity were highly evaluated in alginate/lignin, especially, α – glucosidase inhibition activity of alginate/lignin. Alginate/lignin did not exhibit DPPH free radical scavenging activity and also be typical in four cancer cell lines such as Hep G2, fibroblast, MCF-7, and NCI H460. Alginate/lignin was in a thermally stable regular spherical shape containing both metal and oxide. C, O, Na, and S were typical elements in the alginate/lignin structure. Alginate/lignin crystal did not contain some specific functional groups of alginate and lignin. The acute toxicity test of alginate/lignin in mice presented LD0 (3.91 g/kg) and LD100 (9.77 g/kg).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Mass and atom of elements in alginate/lignin on SEM_EDS mapping.
Author Contributions
Conceptualization, L.T.H.A. and H.T.H.; methodology, N.X.H. and H.T.H.; software, D.X.C.; validation, H.T.H., and N.X.H; formal analysis, H.T.H; investigation, H.T.H. and N.X.H.; resources, L.T.H.A.; data curation, D.X.C.; writing—original draft preparation, L.T.H.A., N.X.H., H.T.H. and D.X.C.; writing—review and editing, L.T.H.A., H.T.H., N.X.H. and D.X.C.; visualization, N.X.H.; supervision, D.X.C.; project administration, N.X.H.; funding acquisition, N.X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Industry and Trade, grant number 114.2021.ĐT.BO/HĐKHCN”.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Ministry of Health (458/QĐ-BYT, February 16, 2012) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thankful for Assoc. Prof. Dr. Do Thi Hong Tuoi – Ho Chi Minh City University of Medicine and Pharmacy
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A brief review on the development of alginate extraction process and its sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Roya, A.-k.; Latifa, T.; Nasim, S.; Anil, K.P.; Slim, A.; Philippe, M. Structures, properties and applications of alginates. Mar Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Savindra, K.; Dinabandhu, S. A comprehensive analysis of alginate content and biochemical composition of leftover pulp from brown seaweed Sargassum wightii. Algal Res. 2017, 23, 233–239. [Google Scholar]
- Vijayalakshmi, K.; Latha, S.; Rose, M.H.; Sudha, P.N. Industrial applications of alginate. In Industrial applications of marine biopolymers; Sudha, P.N., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 545–575. [Google Scholar]
- Niculescu, A.-G.; Grumezescu, A.M. An up-to-date review of biomaterials application in wound management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci 2020, 37, 100672. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Thu, T.M.Q.; Van, T.T.T.; Thanh, V.N.; Shiho, S.; Shinichi, K.; Yoshiaki, Y. Structural characteristics and biological activity of different alginate blocks extracted from brown seaweed Turbinaria ornate. J Carbohydr Chem 2021, 40, 1–18. [Google Scholar]
- Dang, X.C.; Dang, T.T.T.; Do, T.K. Biophysical-chemistry characterization of alginate in brown algae species Sargassum dupplicatum. WJFST. Special Issue: Marine bio-polymer: Bio-activity, extraction and application 2020, 4, 17–22. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vu, N.B.; Nguyen, X.H.; Dang, T.T.T.; Do, T.K.; Nguyen, K.N.; Nguyen, N.B.H.; Pham, T.T.; Pham, T.T.; Dang, X.C. The content, antioxidant activity, and structural characteristics of sodium alginate extracting from Sargassum polycystum grew in Vietnam: Effect of various extraction conditions. J Pharm Res Int 2021, 33, 197–206. [Google Scholar]
- Cör, A.D.; Knez, Ž.; Knez, M.M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front Pharmacol 2022, 13, 934982. [Google Scholar] [CrossRef]
- Maochen, X.; Qi, C.; Yu, W.; Han, X.; Jiarui, Z.; Qing, Z.; Aiguo, J.; Shuliang, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Jiayu, C.; Yu, H.; Lirong, Z.; Yingjian, W.; Shichao, W.; Yanzi, Z.; Haiyan, G.; Degang, J.; Yingtao, W. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front Pharmacol 2017, 8, 623. [Google Scholar] [CrossRef]
- Qingquan, L.; Le, L.; Luqing, Z. Lignins: Biosynthesis and biological functions in plants. Int J Mol Sci 2018, 19, 335. [Google Scholar] [CrossRef]
- Jianming, T.; Sheng, L.; Fayin, Y.; Yun, Z.; Lin, L.; Guohua, Z. Lignin – An underutilized, renewable and valuable material for food industry. Crit Rev Food Sci Nutr 2020, 60, 2011–2033. [Google Scholar]
- Atika, A.; Fahriya, P.S.; Asma, S.; Witta, K.R.; Melati, S.; Nurhani, A.; Widya, F.; Adarsh, K. Current roles of lignin for the agroindustry: Applications, challenges, and opportunities. Int J Biol Macromol 2023, 240, 124523. [Google Scholar]
- Theodoros, V.; George, Z.; Francis, V. Plant food residues as a source of nutraceuticals and functional foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Ngo, D.V.; Hang, T.T.; Nhi, D.B.; Cuong, D.V.; Hung, V.N. Lignin and cellulose extraction from Vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int J Polym Sci 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: properties, applications, analyses, and methods of extraction: a review. Biotechnol Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Jost, R.; Fredrik, H.B.; Gary, C.-C. Functional surfaces, films, and coatings with lignin – a critical review. RSC Adv 2023, 13, 12529–12553. [Google Scholar] [CrossRef]
- Pinar, K.; Sansanee, K.; Marco, P.C.M.; Samir, S.; Timothy, D.H.B.; Gary, J.L. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: linking lignin source and processing with clinical indication. Biomass Conv Bioref 2023. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Sada, K.A.; Ajab, H. 9 - Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic fibre and biomass-based composite materials. processing, properties and applications; Mohammad, J., Paridah, M.T., Naheed, S., Eds.; Woodhead Publishing: Cambridge, United Kingdom, 2017; pp. 165–178. [Google Scholar]
- Roland, E.H.; Nicolas, B.; Laurent, C.; Christian, S.; Poulomi, S.; Arthur, R. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym Degrad Stab 2009, 94, 1632–1638. [Google Scholar]
- Orsavová, J.; Tunde, J.; Růžena, B.; Jiří, M. Total phenolic and total flavonoid content, individual phenolic compounds and antioxi-dant activity in sweet rowanberry cultivars. Antioxidants 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Suming, L.; Moncef, N.; Zouari, N. The brown seaweed Cystoseira schiffneri as a source of sodium alginate: Chemical and structural characterization, and antioxidant activities. Food Biosci 2021, 40, 100873. [Google Scholar] [CrossRef]
- Tao, C.; Haochen, L.; Jie, G.; Guowen, H.; Yuan, Z.; Xiuqin, T.; Xiaobing, H. Efficient removal of methylene blue by bio-based sodium alginate/lignin composite hydrogel beads. Polymers 2022, 14, 2917. [Google Scholar] [CrossRef]
- Xiangpeng, G.; Cheng, G.; Junjie, H.; Zhuo, Z.; Hongming, L.; Mingyang, L. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int J Biol Macromol 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Rita, A.; Federica, M.; Rodolfo, E.; Gerardino, D.; Lucia, P.; Alessandra, N. Recovery of lignins with potent antioxidant properties from shells of edible nuts by a green ball milling/deep eutectic solvent (des)-based protocol. Antioxidants 2022, 11, 1860. [Google Scholar] [CrossRef]
- Huy, T.N.; Thanh-Trung, N.; Thuc-Huy, D.; Nguyen-Minh-An, T.; Chuong, H.N.; Thi-Hong-Anh, N.; Jirapast, S. α-glucosidase inhibitory and antimicrobial benzoylphloroglucinols from Garcinia schomburgakiana fruits: In vitro and in silico studies. Molecules 2022, 27, 2574. [Google Scholar] [CrossRef]
- Bo-wei, Z.; Xia, L.; Wen-long, S.; Yan, X.; Zhi-long, X.; Chun-lin, Z.; Yue-sheng, D. Dietary flavonoids and acarbose synergistically inhibit α-glucosidase and lower postprandial blood glucose. J Agric Food Chem 2017, 65, 8319–8330. [Google Scholar]
- Bin, S.; Hongxu, L.; Ruru, S.; Pai, P.; Yun, J.; Diao, S. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int J Biol Macromol 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Dominika, F.; Zbigniew, H.; Dorota, K. Fabrication, characterization and evaluation of an alginate–lignin composite for rare-earth elements recovery. Materials 2022, 15, 944. [Google Scholar] [CrossRef]
- Guiting, L.; Hongxun, Z.; Hong, W.; Rong, C.; Shaoyun, G. Preparation of alginate hydrogels through solution extrusion and the release behavior of different drugs. J Biomater Sci Polym Ed 2016, 27, 1808–1823. [Google Scholar]
- Tong, H.; Nanta, S.; Ayumu, T.; Olena, S.; Pelle, M.; Weihong, Y. Characterization of lignin at pre-pyrolysis temperature to investigate its melting problem. Fuel 2019, 235, 1061–1069. [Google Scholar]
- Keshaw, R.A.; Harit, J. Physico-chemical properties of lignin–alginate based films in the presence of different plasticizers. Iran Polym J 2016, 25, 661–670. [Google Scholar]
- Köhnke, J.; Gierlinger, N.; Prats-Mateu, B.; Unterweger, C.; Solt, P.; Mahler, A.; Schwaiger, E.; Liebner, F.; Gindl-Altmutter, W. Comparison of four technical lignins as a resource for electrically conductive carbon particles. BioRes 2019, 14, 1091–1109. [Google Scholar] [CrossRef]
- Samer, R.A.; Mutasem, O.T. Enhanced drug encapsulation and extended release profiles of calcium–alginate nanoparticles by using tannic acid as a bridging cross-linking agent. J Microencapsul 2015, 32, 96–105. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem, 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Zhu, Q.; Hackman, R.; Ensunsa, J.; Holt, R.; Keen, C. Antioxidative activities of Oolong tea. J Agric Food Chem 2002, 50, 6929–6934. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.H.; Priyadarshini, G.; Michael, T. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Luo-sheng, W.; Cui-ping, C.; Zuo-qi, X.; Yong-long, Wang; Qiu-xia, M.; Yuedong, Y.; Jiachun, C. In vitro and in vivo anti-diabetic activity of Swertia kouitchensis extract. J Ethnopharmacol 2013, 147, 622–630. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Nguyen, T.V.; Tat, T.T.; Nguyen, T.T.G.; Nguyen, N.H.; Ho, H.T.D. (2007). Standardize the sulforhodamine B (SRB) test to determine the cytotoxicity of the natural compound. In Proceedings of National Scientific Conference 2007 - Basic Research in Life Sciences, Quy Nhon, Vietnam, 10/08/2007 (p. 809).
- My-Nuong, T.N.; Thuy-Duong, H.-H. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects. BMC Complement Altern Med 2016, 16, 202–236. [Google Scholar]
- Do, T.D. Methods for determining acute toxicity of drugs; Medical Publishing House, Ha Noi, Vietnam, 2014.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).