Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
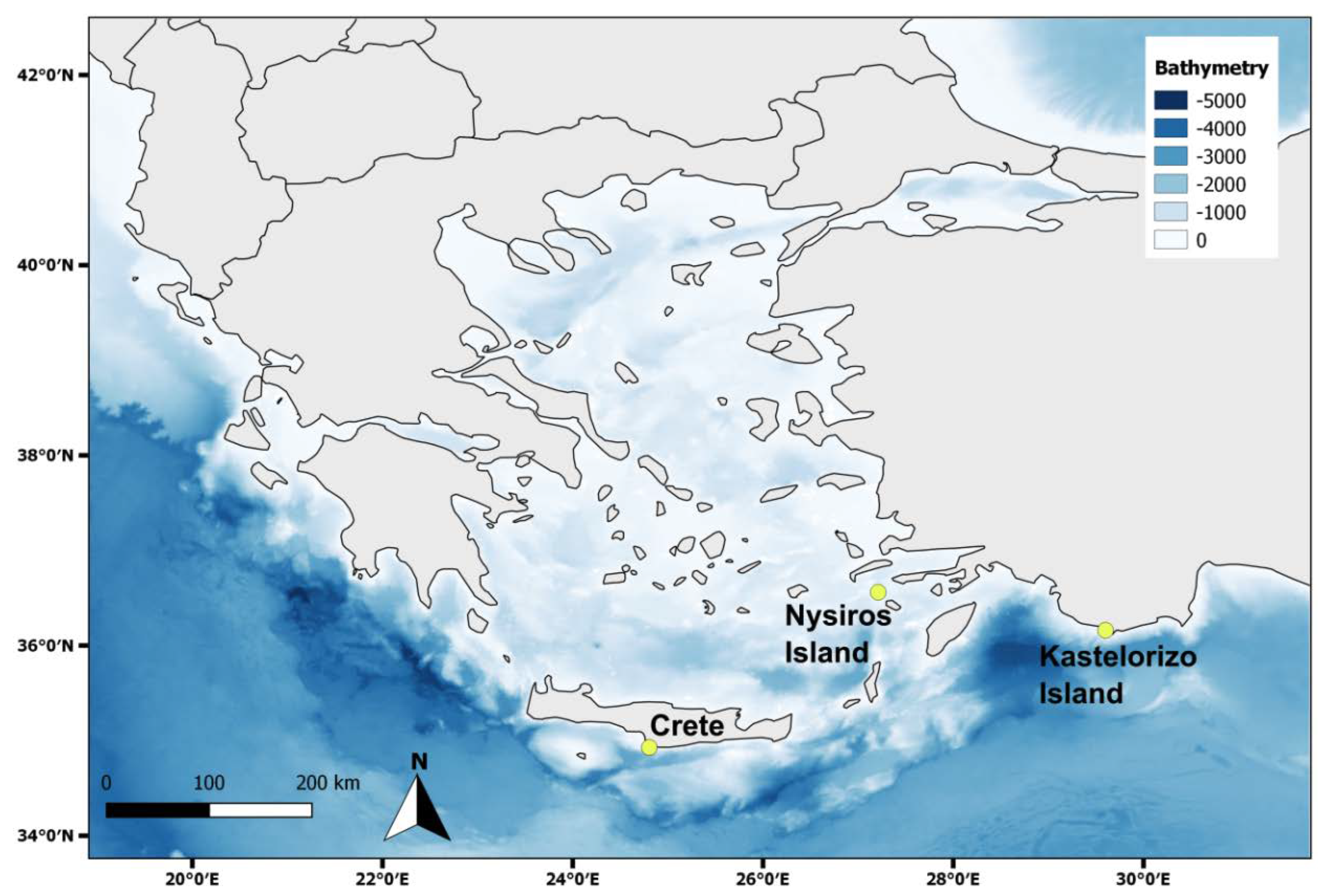
2.2. Sample collection
2.3. Lab work
2.4. Data analysis
3. Results
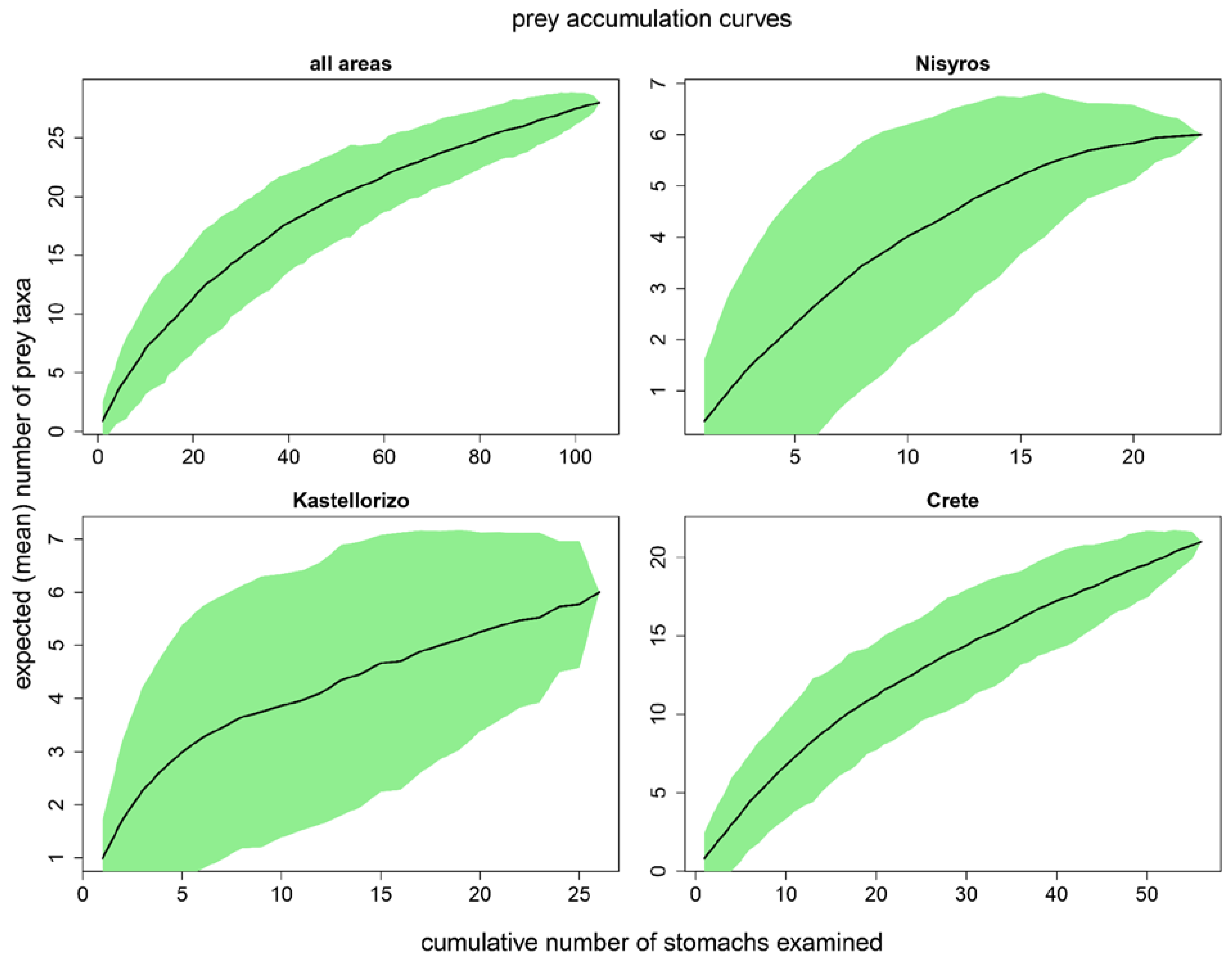
3.1. Sample size adequacy
3.2. Pterois miles feeding intensity
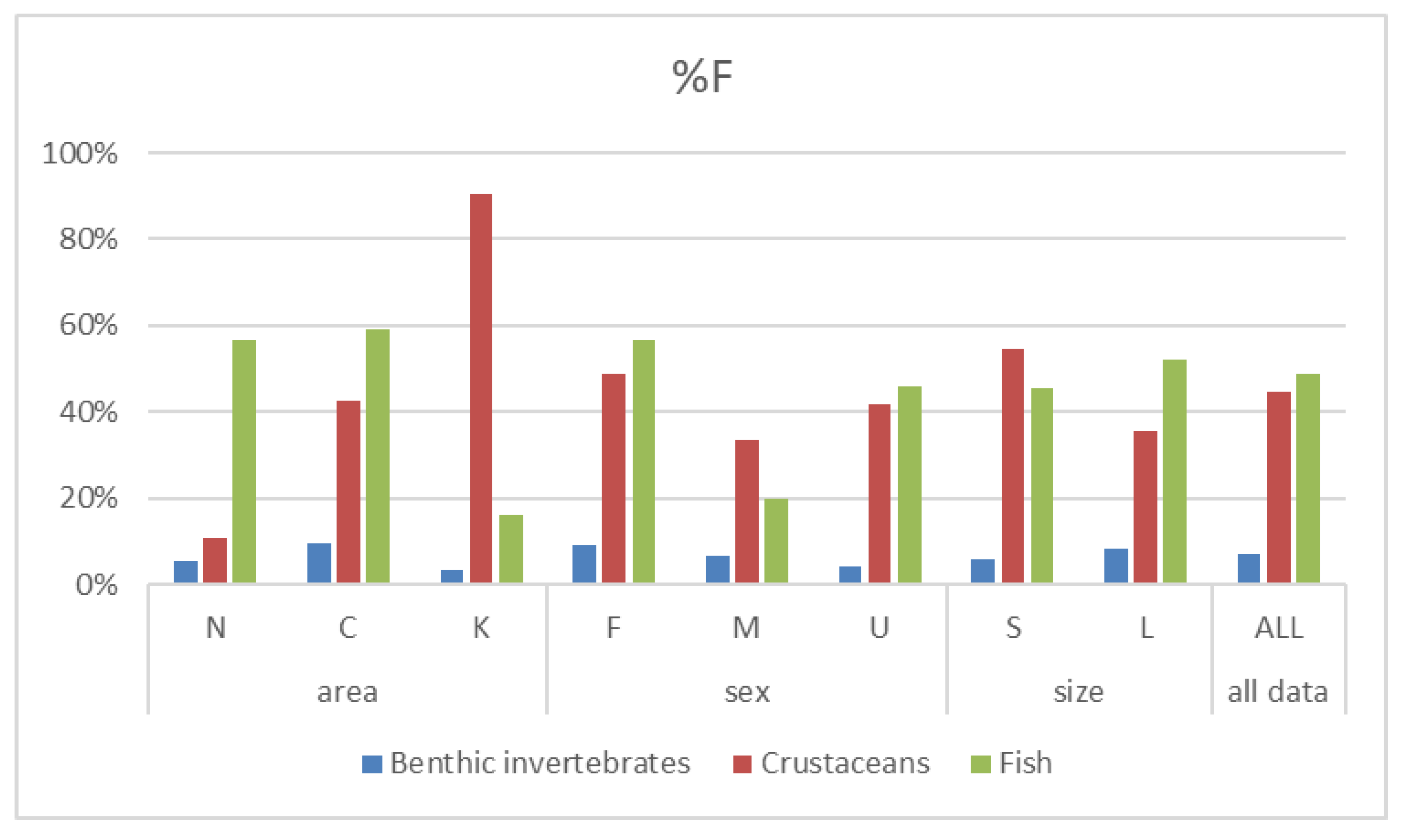
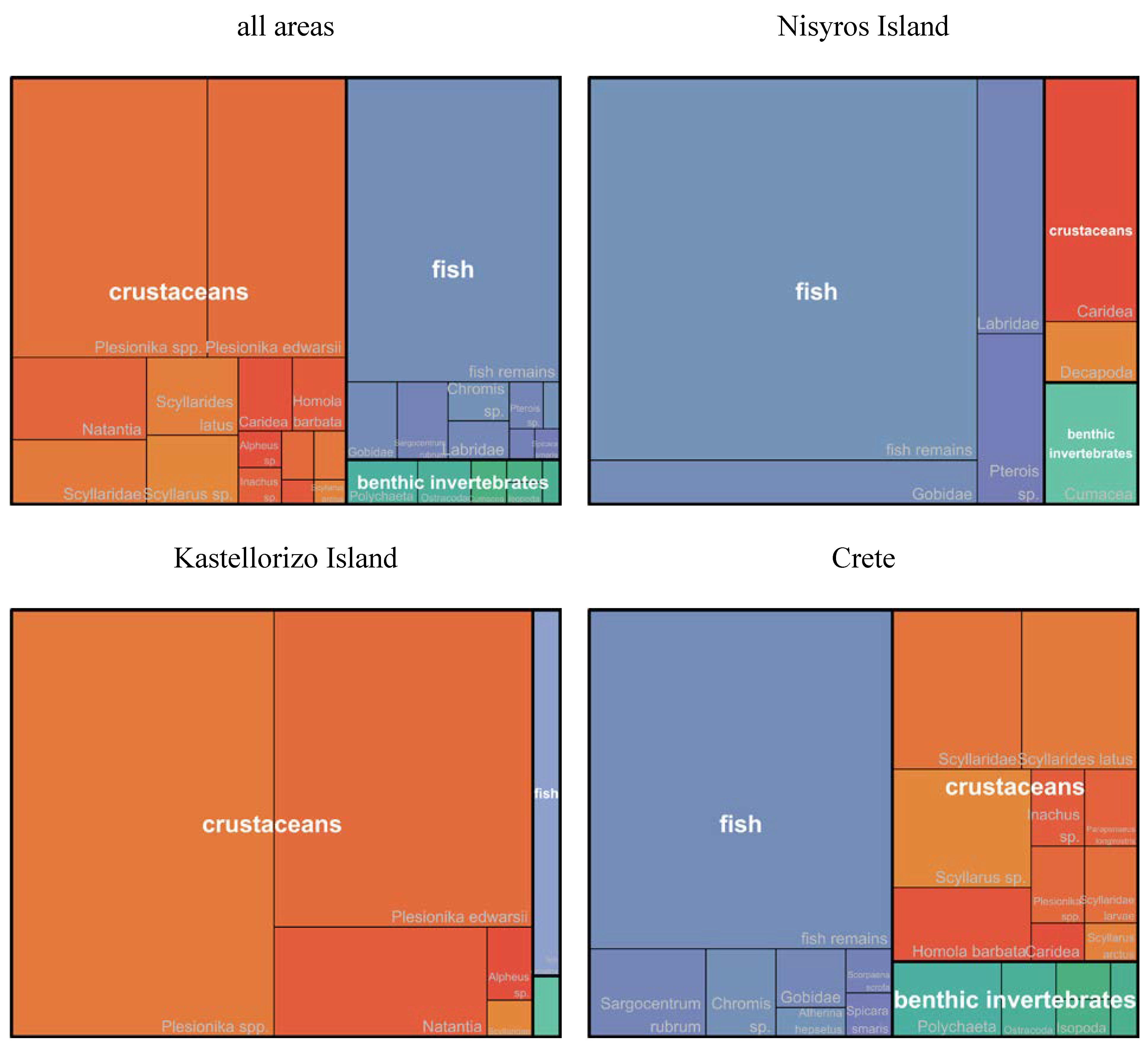
3.3. Contribution of prey taxa to P. miles diet
3.3. Multivariate analysis of P. miles diet composition
3.4. Pterois miles diet overlap between areas, sexes and size classes
3.5. Pterois miles feeding strategy
3.6. Pterois miles diet breadth
4. Discussion
Supplementary Materials
Acknowledgments
References
- Zenetos, A. , Albano, P. G., Lopez Garcia, E., Stern, N., Tsiamis, K., & Galanidi, M. (2022). Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterranean Marine Science, 23(1). [CrossRef]
- Katsanevakis S, Rilov G, Edelist D (2018) Impacts of marine invasive alien species on European fisheries and aquaculture–plague or boon? 1: CIESM Monograph 50.
- Tsirintanis K, Azzurro E, Crocetta F, Dimiza M, Froglia C, Gerovasileiou V, Langeneck J, Mancinelli G, Rosso A, Stern N, Triantaphyllou M, Tsiamis K, Turon X, Verlaque M, Zenetos A, Katsanevakis S (2022) Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. 3: Aquatic Invasions 17(3). [CrossRef]
- Olden, J. , & Poff, N., Douglas, M., Douglas, M., & Fausch, K. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends in ecology & evolution. 19. 18-24. [CrossRef]
- South, J. , Dick, J. T., McCard, M., Barrios-O’Neill, D., & Anton, A. (2017). Predicting predatory impact of juvenile invasive lionfish (Pterois volitans) on a crustacean prey using functional response analysis: effects of temperature, habitat complexity and light regimes. Environmental Biology of Fishes, 100, 1155-1165. [CrossRef]
- Fanelli, E. , Azzurro, E., Bariche, M., Cartes, J. E., & Maynou, F. (2015). Depicting the novel Eastern Mediterranean food web: a stable isotopes study following Lessepsian fish invasion. 2163; 17. [Google Scholar]
- Bezerra, L. A. V. , Angelini, R. I. ( 817, 475–489. [CrossRef]
- Côté, I.M. & Smith N.S. (2018). The lionfish Pterois sp. invasion: Has the worst-case scenario come to pass? Fish Biology. 92(3):660-689. [CrossRef]
- Lesser, M. P. , Slattery, M., 2011. Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biological Invasions, 13 (8), 1855-1868. [CrossRef]
- Green SJ, Akins JL, Cote IM (2011). Foraging behaviour and prey consumption in the Indo-Pacific lionfish on Bahamian coral reefs. Mar Ecol Prog Ser 433:159–167. [CrossRef]
- Côté, I.M. , Green S.J., Hixon M.A. 2013. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biological Conservation 164: 50–61. [CrossRef]
- Dahl, K.A. & Patterson III, W.F. (2014) Habitat-specific density and diet of rapidly expanding invasive red lionfish, Pterois volitans, populations in the Northern Gulf of Mexico. PLoS ONE 9(8): e105852. [CrossRef]
- Green SJ & Coˆte’ IM (2014). Trait-based diet selection: prey behaviour and morphology predict vulnerability to predation in reef fish communities. J Anim Ecol 83(6):1451–1460. [CrossRef]
- Harms-Tuohy, C. , Schizas, N.V., & Appeldoorn, R.S. 2016. Use of DNA metabarcoding for stomach content analysis in the invasive lionfish Pterois volitans in Puerto Rico. Marine Ecology Progress Series 558: 181-191. [CrossRef]
- Dahl, K.A. , Patterson III, W.F., Robertson, A., & Ortmann, A.C. 2017. DNA barcoding significantly improves resolution of invasive lionfish diet in the Northern Gulf of Mexico. Biological Invasions 19(6): 1917-1933. [CrossRef]
- Green SJ, Akins JL, Maljkovi A, & Coˆte’ IM. 2012. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 7(3):e32596. [CrossRef]
- Layman CA, & Allgeier JE. 2012. Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Prog Ser 448:131–141. [CrossRef]
- Golani, D. & Sonin, O., 1992. New records of the Red Sea fishes, Pterois miles (Scorpaenidae) and Pteragogus pelycus (Labridae) from the eastern Mediterranean Sea. Japanese Journal of Ichthyology, 39 (2), 167-169. [CrossRef]
- Albins, M.A. , & Hixon, M.A. (2008) Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Marine Ecology Progress Series 367: 233-238. [CrossRef]
- Bariche, M. Torres, M., & Azzurro, E. (2013). The Presence of the invasive Lionfish Pterois miles in the Mediterranean Sea. Mediterranean Marine Science. 14(2), 292–294. [CrossRef]
- Dimitriadis, C. , Galanidi, M., Zenetos, M., Corsini-Foka, M., Giovos, I., Karachle, P.K., Fournari-Konstantinidou, I., Kytinou, E., Issaris, Y., Azzurro, E., Castriota, L., Falautano, M., Kalimeris, A., & Katsanevakis, S. (2020). Updating the occurrences of Pterois miles in the Mediterranean Sea, with considerations on thermal boundaries and future range expansion. Mediterranean Marine Science, 21(1), 62–69. [CrossRef]
- Peake, J. , Bogdanoff A.K., Layman C.A., Castillo B., Reale-Munroe K., Chapman J., Dahl K., Patterson W.F.III, Eddy C., Ellis R.D., Faletti M., Higgs N., Johnston M.A., Muñoz R.C., Sandel V., VillasenorDerbez J.C., Morris J.A.jr. 2018. Feeding ecology of invasive lionfish (Pterois volitans and Pterois miles) in the temperate and tropical western Atlantic. Biological Invasions 20 (9): 2567–2597. [CrossRef]
- Côté, I.M. , Maljković A. 2010. Predation rates of IndoPacific lionfish on Bahamian coral reefs. Marine Ecology Progress Series 404: 219–225. [CrossRef]
- Ritger, A. L. Fountain, C. T., Bourne, K., Martín-Fernández, J. A., & Pierotti, M. E. (2020). Diet choice in a generalist predator, the invasive lionfish (Pterois volitans/miles). Journal of Experimental Marine Biology and Ecology, 524. [CrossRef]
- Zannaki, K.; Corsini-Foka, M.; Kampouris, T.E.; Batjakas, I.E. 2019. First results on the diet of the invasive Pterois miles (Actinopterygii: Scorpaeniformes: Scorpaenidae) in the Hellenic waters. Acta Ichthyol. Piscat. 2019, 49(3), 311–317. [CrossRef]
- D’Agostino, D.; Jimenez, C.; Reader, T.; Hadjioannou, L.; Heyworth, S.; Aplikioti, M.; Argyrou, M.; Feary, D.A. Behavioural traits and feeding ecology of Mediterranean lionfish and naiveté of native species to lionfish predation. Mar. Ecol. Prog. Ser. 2020, 638, 123–135. [Google Scholar] [CrossRef]
- Savva, I.; Chartosia, N.; Antoniou, C.; Kleitou, P.; Georgiou, A.; Stern, N.; Hadjioannou, L.; Jimenez, C.; Andreou, V.; Hall-Spencer, J.M.; Kletou, D. They are here to stay: the biology and ecology of lionfish (Pterois miles) in the Mediterranean Sea. Fish Biol. 2020, 97(1), 148–162. [Google Scholar] [CrossRef] [PubMed]
- Morris Jr, JA. 2009. The biology and ecology of invasive Indo–Pacific lionfish. PhD Thesis, North Carolina State University, USA. 168 pp.
- Tuset VM, Lombarte A, & Assis CA. 2008. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci Mar 72:7–198. https://doi. org/ 10. 3989/ scimar. 2008.
- Ferry LA, & Cailliet GM. 1996. Sample size and data analysis: are we characterizing and comparing diet properly? In: Shearer KD, MacKinlay DD (eds) (1996) GUTSHOP ‘96. Feeding Ecology and Nutrition in Fish. International Congress on the Biology of Fishes. San Francisco State University, USA, -18, 1996. 14 July.
- Tiralongo F, Messina G, Cazzolla Gatti R, Tibullo D, & Lombardo BM. 2018. Some biological aspects of juveniles of the rough ray, Raja radula Delaroche, 1809 in Eastern Sicily (central Mediterranean Sea). 1: Journal of Sea Research 142. [CrossRef]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, & Wagner H. 2019. vegan: Community Ecology Package. R package version 2.6-4. https://CRAN.R-project. 4 December.
- Hyslop, EJ. 1980. Stomach contents analysis—a review of methods and their application. J Fish Biol 17(4):411–429. [CrossRef]
- Tennekes M (2021). treemap: Treemap Visualization. R package version 2.4-3. https://CRAN.R-project. 4 December.
- Kruskal, J.B. , 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika 29: 115-129.
- Anderson, M. , 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26: 32-46.
- Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143.
- Schoener TW (1970) Nonsynchronous spatial overlap of lizards in patchy habitats. 4: Ecology 51(3).
- Amundsen, P.-A. , Gabler, H.-M., & Staldvik, F. J. 1996. A new method for graphical analysis of feeding strategy from stomach contents data. 48.
- Levins, R. 1968. Evolution in changing environments. Princeton Univ. Press, Princeton, N. J. 120 p.
- Krebs, C.J. 2014. Ecological Methodology, 3nd ed. Addison-Wesley Educational Publishers, Inc.
- R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.
- Clarke, K.R. , & Warwick, R.M. 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Second edition.
- Clarke, Κ.Ρ. & Gorley, R.N., 2006. PRIMER v6: User Manual/Tutorial.
- Anderson, M.J. , Gorley, R.N. & Clarke, K.R., 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods.
- Fishelson, L. 1975. Ethology and reproduction of pteroid fishes found in the Gulf of Aqaba (Red Sea), especially Dendrochirus brachypterus (Cuvier), (Pteroidae, Teleostei). Pubblicazioni della Stazione Zoologica di Napoli 39 (Suppl.): 635–656.
- Khalaf MA, Disi AM (1997) Fishes of the Gulf of Aqaba.
- Morris JA, & Akins JL 2009. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ Biol Fishes 86(3):389–398. [CrossRef]
- Arredondo-Chávez, A.F. , Sánchez-Jimenez, J.A., Ávila-Morales, O.G., TorresChávez, P., Herrerias-Diego, Y., Medina-Nava, M., Madrigal-Guridi, X., Campos-Mendoza, A., Domínguez- Domínguez, O., & Caballero-Vázquez, J.A. 2016. Spatio-temporal variation in the diet composition of red lionfish, Pterois volitans, (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Mexican Caribbean: insights into the ecological effect of the alien invasion. Acta Ichthyologica et Piscatoria 46(3): 185-200.
- Valdez-Moreno, M.; Quintal-Lizama, C.; Gómez-Lozano, R.; & del Carmen García-Rivas, M. 2012. Monitoring an Alien Invasion: DNA Barcoding and the Identification of Lionfish and Their Prey on Coral Reefs of the Mexican Caribbean. PLoS ONE 2012, 7(6), e36636. [CrossRef]
- Sandel V, Martinez-Fernandez D, Wangpraseurt D, & Sierra L 2015. Ecology and management of the invasive lionfish Pterois volitans/miles complex (Perciformes: Scorpaenidae) in Southern Costa Rica. 2: Rev Biol Trop 63(1).
- Mun˜oz RC, Currin CA, & Whitfield PE. 2011. Diet of invasive lionfish on hard bottom reefs of the Southeast USA: insights from stomach contents and stable isotopes. Mar Ecol Prog Ser 432:181–193. [CrossRef]
- Šantić, M.; Pallaoro, A.; Stagličić, N.; Markov-Podvinski, M. Feeding Habits of the red scorpionfish, Scorpaena scrofa (Osteichthyes: Scorpaenidae) from the eastern central Adriatic Sea. Cah. Biol. Mar. 2011, 52(2), 217–226. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Koutsoubas, D.; Milenkova, D.; Economidis, G.; Tamvakidis, S.; & Batjakas, I.E. 2020. New Data on the Biology and Fisheries of the Threatened Palinurus elephas (Fabricius, 1787) (Decapoda, Achelata, Palinuridae) from the North-West Aegean Sea, Greece. Water 2020, 12(9), 2390. [CrossRef]
- Kleiven, A.R. , Olsen, E. M. & Vølstad, J.H. 2012. Total catch of a red-listed marine species is an order of magnitude higher than official data. PLoS ONE 2012, 7, e31216. [Google Scholar] [CrossRef]
- La Mesa, G. , La Mesa, M. , & Tomassetti, P.2007. Feeding habits of the Madeira rockfish Scorpaena maderensis from central Mediterranean Sea. Mar. Biol. 2007, 150, 1313–1320. [Google Scholar] [CrossRef]
- Ordines, F.; Valls, M.; Gouraguine, A. Biology, feeding and habitat preferences of Cadenat’s rockfish, Scorpaena loppei (Actinopterygii: Scorpaniformes: Scorpanidae), in the Balearic Islands (western Mediterranean). Acta Ichthyol. Piscat. 2012, 42(1), 21-30. Acta Ichthyol. Piscat. 2012, 42(1), 21–30. [Google Scholar] [CrossRef]
- Demirhan, S.A. & Can, M. F. 2009. Age, growth and food composition of Scorpaena porcus (Linnaeus, 1758) in the southeastern Black Sea. J. Appl. Ichthyol. 2009, 25, 215–218. [Google Scholar] [CrossRef]
- Compaire, J.C.; Casademont, P.; Cabrera, R.; Gómez-Cama, C.; & Soriguer, M.C. 2018. Feeding of Scorpaena porcus (Scorpaenidae) in intertidal rock pools in the Gulf of Cadiz (NE Atlantic). J. Mar. Biolog. Assoc. U.K. 2018, 98(4), 845-853. [CrossRef]
- Omri, N.; Derbal, F.; & Kara, M.H. 2019. Diet of the black scorpionfish Scorpaena porcus (Scorpaenidae) of the gulf of Annaba, Algeria. Cybium 2019, 43(2), 179-186. [CrossRef]
- Aydin, M.; & Mazlum, R.E. ; & Mazlum, R. E. 2020. Feeding ecology of black scorpion fish (Scorpaena porcus Linnaeus, 1758) in SE Black Sea region, (Ordu) Turkey. J. Mar. Biolog. Assoc. U.K. 2020, 100, 435–444. [Google Scholar] [CrossRef]
- Sahin, C.; Erbay, M.; Kalayci, F.; Ceylan, Y.; & Yesilcicek, T. 2019. Life-History Traits of the Black Scorpionfish (Scorpaena porcus) in Southeastern Black Sea. Turk. J. Fish. & Aquat. Sci. 2019, 19(7), 571-584. [CrossRef]
- Eddy C, Pitt J, Morris JA Jr, Smith S, Goodbody-Gringley G, & Bernal D. 2016. Diet of invasive lionfish (Pterois volitans and P. miles) in Bermuda. Mar. Ecol. Prog. Ser. 2016, 558, 193–206. [CrossRef]
- Galanidi, M. , Zenetos A., Bacher S. 2018. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterranean Marine Science 19 (1): 107–123. [CrossRef]
- Albins, M.A. & M.A. Hixon. 2011. Worst case scenario: potential long—term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral—reef communities. [CrossRef]
- Morris, J.A., Jr. L. Akins, A. Barse, D. Cerino, D.W. Freshwater, S.J. Green, R.C. Muñoz, C. Paris, and P.E. Whitfield. 2009. Biology and ecology of the invasive lionfishes, Pterois miles and Pterois volitans. Proceedings of the Gulf and Caribbean Fisheries Institute 61:409—414.
- Arias—González, J.E., C. González—Gándara, J.L. Cabrera, & V.Christensen. 2011. Predicted impact of the invasive lionfish Pterois volitans on the food web of a Caribbean coral reef. Environmental Research 111:917—925. [CrossRef]
- Rocha, L.A. , Rocha C.R., Baldwin C.C., Weigt L.A., & McField M. 2015. Invasive lionfish preying on critically endangered reef fish. Coral Reefs 34 (3): 803–806. [CrossRef]
- Ballew NG, Bacheler NM, Kellison GT, & Schueller AM. 2016. Invasive lionfish reduce native fish abundance on a regional scale. Sci Rep 6:32169. [CrossRef]
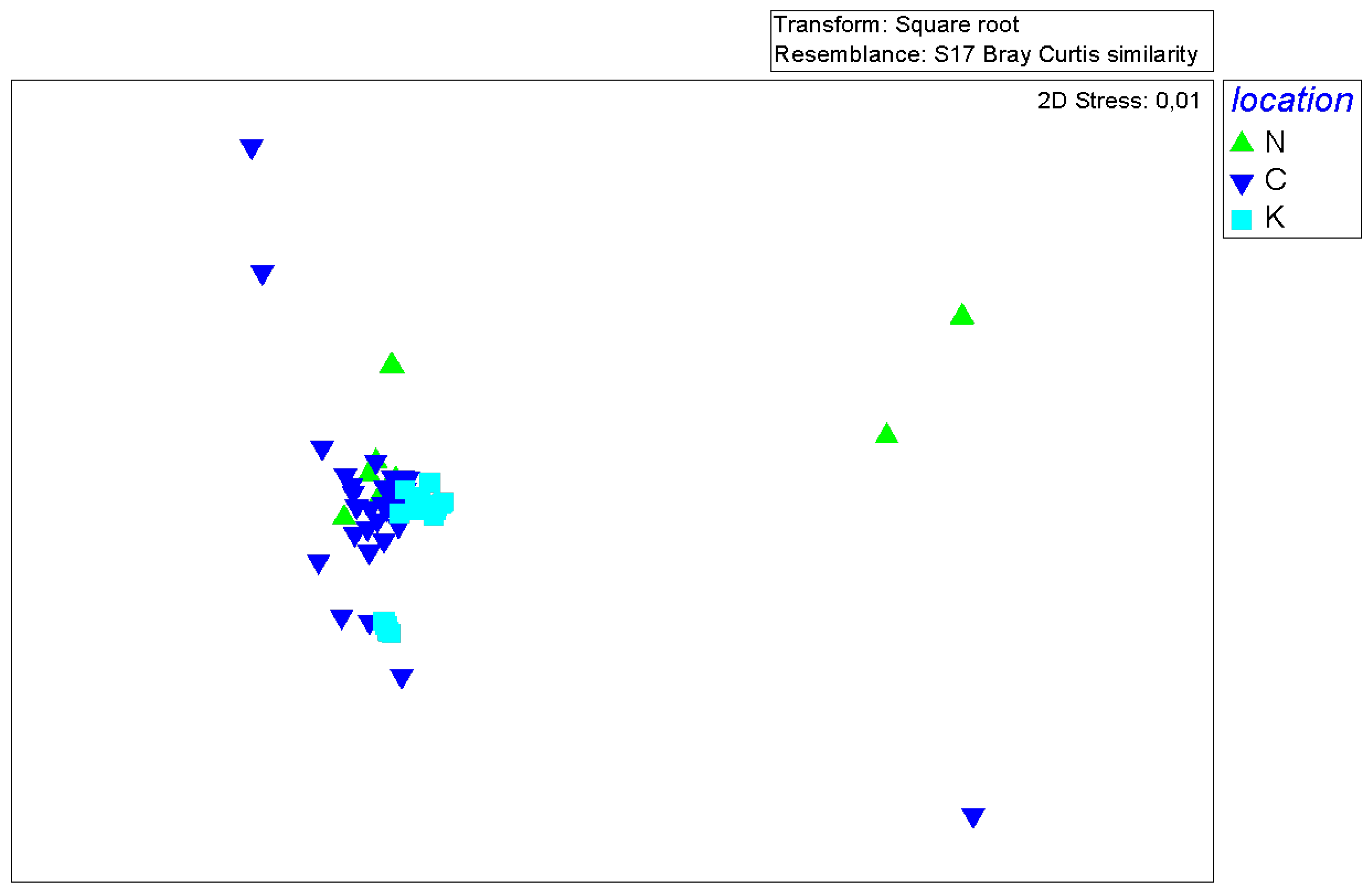
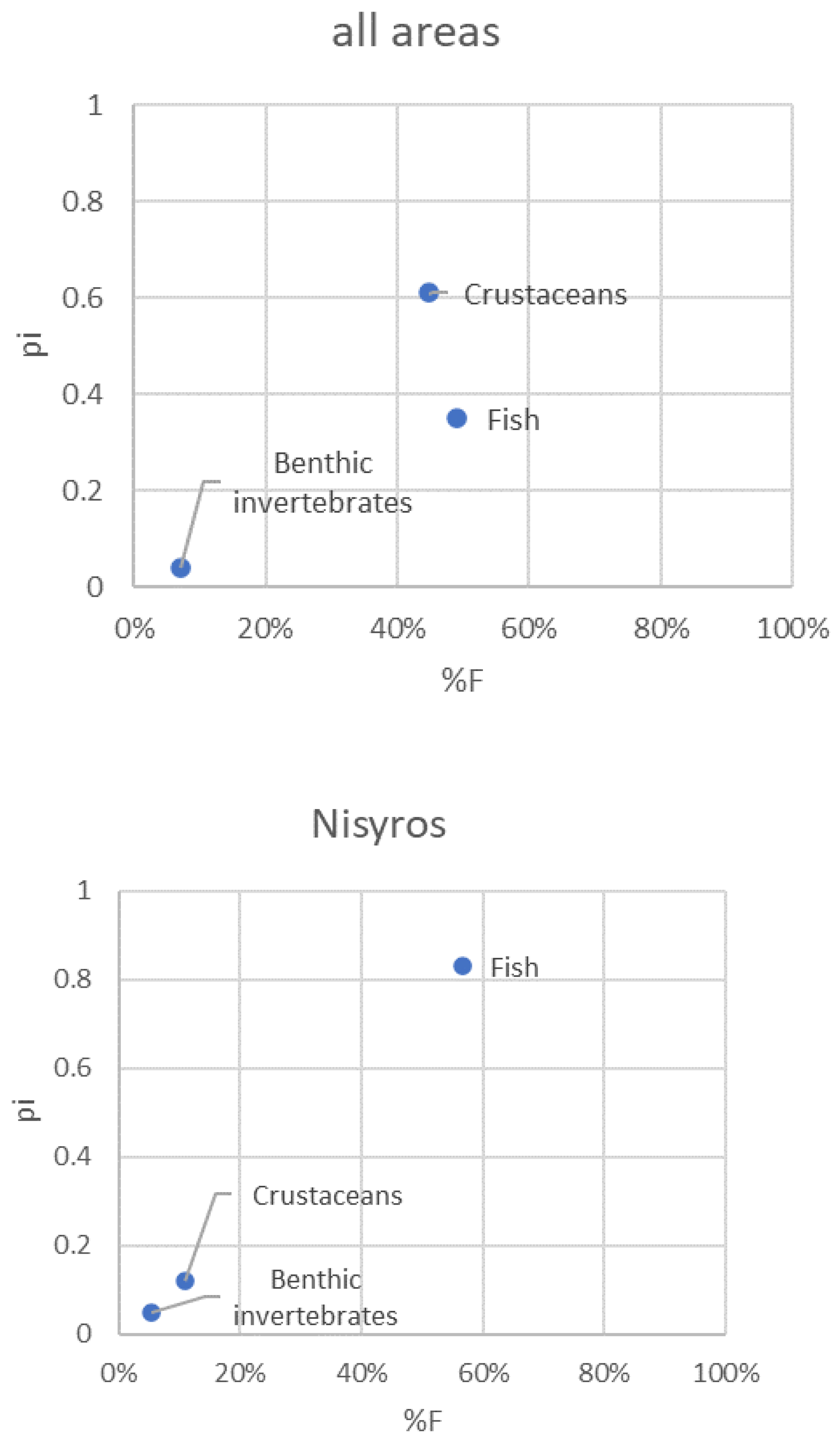
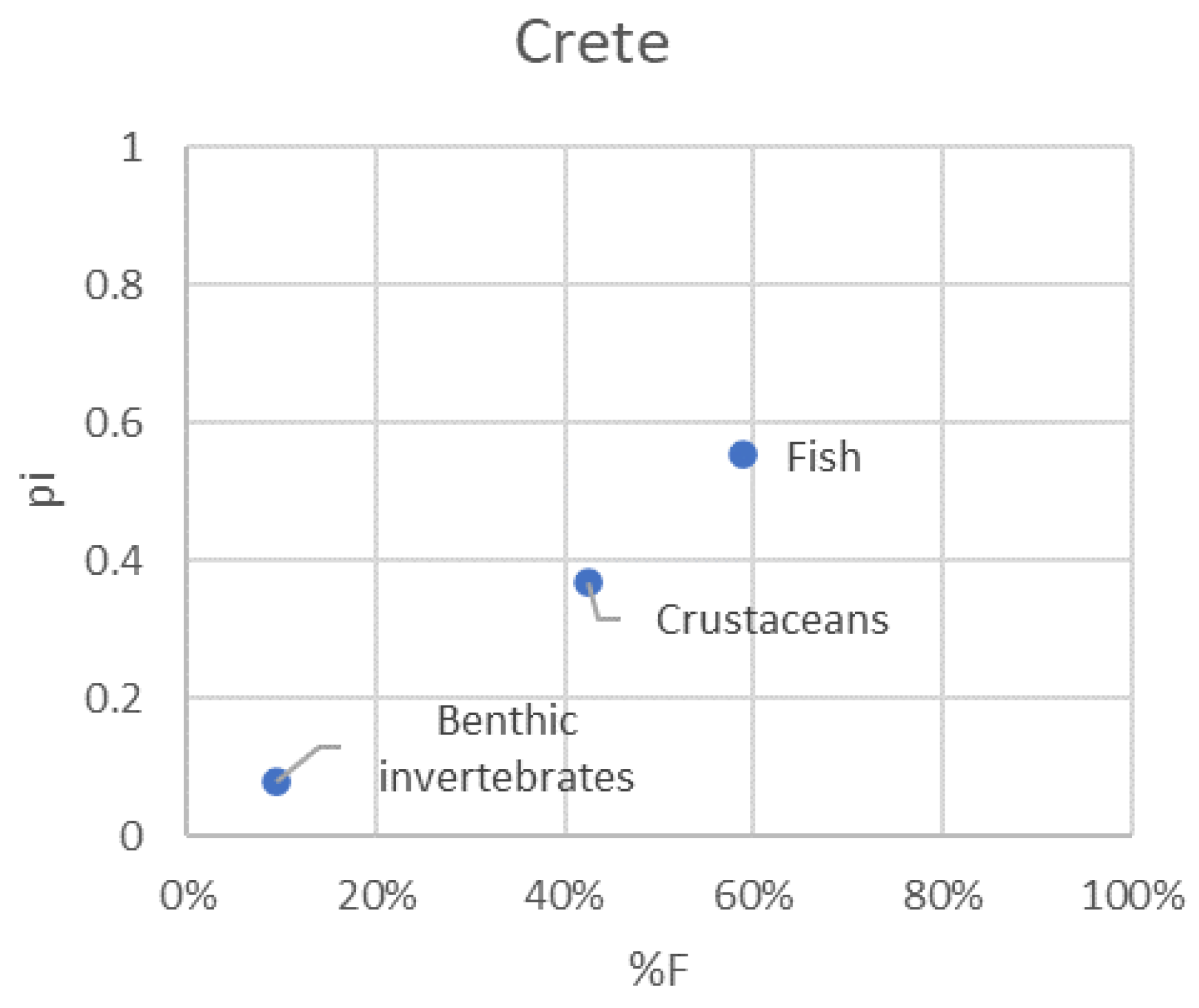
| Factor | Levels | Total | Full % | Empty % (= VI) |
|---|---|---|---|---|
| Area | Crete | 73 | 77% | 23% |
| Kastellorizo | 31 | 84% | 16% | |
| Nisyros | 37 | 62% | 38% | |
| Sex | F | 78 | 78% | 22% |
| M | 15 | 60% | 40% | |
| U | 48 | 73% | 27% | |
| Size | S | 68 | 76% | 24% |
| L | 73 | 73% | 27% | |
| Grand Total | 141 | 74% | 26% |
| Sex | Size | Nisyros | Kastellorizo | Crete |
|---|---|---|---|---|
| F | 31.83 | 315.07 | 171.12 | |
| S | 27.66 | 294.77 | 210.83 | |
| L | 32.66 | 330.30 | 103.39 | |
| M | 27.19 | 187.33 | ||
| S | 126.32 | |||
| L | 27.19 | 614.39 | ||
| U | 96.94 | 230.66 | 83.19 | |
| S | 23.50 | 166.26 | 100.78 | |
| L | 121.42 | 311.17 | 61.20 | |
| Area | 52.07 | 257.60 | 138.60 | |
| One-way SIMPER analysis | ||||||
| Group N (Average similarity: 34,59) | ||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |
| fish remains | 0,80 | 32,91 | 0,79 | 95,14 | 95,14 | |
| Group C (Average similarity: 29,05) | ||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |
| fish remains | 0,73 | 27,19 | 0,74 | 93,60 | 93,60 | |
| Group K (Average similarity: 26,60) | ||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |
| Plesionika edwarsii | 0,91 | 15,56 | 0,53 | 58,51 | 58,51 | |
| Plesionika spp. | 0,91 | 9,28 | 0,35 | 34,90 | 93,41 | |
| Groups N & C (Average dissimilarity = 69,17) | ||||||
| Group N | Group C | |||||
| Species | Av.Abund | Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% |
| fish remains | 0,80 | 0,73 | 23,30 | 1,09 | 33,69 | 33,69 |
| Caridea | 0,15 | 0,02 | 5,05 | 0,39 | 7,30 | 40,98 |
| Scyllarus sp. | 0,00 | 0,13 | 4,02 | 0,35 | 5,81 | 46,80 |
| Scyllaridae | 0,00 | 0,14 | 3,89 | 0,37 | 5,63 | 52,42 |
| Pterois sp. | 0,09 | 0,00 | 3,53 | 0,30 | 5,10 | 57,52 |
| Cumacea | 0,09 | 0,00 | 3,53 | 0,30 | 5,10 | 62,63 |
| Gobidae | 0,10 | 0,04 | 2,96 | 0,34 | 4,29 | 66,91 |
| Scyllarides latus | 0,00 | 0,12 | 2,89 | 0,29 | 4,18 | 71,09 |
| Labridae | 0,10 | 0,00 | 2,79 | 0,29 | 4,04 | 75,13 |
| Homola barbata | 0,00 | 0,08 | 2,11 | 0,26 | 3,04 | 78,18 |
| Decapoda | 0,04 | 0,00 | 1,76 | 0,21 | 2,55 | 80,73 |
| Polychaeta | 0,00 | 0,06 | 1,65 | 0,23 | 2,39 | 83,12 |
| Chromis sp. | 0,00 | 0,04 | 1,50 | 0,18 | 2,18 | 85,29 |
| Parapenaeus longirostris | 0,00 | 0,04 | 1,29 | 0,18 | 1,87 | 87,16 |
| Inachus sp. | 0,00 | 0,04 | 1,19 | 0,18 | 1,72 | 88,88 |
| Ostracoda | 0,00 | 0,03 | 0,95 | 0,13 | 1,38 | 90,26 |
| Groups N & K (Average dissimilarity = 93,62) | ||||||
| Group N | Group K | |||||
| Species | Av.Abund | Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% |
| Plesionika edwarsii | 0,00 | 0,91 | 26,14 | 0,91 | 27,92 | 27,92 |
| Plesionika spp. | 0,00 | 0,91 | 23,09 | 0,71 | 24,67 | 52,59 |
| fish remains | 0,80 | 0,21 | 21,39 | 1,12 | 22,84 | 75,43 |
| Natantia | 0,00 | 0,27 | 6,06 | 0,40 | 6,48 | 81,91 |
| Caridea | 0,15 | 0,00 | 3,72 | 0,37 | 3,97 | 85,88 |
| Pterois sp. | 0,09 | 0,00 | 2,86 | 0,29 | 3,06 | 88,94 |
| Cumacea | 0,09 | 0,00 | 2,86 | 0,29 | 3,06 | 92,00 |
| Groups C & K (Average dissimilarity = 93,68) | ||||||
| Group C | Group K | |||||
| Species | Av.Abund | Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% |
| Plesionika edwarsii | 0,00 | 0,91 | 24,18 | 0,90 | 25,81 | 25,81 |
| Plesionika spp. | 0,03 | 0,91 | 21,79 | 0,72 | 23,26 | 49,08 |
| fish remains | 0,73 | 0,21 | 19,08 | 1,07 | 20,37 | 69,44 |
| Natantia | 0,00 | 0,27 | 5,63 | 0,40 | 6,00 | 75,45 |
| Scyllaridae | 0,14 | 0,04 | 3,57 | 0,40 | 3,81 | 79,26 |
| Scyllarus sp. | 0,13 | 0,00 | 3,14 | 0,35 | 3,35 | 82,61 |
| Scyllarides latus | 0,12 | 0,00 | 2,35 | 0,29 | 2,51 | 85,12 |
| Homola barbata | 0,08 | 0,00 | 1,68 | 0,26 | 1,79 | 86,91 |
| Polychaeta | 0,06 | 0,00 | 1,33 | 0,23 | 1,42 | 88,33 |
| Ostracoda | 0,03 | 0,04 | 1,27 | 0,21 | 1,36 | 89,69 |
| Chromis sp. | 0,04 | 0,00 | 1,16 | 0,18 | 1,24 | 90,93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





