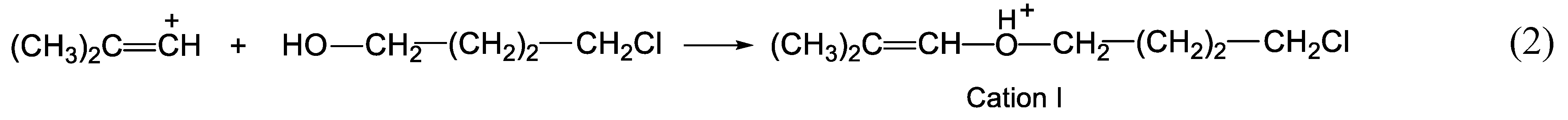1. Introduction
Until recently, vinyl carbocations have been thought to be highly reactive and therefore difficult to investigate,1-3 although recent studies showed that their reactivity is exaggerated.4-6 The first vinyl cations whose salts were isolated in pure form and analyzed by X-ray diffraction and NMR methods were stabilized by such substituents as methyl, cycloalkyl, phenyl, and R3Si-groups.7-10 Stabilization occurs due to a scattering of the charge of the cation over atoms of these substituents, which act as electron donors. It has turned out that the supply of electron density from substituents increases electron density on the C=C bond so much that it approaches triple-bond status.8 Later, salts of unstabilized vinyl cations С3Н7+ and С4Н9+ have been obtained.11-13 In them, the positive charge is concentrated mainly on the C=C bond, thereby greatly reduceing its CC stretch frequency to that corresponding to one-and-half-bond status. Interaction of cations with surrounding anions with the formation of contact ion pairs or the introduction of Cl-substituents, also contributes to better scattering of the positive charge and its decrease in C=C bonds. The use of carborane superacids has made it possible to obtain and study solid salts of unstabilized vinyl and acetylene carbocations, which are stable at room and elevated temperatures.11-14
In the present work, two goals were set: (1) to establish how the nature of the C=C bond of a vinyl cation is affected by bonding of its various C atoms to one weak nucleophile, a Cl atom, or two Cl atoms, and (2) how the carbocation changes when a stronger nucleophile (an oxygen atom) is introduced. The salts of carbocations were studied by X-ray crystallography and IR spectroscopy. As a counterion, the undecachlorocarborane anion, CHB
11Cl
11−, was chosen (
Figure S1 in SI) because its extreme stability and low basicity promote the formation of stable ionic salts with highly acidic cations.
15 In what follows, this anion will be denoted as {Cl
11−}.
2. Results
Crystals of a salt of the dichlorovinyl cation, C
4H
5Cl
2+{Cl
11-}, were obtained as follows: an aliquot of a dichloromethane (DCM) solution of CH
3С(CH
2Cl)
3 was introduced into a freshly prepared solution of acid H{Cl
11} in DCM in such an amount that the molar ratio of CH
3С(CH
2Cl)
3 to H{Cl
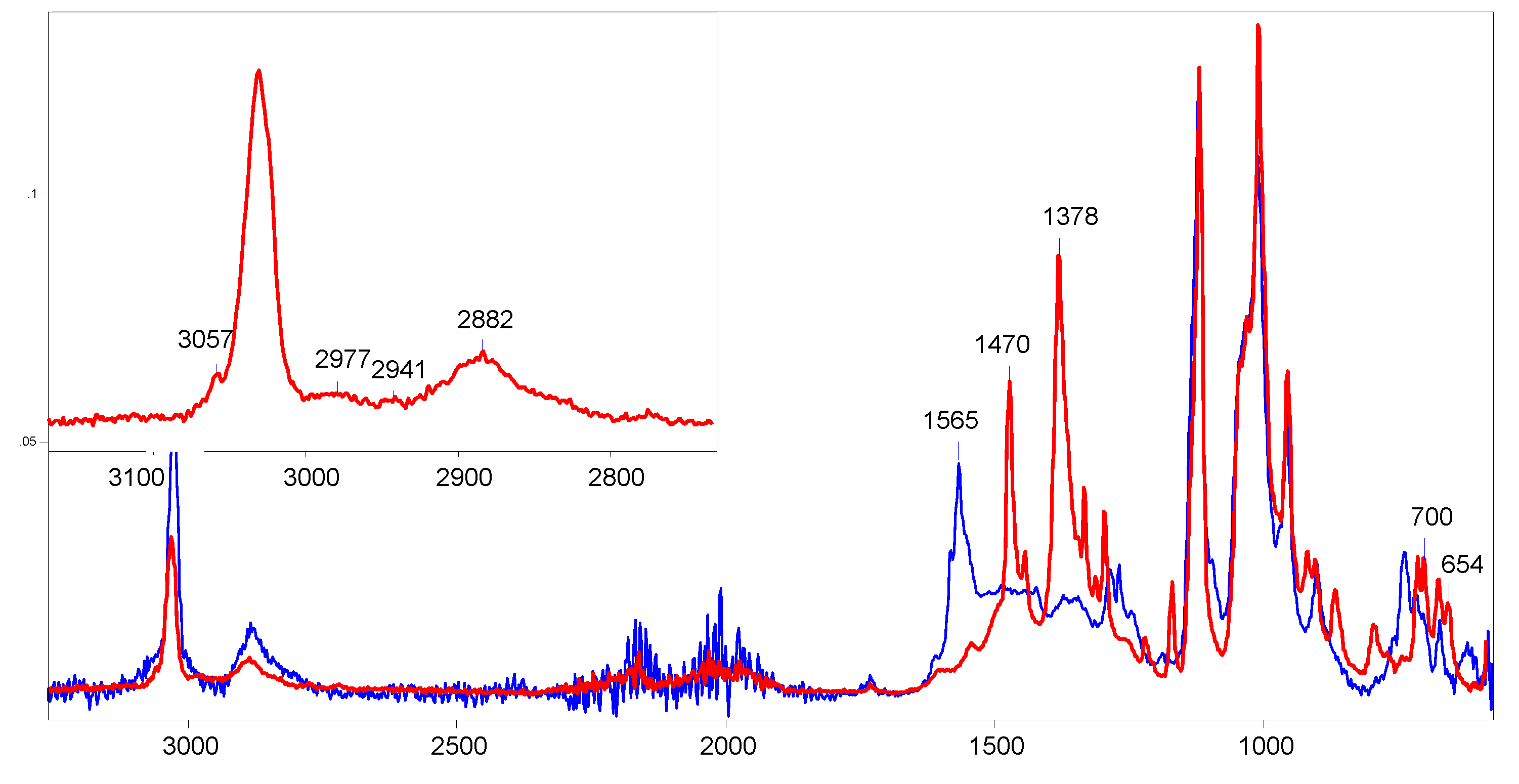
11} did not exceed 1.0. The solution first turned cloudy, then, within 15 min, it darkened strongly and became transparent. That is, sequential reactions took place. A drop of the solution was evaporated on the surface of a crystal of the ATR accessory. The recorded ATR IR spectrum contains strong bands of C=C stretch vibrations at 1565 cm
-1, which are characteristic of vinyl cations (
Figure 1), and does not contain absorption bands of chloronium cation (CH
2Cl)
2Cl
+, which forms upon dissolution of the acid H{Cl
11} in DCM.
16 It was found that when interacting with chloroalkanes, the chloronium cation, as its neutral analog CH
2Cl{Cl
11}, takes away a chlorine atom from them, turning into DCM.
11,12 Therefore, when mixing solutions at the first stage, the reaction of dechlorination of chloroalkane should occur giving rise to chlorinated carbocation С
5Н
7Cl
2+ (Eq. 1), which is unstable and spontaneously decompose to the vinyl carbocation (with the band of C=C stretch frequency at 1565 cm
-1,
Figure 1) with a release of HCl:
Incubation the solution for 2 days led to the growth of crystals from it. Their IR spectrum differs from the spectrum of products arising in the initial solution (
Figure 1) in that it lacks the bands of the double C=C
+ bonds at ≥ 1490 cm
-1.
13 X-ray diffraction analysis of the crystals indicated that this is an ionic salt with discrete {Cl
11-} anions and two types of crystallographically independent 1,1-dichlorobutylene cations that are very similar in geometry (
Figure S2 in SI, the structure of cations with averaged CC distances is given in
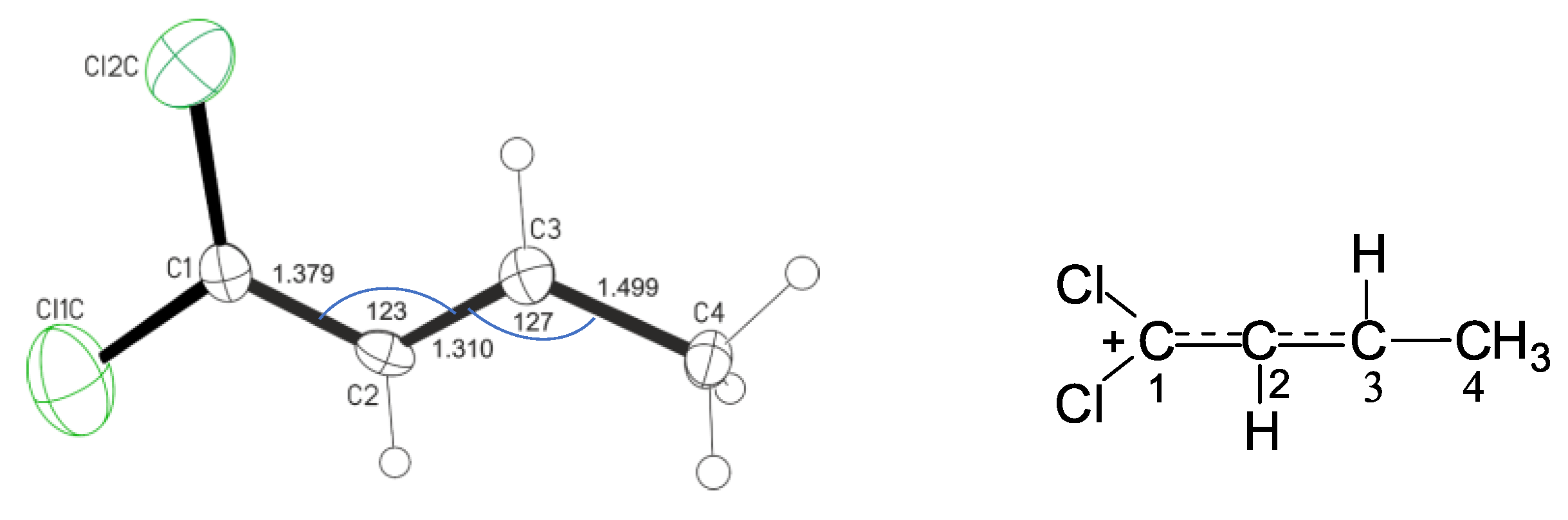
Figure 2). Their two Cl atoms and four C atoms are actually in the same plane with an average deviation from the least-squares plane of 0.029 Å for one cation and 0.075 Å for the other. Lengths of the two CC bonds (averaged 1.34 Å) are close to aromatic, and angles C1C2C3 and C2C3C4 are close to 120° (
Figure 2). This means that the atoms С1, С2 and С3 have sp
2 hybridization and belong to groups СCl
2, СH and СH, respectively. It is expected that the positive charge is localized mainly on the atoms of the СCl
2 group, which forms the С1=С2 double bond. Nevertheless, the electron density is evenly distributed over two equivalent bonds of the С1С2С3 group, thereby equalizing their multiplicity to one and a half.
The IR spectrum of the 1,1-dichlorobutylene cation shows two intense bands of CC stretch vibrations at 1470 and 1378 cm
−1, which may belong to ν
as and ν
as frequencies of the
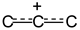
group. These frequencies are higher than those of allyl cation

(1303 and 1265 cm
-1), indicating a decrease in the positive charge on this group
13 owing to transfer of the charge to Cl atoms. The spectrum also contains two bands of symmetric and asymmetric C-Cl stretch vibrations at 700 and 654 cm
-1 in accordance with the presence of two C-Cl bonds at one carbon atom in the cation.
Previously, we have researched the interaction of vinyl carbocations, C
3H
5+ and C
4H
7+, with water molecules in solutions of their salts in C
6HF
5.
17 One of the objectives of the current work is to check how the chlorination of vinyl carbocations affects their interaction with water molecules. For their preparation cyclobutylchloronium (CH
2CH
2)
2Cl
+ and divinylchloronium (СН
2=СН)
2Cl
+ salts were used. The salt of cyclobutylchloronium is poorly soluble in C
6HF
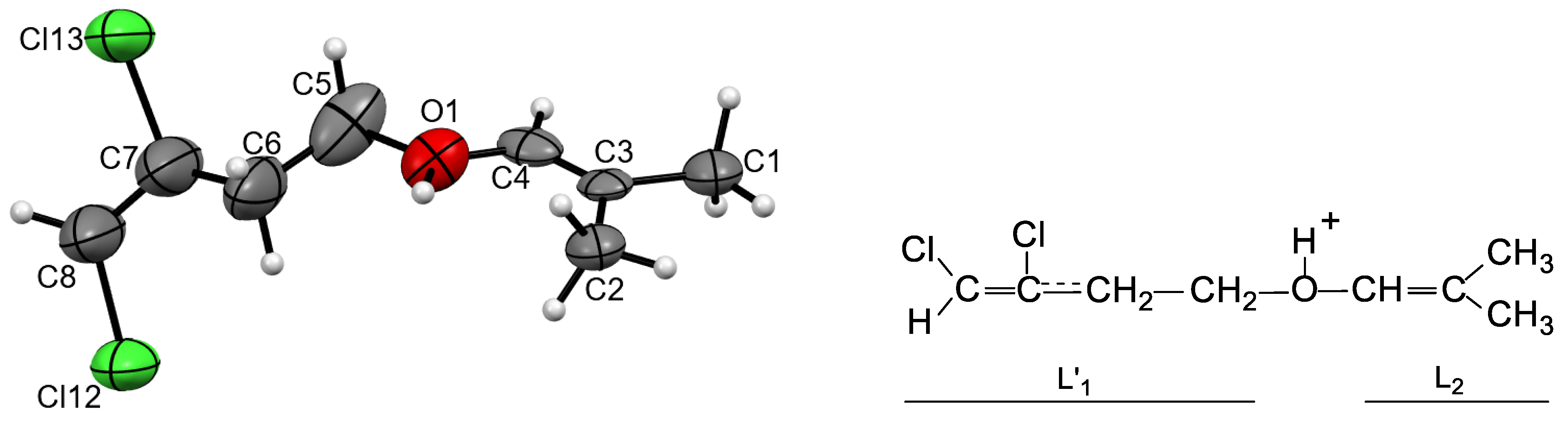
5 if it contains trace amounts of water (the solvent was pre-dried with molecular sieves). Keeping this solution over hexane vapor led to the isolation of 2-3 crystals from it after a few days. The center of each crystal contained a disordered crystallite, around which a regular crystal grew. This finding indirectly indicates that the crystals are formed by one of products of reactions occurring in solution. A piece of its outer part that was broken off from the crystal showed a good diffraction pattern. X-ray analysis showed that the crystal consists of discrete {Cl
11-} anions and two crystallographically independent cations (
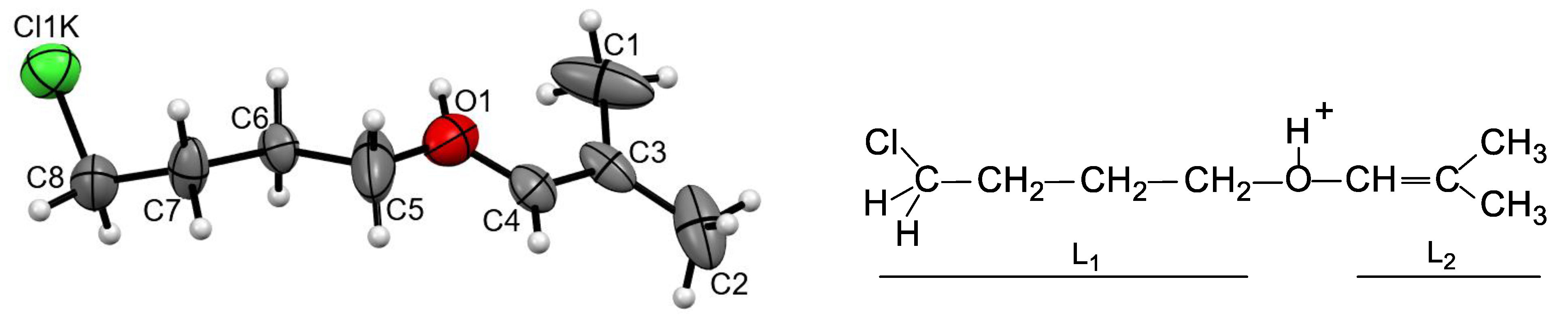
Figure S3 in SI). The cation are protonated ether L
1-O(H
+)-L
2, in which both L
1 and L
2 contain four carbon atoms each (
Figure 3). Its chloropropane moiety L
1 contains single C-C bonds and a terminal CH
2Cl group. The second group, L
2 is isobutylene with one short CC bond of 1.274 and 1.278 Å for two crystallographically independent cations, which corresponds to the C=C double bond. All its four C atoms and an oxygen atom deviate from the least-squares plane by 0.011 and 0.010 Å for both cations with the C–C–O angle close to 120 °, confirming that it is an isobutylene group: (CH
3)
2C=CH-. Hereafter, this cation is referred to as I.
Using divinylchloronium salt (CH
2=CH)
2Cl
+{Cl
11-} (preparation is given in ref.
14), another similar cation was obtained. A solution of this salt in C
6HF
5 was kept over hexane vapor, and after 2 weeks, small crystals appeared. The first selected crystal (0.1 mm) and the second smaller one (0.05 mm) yielded no diffraction pattern. A third very small crystal (0.005 × 0.01 × 0.30 mm) showed a diffraction pattern and an X-ray pattern was obtained with long exposure. Such a small crystal size (less than 0.005 mm in one of the dimensions) led to the interpretation of the structure with a poor R
f factor (11.8%). Therefore, we do not discuss the geometric parameters of the cation. Nonetheless, the topology of the cation and its geometry were determined (
Figure 4). It turned out to be protonated ether L′
1-O(H
+)-L
2, with exactly the same isobutylene group L
2 as in cation I. The L′
1 group is chain butylene, which differs from the corresponding L
1 group in I in that it contains two Cl atoms that are attached to atoms C7 and C8. All these four atoms, together with the C6 atom, are in the same plane, with the mean deviation from the least-squares plane of 0.121 Å. This means that atoms C7 and C8 have sp
2 hybridization and form CCl and CHCl groups, respectively, with a C7=C8 double bond. The C5C6C7 angle at 108° is close to tetrahedral, i.e., the C6 atom forms a CH
2 group. Thus, the scheme of the structure of this cation was established (
Figure 4), and in the text below, we will designate it as cation II.
3. Discussion
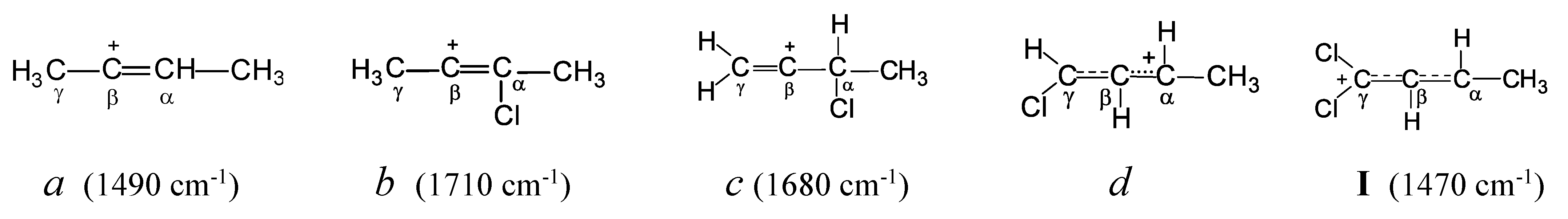
Let us examine how the attachment of a Cl atom to different carbon atoms of the butylene cation affects the nature of its CC bonds, by means of also the known data on chlorobutylene carbocations.
13 In symmetrical vinyl cation
a (
Scheme 1), which does not contain Cl atoms, the positive charge is locaed mainly on the C=C double bond. Therefore, its C=C stretching frequency (1490 cm
-1) is much lower than that of the neutral 2-butene molecule (1645 cm
-1). Replacing the H atom in the -СH=С
+- group of this cation with a Cl atom (cation
b) leads to such a substantial supply of electron density from the Cl atom to the С=С bond that the C=C stretch frequency increases by 220 cm
-1 (up to 1710 cm
-1) exceeding that of neutral 2-butene. Obviously, the Cl atom completely accepts the positive charge, extinguishing it on the C=C bond. If the Cl atom is attached to the С
α atom adjacent to the C=C bond (cation
c), then its effect on the weakening of the charge on it is weaker (its effect on reducing its charge is weaker) (C=C stretch frequency increases by 190 cm
-1). Attachment of the Cl atom to the С
γ atom of the terminal C=C bond (cation
d) leads to a decrease in the multiplicity of the С
γС
β bond owing to the partial transfer of π-electron density to the С
βС
α bond (according to NMR data). Finally, the addition of the second Cl atom to the terminal С
γ atom with sp
2 hybridization (cation I under study) leads to the alignment of multiplicities of С
γС
β and С
βС
α bonds and the transfer of the positive charge to two Cl atoms. This event leads to an increase in the frequencies of С
γС
βС
α stretch vibrations as compared to the unstabilized allyl cation (CH
2CHCH
2)
+ (by 170 cm
-1), bringing them closer to aromatic ones in neutral molecules.
Thus, in neutral CH2=CCl-R molecules, the chlorine atom has properties of an electron acceptor, slightly lowering the C=C stretch frequency by 20 cm-1,18 whereas in carbocations it acts as an electron donor, extinguishing the positive charge on the C=C bond to a greater extent with decreasing distance to the double C=C bond. The chlorine atom also contributes to the delocalization of π-electron density to the neighboring CC bond.
We do not have necessary data to discuss a possible mechanism of formation of C4-cation C4H5Cl2+ from C5-cation C5H7Cl2+ emerging at the first stage of the reaction, according to Eq. 1. This reaction requires spontaneous cleavage of the C-C bond in the С5Н7Cl2+ cation with the formation of the C4-cation and other unidentified products and therefore requires additional research.
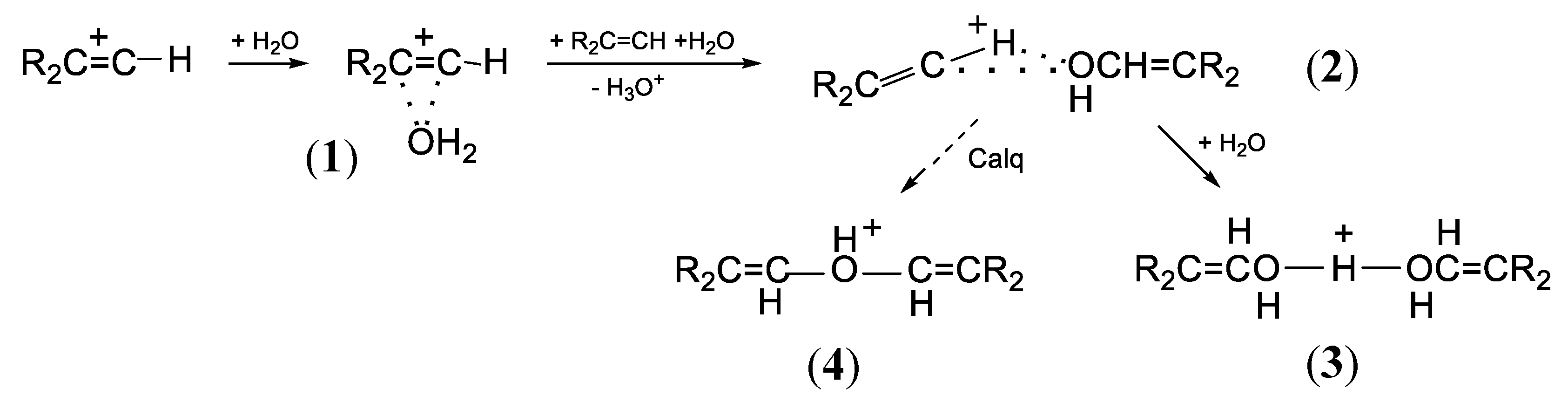
Cations
I and
II are products of the interaction of chlorinated vinyl carbocations with water molecules. In the case of non-chlorinated vinyl cations С
3Н
5+ and С
4Н
7+, their interaction with water molecules in solutions of their salts in C
6HF
5 proceeds in three stages as the water content increases (
Scheme 2):
17 (i) adduct
1 arises; (ii) an alcohol molecule is formed from it, which attaches to the vinyl cation giving rise to adduct
2; (iii) next, it transforms into proton disolvate
3. According to quantum chemical calculations, adduct 2 is unstable and was likely to transform into protonated ether cation 4. Nevertheless, in solutions in C
6HF
5, it is cation 3 that is the final product.
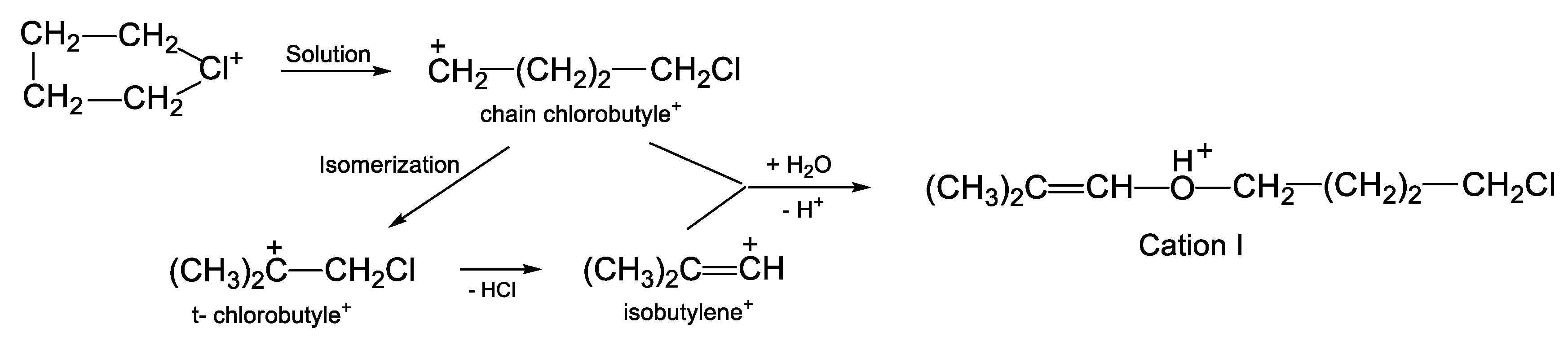
On the other hand, if cyclobutylchloronium or divinylchloronium salts are dissolved in C
6HF
5, then the chlorinated vinyl cations arising from them—by interacting with H
2O after that—form precisely the protonated esters I and II, respectively. The formation of cation I from cyclobutylchloronium occurs in a high yield, and a rather obvious formation mechanism can be proposed (
Scheme 3). In solutions in DCM, the butylene ring of the chloronium cation (CH
2CH
2)
2Cl
+ breaks, followed by its isomerization to tertiary chlorobutyle
+, which, by releasing HCl, transitions into the vinyl isobutylene
+ cation. Subsequently, the two nascent cations, chain chlorobutylene
+ and isobutylene
+, interact with a water molecule, thereby generating the final product: protonated ether I:
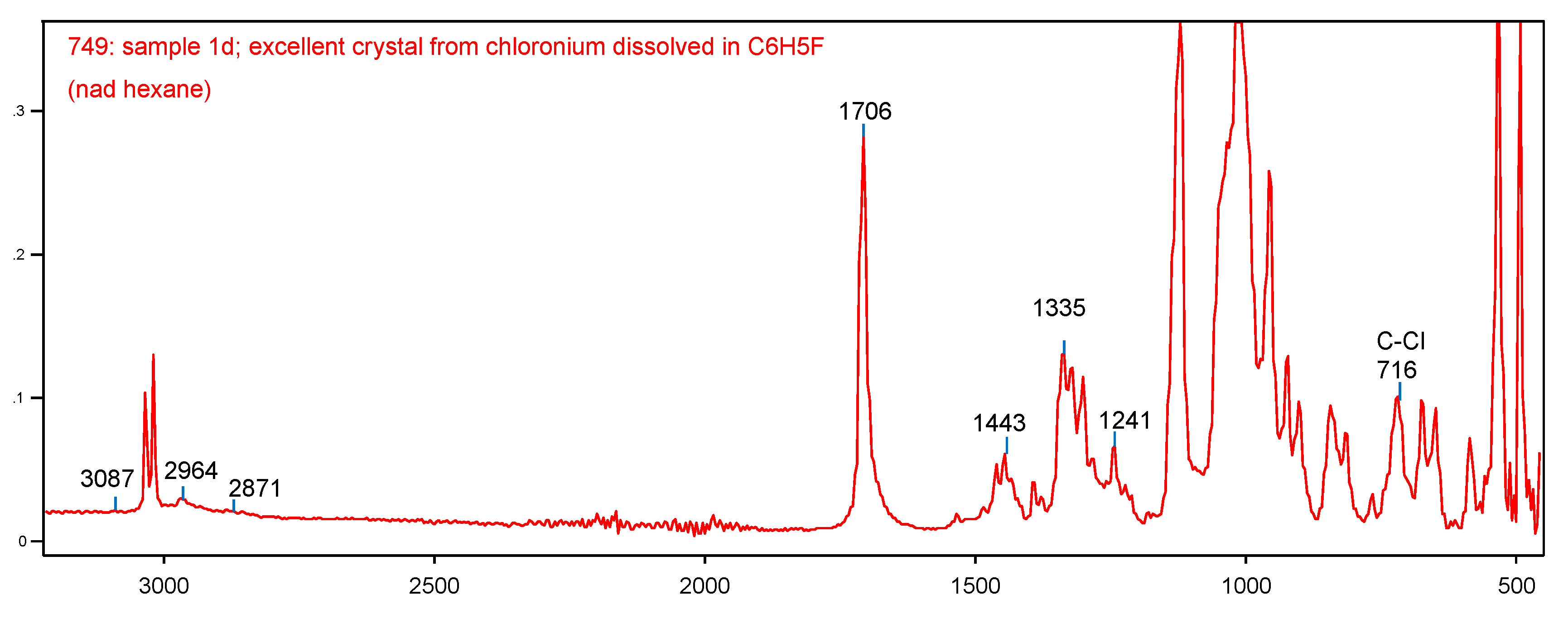
In the IR spectrum of the salt of cation I, there is a very intense band of C=C stretch at 1706 cm
-1, whose frequency exceeds that of the neutral (CH
3)
2C=CHOH molecule (
Figure 5). Obviously, the positive charge from the OH
+ group almost does not reach the C=C bond. Besides, vibration frequencies of CH
3 groups (2964 and 2871 cm
-1) and of C-Cl at 716 cm
-1 match those of the neutral molecules.
18 Therefore, the positive charge is actually situated on the OH
+ group with the νOH frequency at 3087cm
-1.
The formation of cation II from divinylchloronium cation proceeds in a low yield. Obviously, it is one of a number of emerging products, and it is difficult to propose a sufficiently substantiated mechanism for its formation. We failed to obtain an IR spectrum of cation II. It should differ from the spectrum of I only in the frequencies of C-Cl and CC bonds of 1,1-dichlorobutylene.
Cations I and II are not carbocations but protonated esters, which were produced by the interaction of vinyl carbocations with water molecules. In fact, they are the product of the addition of an alcohol molecule to an isobutylene carbocation, as shown in Eq. 2 using cation I as an example:
The formation of carbocations from cations I and II can be formally represented as the elimination of an H
2 molecule from the latter, as shown for cation I in
Scheme 4:
Whether such a type of O-containing carbocations exists and whether they can be obtained will be determined by future research.
4. Conclusions
If uncharged hydrocarbons have such substituents as Cl and Ph, which possess weak electron-withdrawing properties or, just as alkylsilylium (-SiR3) do not possess them at all, then the binding of these substituents to a positively charged center of the vinyl carbocations causes their transformation into strong electron donors, which quench the positive charge on the C=C bonds, taking it upon themselves. The influx of electron density from substituents to the C=C bond is so considerable that it exceeds that of neutral analogs and the C=C bond’s multiplicity exceeds double and can approach triple. Because this bond involves C atoms with sp1 and sp2 hybridization, it is expected that some δ− charge appears on it. A detailed study on these features of carbocations by quantum-chemical methods is necessary.
If an O atom is added to the carbocation, then H+ can be transferred to it, with almost the entire charge of the cation concentrated on the =O-H+ group. Such entity becomes a protonated O molecule. On the other hand, it is quite possible that in the future, it will be possible to obtain vinyl carbocations containing an unprotonated O-atom.
Thus, the incorporation of any substituents into vinyl cations turns them into electron donors. If these are X heteroatoms with sufficiently high basicity that allows them to be protonated, then protonated molecules containing the XH+ group are generated with a high probability.
5. Methods and Materials
To obtain the C4H5Cl2+{Cl11-} salt, we used 1,3-dichloro-2-(chloromethyl)-2-methylpropane (98%, Sigma-Aldrich) without further purification as well as carborane acid H(CHB11Cl11), which was prepared as described previously.19 Details of the preparation of the C4H5Cl2+{Cl11-} salt are given in the Results section, as they are necessary to describe the results of the work. In the body of the Results section, details of preparation of protonated ester salts I and II are given as well. The salts of cyclobutylchloronium (CH2CH2)2Cl+ and divinylchloronium (CH2=CH)2Cl+ cations used for their preparation were obtained as described previously.13,14 Solvent C6HF5 from Sigma-Aldrich was not subjected to additional purification.
All sample handling was carried out in an atmosphere of argon (H2O, [O2] < 0.5 ppm) in a glove box. ATR IR spectra were recorded on a Shimadzu IRAffinity-1S spectrometer housed inside the glove box in the 4000−400 cm−1 frequency range using an ATR accessory with a diamond crystal. The spectra were processed in the GRAMMS/A1 (7.00) software from Thermo Scientific, Waltham, MA, USA.
X-ray diffraction data were collected on a Bruker Kappa Apex II CCD diffractometer using φ,ω-scans of narrow (0.5°) frames with Mo Kα radiation (λ = 0.71073 Å) and a graphite monochromator at temperature 200 K. The structures were solved by direct methods with the help of SHELXT-2014/5,20 and refined by the full-matrix least-squares method against all F2 in an anisotropic-isotropic (for H atoms) procedure using SHELXL-2018/3.20 Absorption corrections were applied by the empirical multiscan method in the SADABS software.21 Hydrogen atom positions were calculated using the riding model.
The crystallographic data and details of the refinements for all structures are summarized in
Table S1 in SI.
The independent part of the unit cell of the crystal lattice of salts of cations 1,1-dichlorobutylene
+ and I includes two anions and two cations (
Figures S2 and S3 in SI). In both independent cations I, the C6, C7, C14, and C15 atoms are disordered over two positions with occupancy ratios of 0.63:0.37 (C6’, C7’) and 0.54:0.46 (C14’, C15’); they are perfectly planar with standard deviations from the mean plane of 0.029 and 0.075Å, respectively. The geometry of the 1,1-dichlorobutylene
+ and I cations is given in
Tables S2 and S3 in SI, as is the average of their two independent positions. A quite high R-factor for II is explained by the very small size of the analyzed crystal (0.005× 0.01 × 0.30 mm).
CCDC 2258356, 2258357 and 2258358 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at
http://www.ccdc.cam.ac.uk/data_request/cif (accessed on May 10, 2023).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization and methodology, E.S.S.; validation, I.Y.B.;formal analysis, I.Y.B and I.V.S.; writing—review and editing, E.S.S.; supervision, E.S.S.; projectadministration, E.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported the Ministry of Science and Higher Education of the Russian Federation (state registration No. 1021052806375-6-1.4.3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radom, L.; Hariharan, P.C.; Pople, J.A.; Schleyer, P.R. Molecular orbital theory of the electronic structure of organic compounds. XIX. G eometries and energies of C3H5 cations. Energy relations among allyl, vinyl, and cyclopropyl cations. J. Am. Chem. Soc. 1973, 95, 6531–6544. [Google Scholar] [CrossRef]
- Müller, T.; Juhasz, M.; Reed, C.A. The X-ray structure of a vinyl cation. Angew. Chem. Int. Ed. 2004, 43, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Siehl, H.-U.; Müller, T.; Gauss, J. NMR Spectroscopic and quantum chemical characterization of the (E )− and (Z )− isomers of the penta-1,3-dienyl-2-cation. J. Phys. Org. Chem. 2003, 16, 577–581. [Google Scholar] [CrossRef]
- Byrne, P.A.; Kobayashi, S.; Wrthwein, E.-U.; Ammer, J.; Mayr, H. Why Are Vinyl Cations Sluggish Electrophiles? J. Am. Chem. Soc. 2017, 139, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, M.; Gao, S. Are Vinyl Cations Finally Coming of Age? Angew. Chem. Int. Ed. 2018, 57, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E.S.; Stoyanova, I.V. The Mechanism of High Reactivity of Benzyl Carbocation, C6H5CH2+, during Interaction with Benzene. Chem. Sel. 2020, 5, 9277–9280. [Google Scholar] [CrossRef]
- Müller, T.; Juhasz, M.; Reed, C. A. The X-ray Structure of a Vinyl Cation. Angew. Chem., Int. Ed. 2004, 43, 1543−1546. [Google Scholar] [CrossRef] [PubMed]
- Klaer, A.; Saak, W.; Haase, D.; Müller, T. Molecular Structure of a Cyclopropyl Substituted Vinyl Cation. J. Am. Chem. Soc. 2008, 130, 14956−14957. [Google Scholar] [CrossRef] [PubMed]
- Klaer, A.; Syha, Y.; Nasiri, H. R.; Müller, T. Trisilyl-Substituted Vinyl Cations. Chem.−Eur. J. 2009, 15, 8414−8423. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Margraf, D.; Syha, Y. σ-Delocalization versus π-Resonance in α-Aril-Substituted Vinyl Cations. J. Am. Chern. Soc. 2005, 127, 10852–10860. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E.S.; Bagryanskaya, I.Y.; Stoyanova, I.V. Unsaturated Vinyl-Type Carbocation [(CH3)2C=CH]+ in Its Carborane Salts. ACS Omega 2021, 6, 15834–15843. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E.S.; Bagryanskaya, I.Y.; Stoyanova, I.V. Isomers of the Allyl Carbocation C3H5+ in Solid Salts: Infrared Spectra and Structures. ACS Omega 2021, 6, 23691–23699. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E.S.; Bagryanskaya, I.Y.; Stoyanova, I.V. IR-Spectroscopic and X-ray- Structural Study of Vinyl-Type Carbocations in Their Carborane Salts. ACS Omega 2022, 7, 27560–27572. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E. S. , Stoyanova I.V. The Chloronium Cation, (C2H3)2Cl+, and Unsaturated C4-Carbocations with C=C and C≡C Bonds in Their Solid Salts and in Solutions: An H1/C13 NMR and Infrared Spectroscopic Study. Int. J. Mol. Sci. 2022, 23, 9111. [Google Scholar] [CrossRef] [PubMed]
- Reed, C. Carborane Acids. New “Strong yet Gentle” Acids for Organic and Inorganic Chemistry. Chem. Com. 2005; 1669–1677. [Google Scholar]
- Stoyanov, E. S. The salts of chloronium ions R–Cl+–R (R = CH3 or CH2Cl): formation, thermal stability, and interaction with chloromethanes. Phys. Chem. Chem. Phys., 2016, 18, 12896–12904. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E. S. , Bagryanskaya I.Yu., Stoyanova I.V. Interaction of Vinyl-Type Carbocations, C3H5+ and C4H7+ with Molecules of Water, Alcohols, and Acetone. Molecules 2023, 28, 1146. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.J. , Advances in Infrared Group Frequencies. Methuen & Co. LTD. Bungay, Suffolk, 1968.
- Juhasz, M.; Hoffmann, S.; Stoyanov, E.; Kim, K.-C.; Reed, C. A. The strongest isolable acid. Angew. Chem., Int. Ed. 2004, 43, 5352−5355. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXT. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- SADABS. v. 2008-1; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
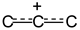 group. These frequencies are higher than those of allyl cation
group. These frequencies are higher than those of allyl cation  (1303 and 1265 cm-1), indicating a decrease in the positive charge on this group13 owing to transfer of the charge to Cl atoms. The spectrum also contains two bands of symmetric and asymmetric C-Cl stretch vibrations at 700 and 654 cm-1 in accordance with the presence of two C-Cl bonds at one carbon atom in the cation.
(1303 and 1265 cm-1), indicating a decrease in the positive charge on this group13 owing to transfer of the charge to Cl atoms. The spectrum also contains two bands of symmetric and asymmetric C-Cl stretch vibrations at 700 and 654 cm-1 in accordance with the presence of two C-Cl bonds at one carbon atom in the cation.