1. Introduction
The treatment of nonunions is one of the most challenging areas in small animal orthopedics. The diagnosis of fracture nonunion is made using radiographic evidence of bone hypertrophy or atrophy of bone fragments, defects between the fracture ends, sclerosis, and a closed medullary cavity [
1]. Most nonunions are the result of inadequate decision-making and technical problems of the surgeon, rather than factors associated with the patient or its owner [
2]. In small-breed dogs, nonunion has the highest incidence in the radius, followed by the tibia, whereas the femur has the lowest occurrence, which can be attributed to the amount of soft tissue and blood supply [
3]. An optimal bone graft supplies not only osteogenic cells for new bone formation, but also contains osteoinductive cells and an osteoconductive matrix that stimulates the differentiation of undifferentiated mesenchymal cells. The osteoconductive matrix also provides mechanical support for the internal growth and organization of the new bone structure [
4]. Autogenous cancellous bone grafting is most effective treatment and accepted as the gold standard for stimulation of fracture healing in orthopedics [
5]. Autogenous cancellous bone grafting is effective in most cases; however, if the defect is large owing to bone resorption or infection, it can be challenging to fill the cavity with cancellous bone. Because cancellous bone grafts do not provide mechanical stability. In cases with large defects, cortical bone grafts provide mechanical stability to the defect area [
6].
2. Case Presentation
2.1. History and Clinical Examination
An 11-month-old, castrated male Pomeranian presented with non-weight-bearing lameness of the left hind limb. The dog underwent femoral head and neck osteotomy at another hospital when he was six months old due to a femoral head fracture. Approximately one month later, the dog fractured his proximal femur. The dog underwent fracture repair surgery with a plate and screws; however, the bone fracture failed to heal. Consequently, the patient underwent a second surgery, which again failed, resulting in severe atrophic nonunion. Therefore, the dog was referred for revision surgery.
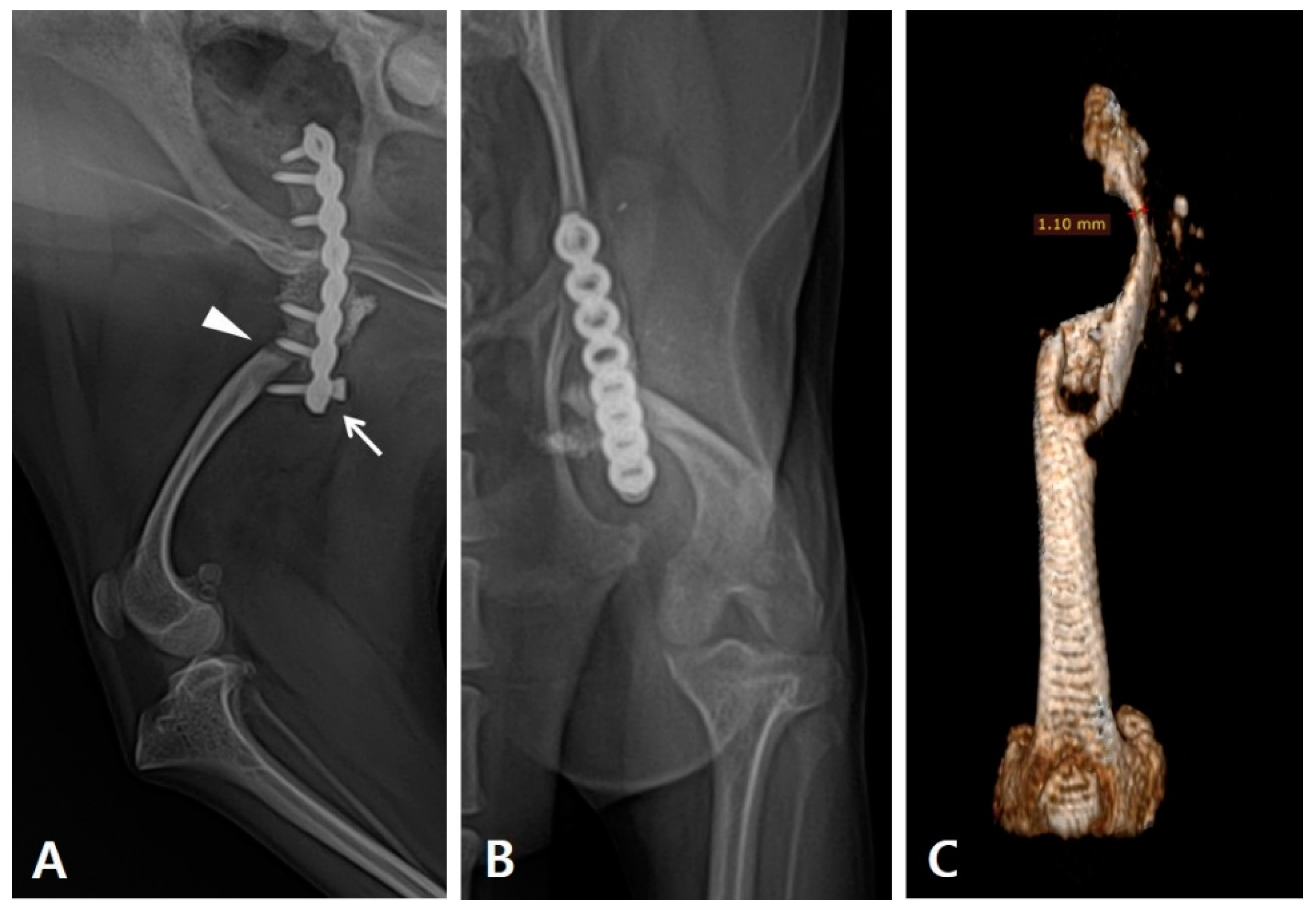
During the physical examination, the range of motion of the stifle and hock joints was found to be severely decreased. Radiography revealed implant failure in the left femur, severe atrophy of the proximal bone fragment, and retardation of the ipsilateral distal bone fragment of the femur and tibia compared to the contralateral side. (
Figure 1A). The failed implants were removed and computed tomography (CT) was performed for preoperative surgical planning (
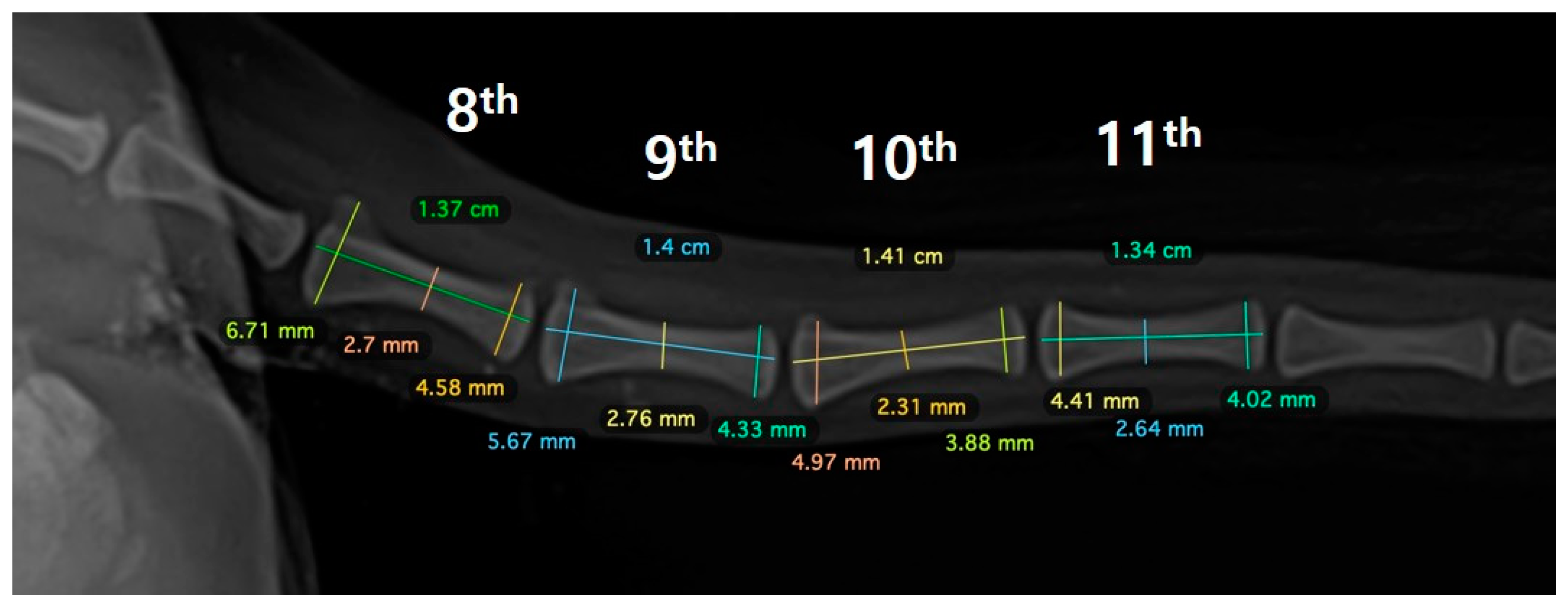
Figure 1B). An autogenous corticocancellous bone graft was planned and the coccygeal bone was ultimately chosen as the autograft (
Figure 2).
2.2. Anesthesia and Surgical Treatment
Before the surgical procedure, the patient received intravenous (IV) administration of premedicants and prophylactic antibiotics containing acepromazine (0.05 mg/kg, IV), ketamine (5 mg/kg, IV) and cefazolin (25 mg/kg, IV). Anesthesia was induced with propofol (6 mg/kg, IV) and maintained with isoflurane.
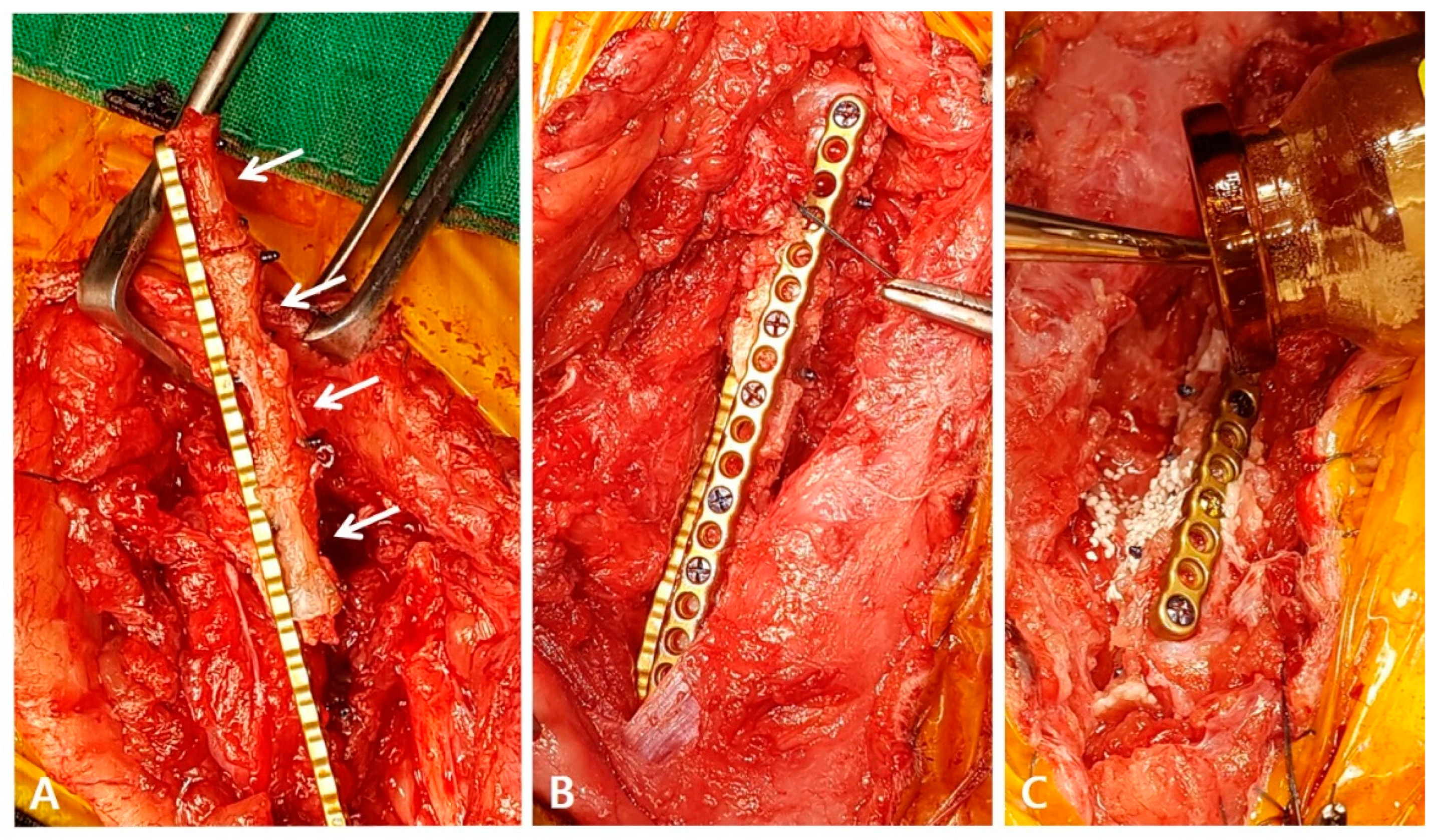
To harvest the planned 8th to 11th coccygeal bones, caudectomy was first performed, and the bone was separated from the surrounding soft tissues. The articular cartilage at both ends of the bone was resected using an oscillating saw. During the surgical procedure, the soft tissue contracture was severe and required stretching. A lateral incision was made to access the left femur, revealing a poorly vascularized proximal end of the distal bone fragment. To ensure proper healing and an adequate blood supply, the end of the bone was decorticated, rimmed, and trimmed. The medial surface of the proximal bone was carefully debrided with an orthopedic rasp to promote the healing of the coccygeal bone. To apply the graft, three-and-a-half coccygeal bones were prepared in series and fixed with a 1.2 polyaxial locking plate system (ARIX
®, Jeil Medical Co., South Korea) for mechanical stability (
Figure 3A). Orthogonal plating was performed laterally and cranially to ensure the stability of the proximal, distal, and coccygeal bones (
Figure 3B). Each coccygeal bone was stabilized with two or three screws, and screws inserted through the lateral side fixed both the proximal and coccygeal bones together. After thoroughly washing the area, recombinant human bone morphogenetic protein-2 (BMP) and biphasic calcium phosphate (BCP) (Cowell BMP
®, Cowellmedi, South Korea) were applied around the gap between the coccygeal bones and the coccygeal and distal bone fragments to encourage healing (
Figure 3C). The surgical site was closed in a standard manner without using a bandage to promote movement.
2.3. Post-Operative Management and Prognosis
During anesthesia, 10 ml of blood with citrate was collected from the patient to prepare platelet-rich plasma (PRP) (Sell Neo PRP 10cc kit®, NeoGenesis Co., Ltd, South Korea) for application to the soft tissue around the fracture sites to enhance the healing environment. PRP was administered three times with a one-week interval between each application, resulting in the administration of 1.5 mL of PRP each time. Transcutaneous electrical nerve stimulation (TENS) and neuromuscular electrical stimulation (NMES) were performed twice per week, and low level laser therapy (LLLT) was performed daily until hospital discharge. Additionally, passive range of motion (PROM) exercises were conducted on the hip, stifle, and hock joints four times a day until the patient was able to bear weight.
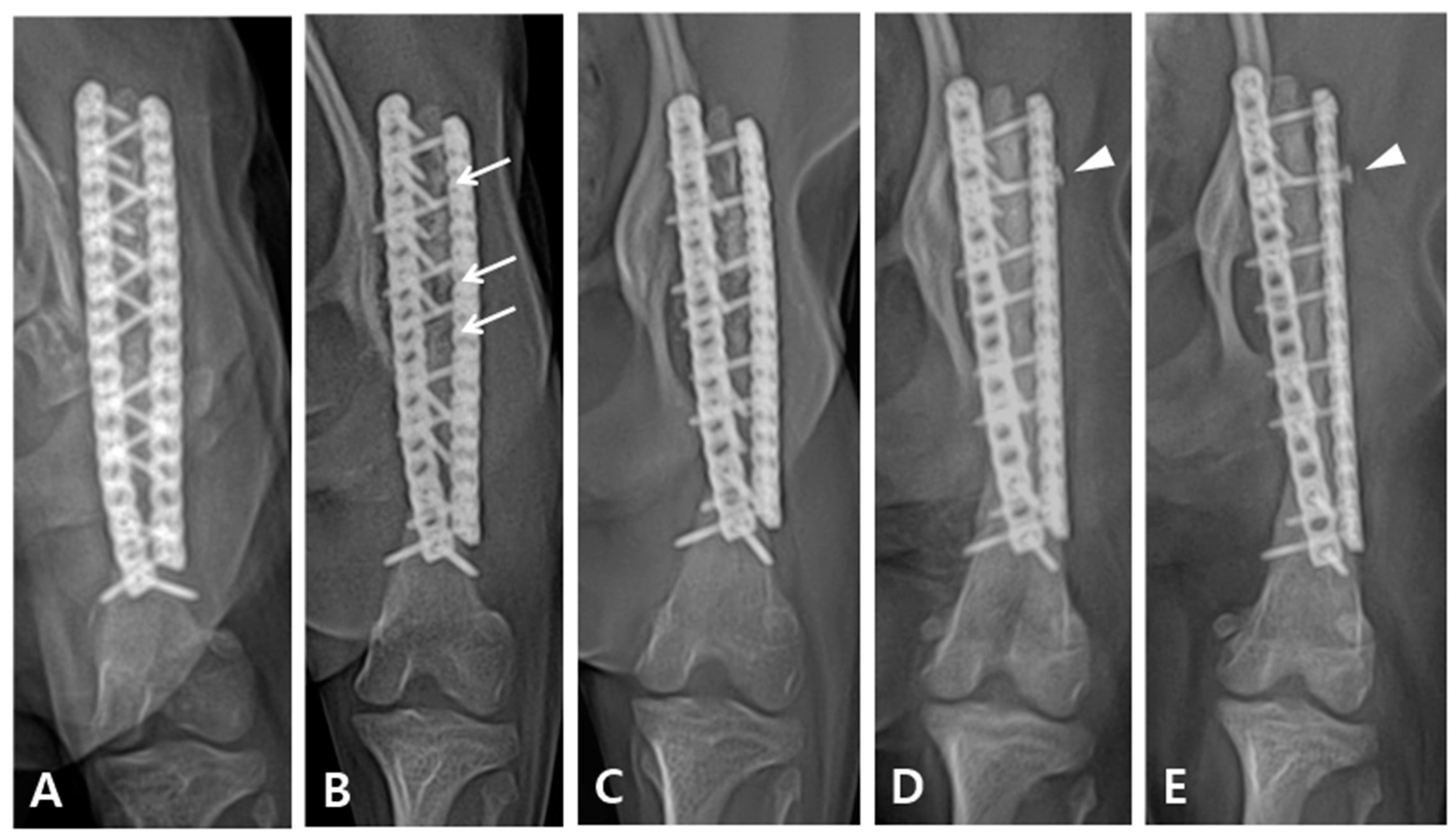
Immediately after surgery, the patient exhibited knuckling and was unable to bear weight. However, a neurological examination revealed no nerve damage. Limb shortening compared with the contralateral side was observed after the operation. Although the patient could partially bear weight, knuckling still persisted for six weeks after the operation. Three months post-operatively, the patient was able to fully bear weight in the limb extended position, with complete resolution of knuckling. On radiographic follow-up, the grafted bone showed temporarily increased lucency four weeks postoperatively, which recovered in three months after the operation (
Figure 4B,C). Eighteen months postoperatively, radiographic examination revealed complete bone healing; however, loosening of the proximal second screw in the lateral plate was observed (
Figure 4D). The patient was followed up for four years after the operation and exhibited satisfactory gait during walking and trotting; however, some lameness was noted following intense exercise or galloping.
3. Discussion
The patient in this case had hip contractures and muscle atrophy from two previously failed surgeries, which made the biological factors unfavorable. Autogenous bone grafting was performed to aid bone healing. While there are no large studies on the complications following autologous bone harvesting in veterinary medicine, complications as high as 25% have been reported in humans, including pain, sepsis, fatigue fractures, intraoperative bleeding, prolonged surgery, and insufficient bone harvesting [
7]. In dogs, the iliac crest, proximal humerus, and proximal tibia are considered the most abundant sources of autogenous cancellous bone [
8]. Owing to the substantial size of the defect, we needed to determine the donor site to harvest the largest bone with least complications, while still being able to fill the large defect with autologous bone. Hence, the coccygeal bone was selected as the autogenous bone graft. Furthermore, due to the differences in the coccygeal bone from that in humans, dogs with short or almost no tails due to breed characteristics, tumors, or fractures, can live without major inconvenience.
Successful use of the coccygeal bone to fill large defects, including the tibia and radius, has also been reported. One case report demonstrated a radial fracture nonunion, in which two coccygeal bones similar in diameter to the radius were selected and fixed in series [
9]. In our case, we also arranged the bones in series, as described by Choi and Yoon (2022); however, unlike the previous study, we inserted screws into the grafted bone itself to increase fixation because there was no place for the plate to be fixed at the proximal fragment, and the length of the graft was too long. By applying the orthogonal double-locking plate to the patient, we could prevent the fixation from collapsing and strengthen the mechanical structure. Another case report presented a tibial fracture nonunion, in which the coccygeal bone was placed parallel to fill the defect owing to the difference in thickness between the tibia and coccygeal bone [
10]. In the present case, we grafted the coccygeal bone onto the femur. Although the thickness and diameter of the coccygeal bone are usually much smaller than those of the femur, in our case, the diameter of the femur was significantly reduced owing to bone atrophy and retardation, resulting in a small difference in diameter between the coccygeal bone and femur.
Cancellous bone grafts have a viability rate of 85
–100%, which decreases over time. Therefore, the graft bone should be harvested immediately before transplantation to ensure maximal graft viability. In experiments with rabbit cancellous bone, viability decreased to 57% 3 h after harvesting and was further reduced when preserved in cooled saline [
11]. In our case, we performed a caudectomy immediately before femoral fracture surgery to improve the viability of the grafted coccygeal bone. Two operators performed the surgery simultaneously to expedite transplantation, one approaching the femoral surgical site and the other separating the coccygeal bone from the surrounding soft tissues. By minimizing the time between bone harvesting and grafting, we may have ensured the survival of the grafted bone.
In some cases, autogenous bone grafting procedures may fail to achieve proper healing because of sequestration or resorption of the grafted bone [
2]. To address this issue, various methods have been proposed to promote successful engraftment, including the use of BMP, BCP, and PRP [
2,
12,
13,
14,
15]. BMP enhances the osteoinductivity of autologous bone grafts and stimulates osteoblast proliferation, whereas BCP exhibits osteoconductivity and promotes the proliferation of osteogenic cells [
2,
14]. Combining BMP with autologous bone grafting can improve bone healing more effectively than using them separately [
14]. Furthermore, clinical studies have demonstrated that PRP can enhance the blood supply and increase biological activity for accelerated bone recovery [
13]. To increase the engraftment success rate, we applied BMP and BCP along with autologous coccygeal bone and injected PRP into the surgical site. In addition, we also administered rehabilitation treatments, such as TENS, NMES, LLLT, and PROM, to improve blood supply and enhance biological activity, thereby promoting bone healing.
In the early stages of direct bone healing, also known as primary bone healing, radiographic fracture lines remain visible. During contact healing, there was no resorption of the fragment tip, and the cutting cone progressed across the fracture gap and became radiographically opaque. In clinical cases of simple transverse fractures treated with rigid fixation with compression, the fracture line may slowly disappear without forming callus [
16,
17]. In our case, as shown in
Figure 3B, partial absorption of the grafted bone was observed 4 weeks after surgery, and the osteotomy line between the grafted coccygeal bone and the distal bone fragment was visible radiographically. However, in
Figure 3C, taken 3 months later, the previously seen lucency became opaque, and in
Figure 3D, taken after 8 months, the osteotomy line completely disappeared. Despite the extended time required for complete bone healing, rigid fixation was maintained for an extended period using locking plates and screws, which facilitated healing without callus formation.
According to D. Franczuszki [
18], >20% difference in femur length can lead to gait problems. Following the operation, the patient exhibited a 22.8% reduction in femur length, 12.0% decrease in tibia length, and 40.0% reduction in tibia diameter compared with the normal contralateral limb (Figure 5). This was because a fracture and nonunion that occurred during the juvenile period. The patient was able to walk, but due to the overall shortening of the limb, the limb was extended, and although there was significant improvement with rehabilitation, the range of motion remained limited due to the presence of joint contracture.