Submitted:
25 April 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of virus Stock
2.2. Mice and Infection Protocol
2.3. BALF Cells, Bioplex Analysis, And virus Titration
2.4. RNA Extraction and Gene Expression Analysis
2.5. In Vivo Micro-CT Imaging and Analysis
2.6. Imaging Processing and Analysis
2.7. Lung Histology
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
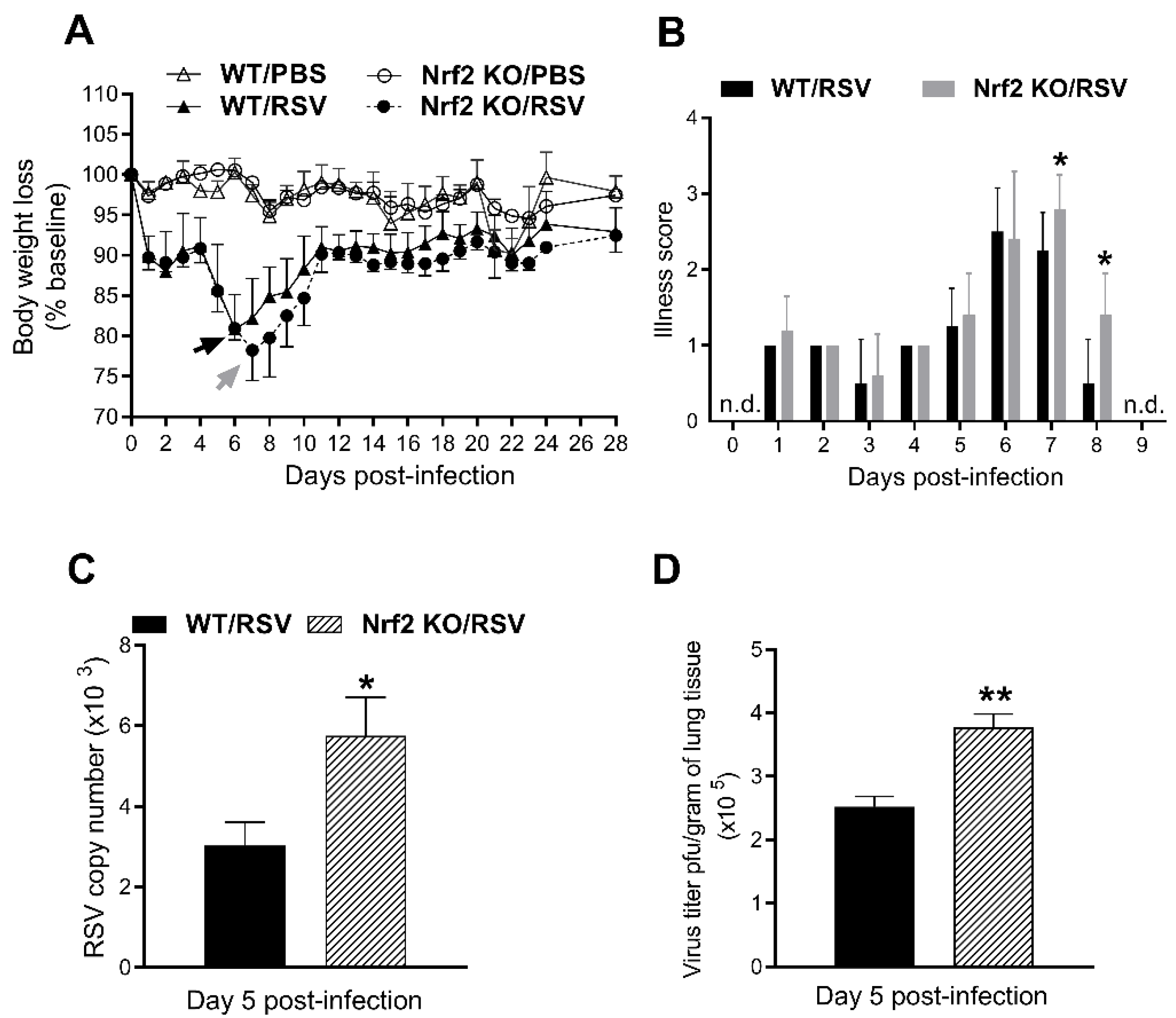
3.1. Enhanced Disease and Viral Replication in Nrf2 Deficient Mice
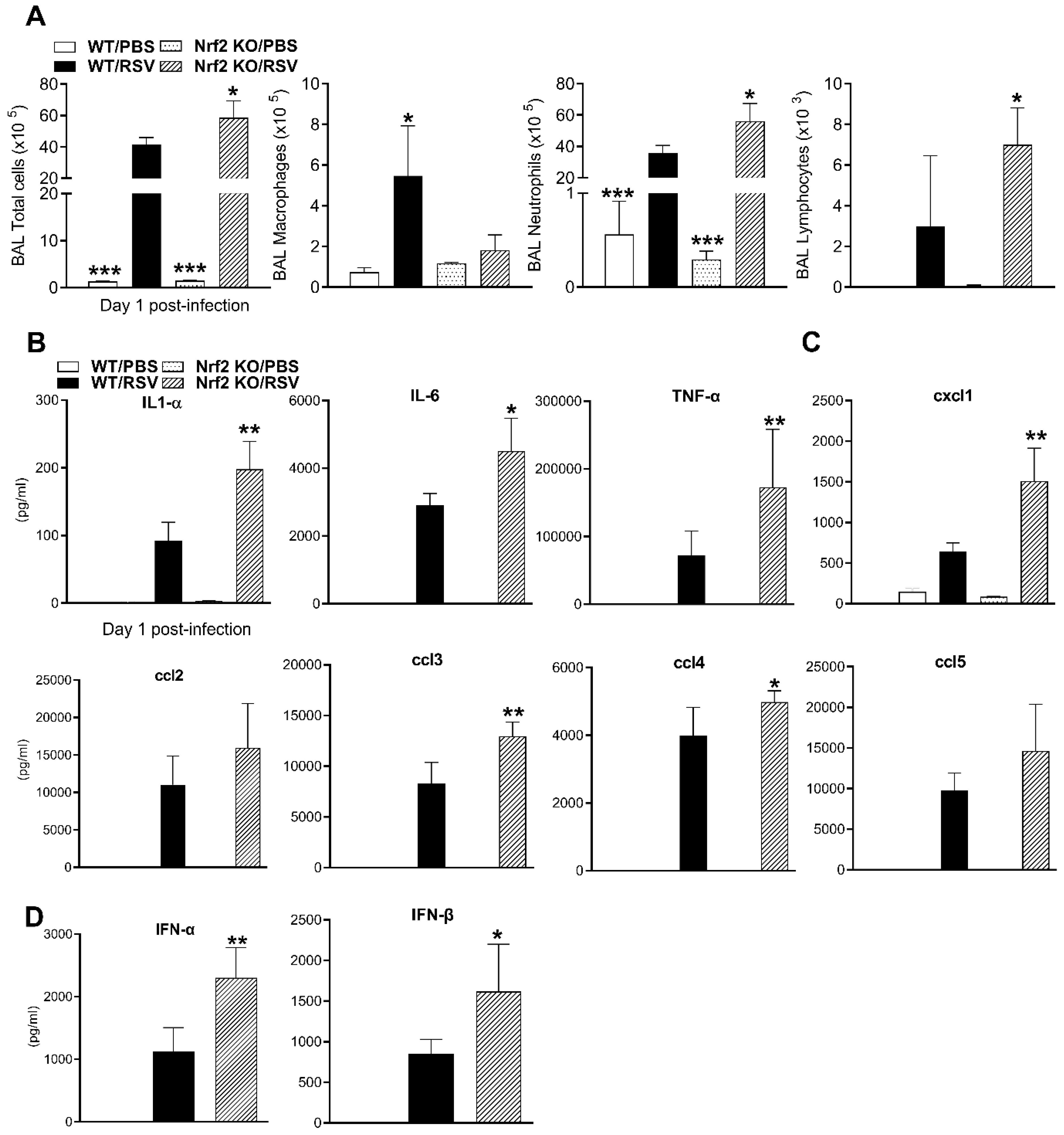
3.2. BALF Cellularity and Cytokines are Increased at Early Time Points in Absence of Nrf2
3.3. Inflammatory and Immunoregulatory Cytokine Gene Expression in the Lung
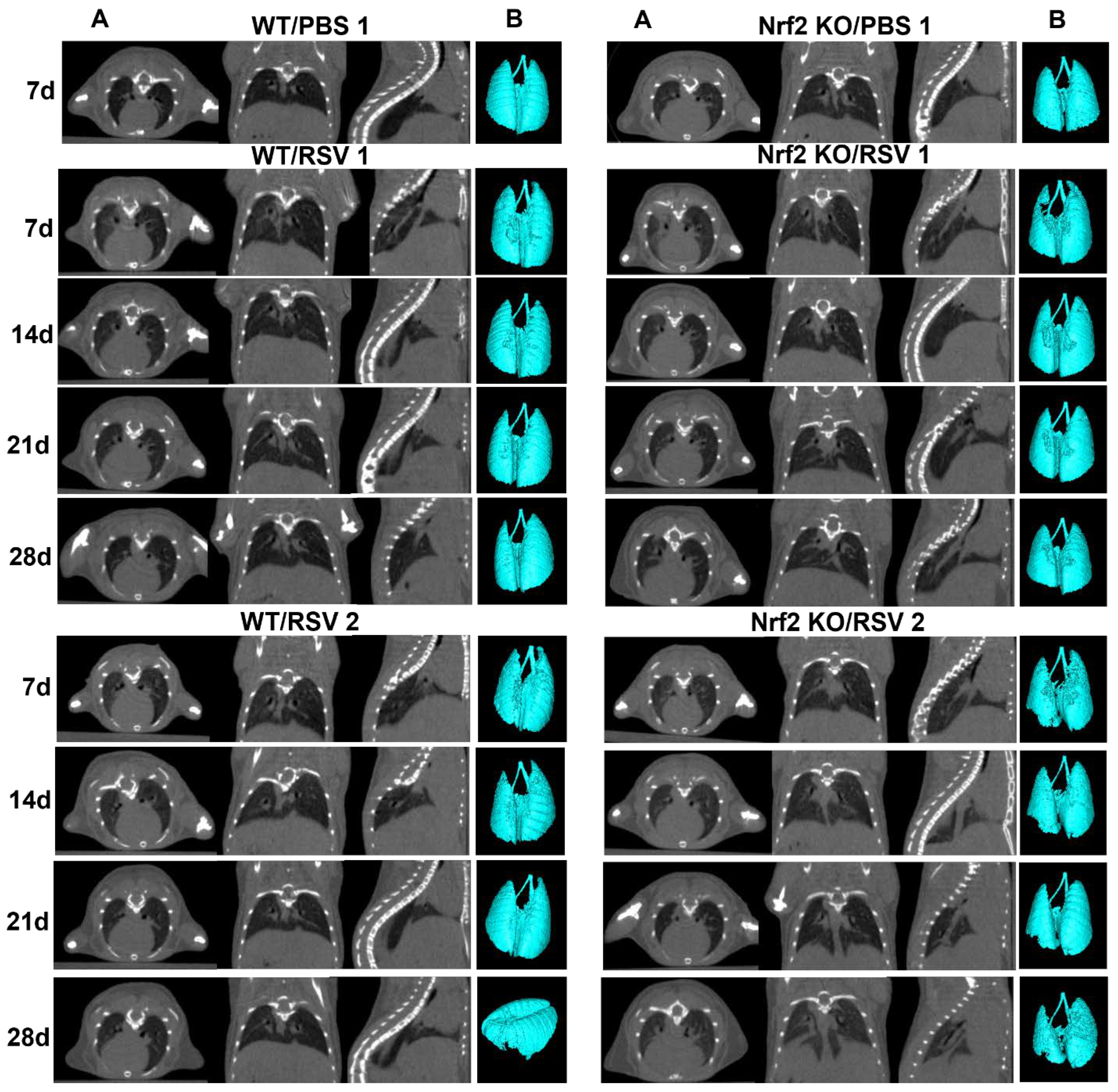
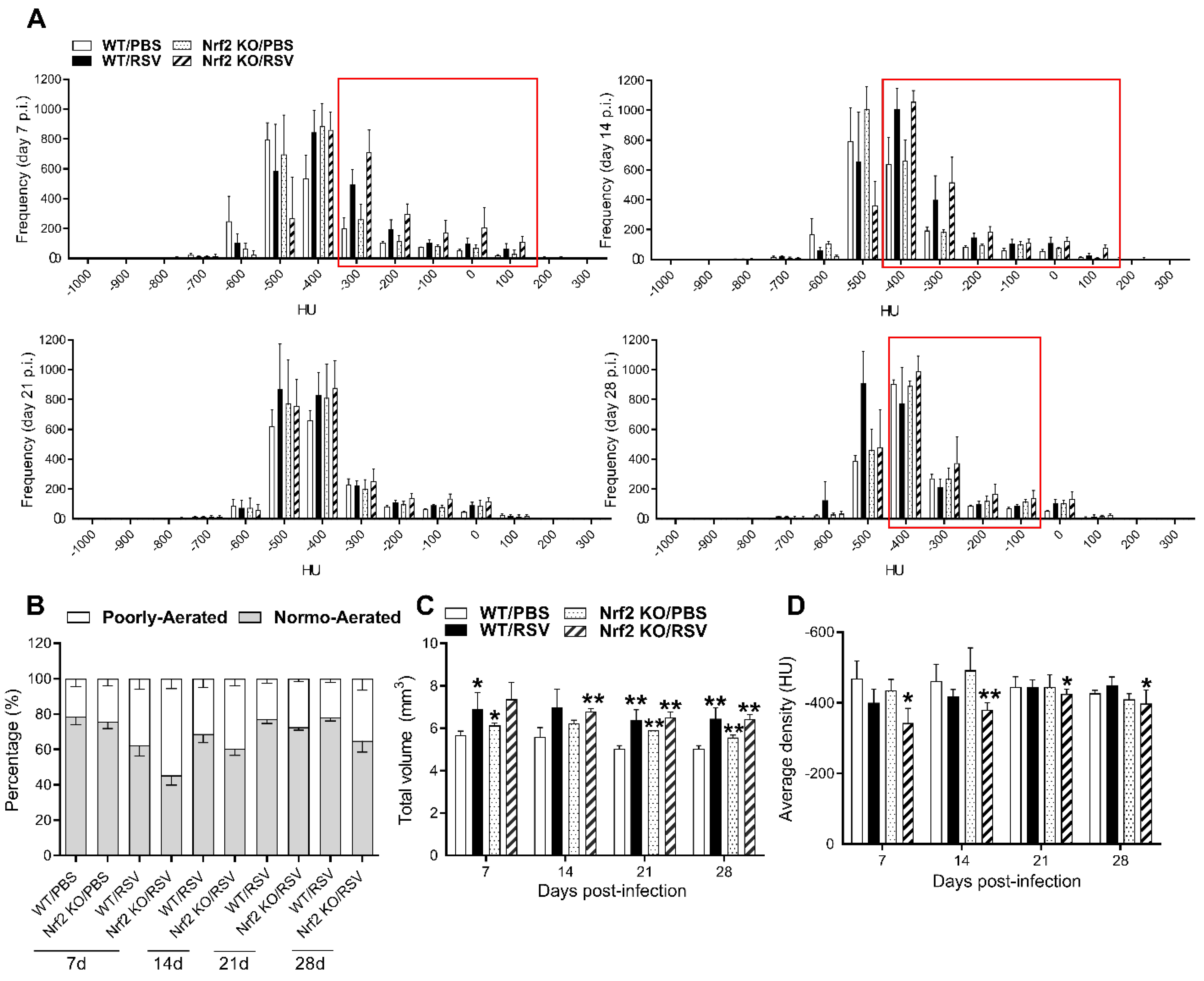
3.4. Longitudinal Assessment of Infected Mouse Lung by Micro-CT Imaging, 3D Reconstruction and Quantitative Analysis
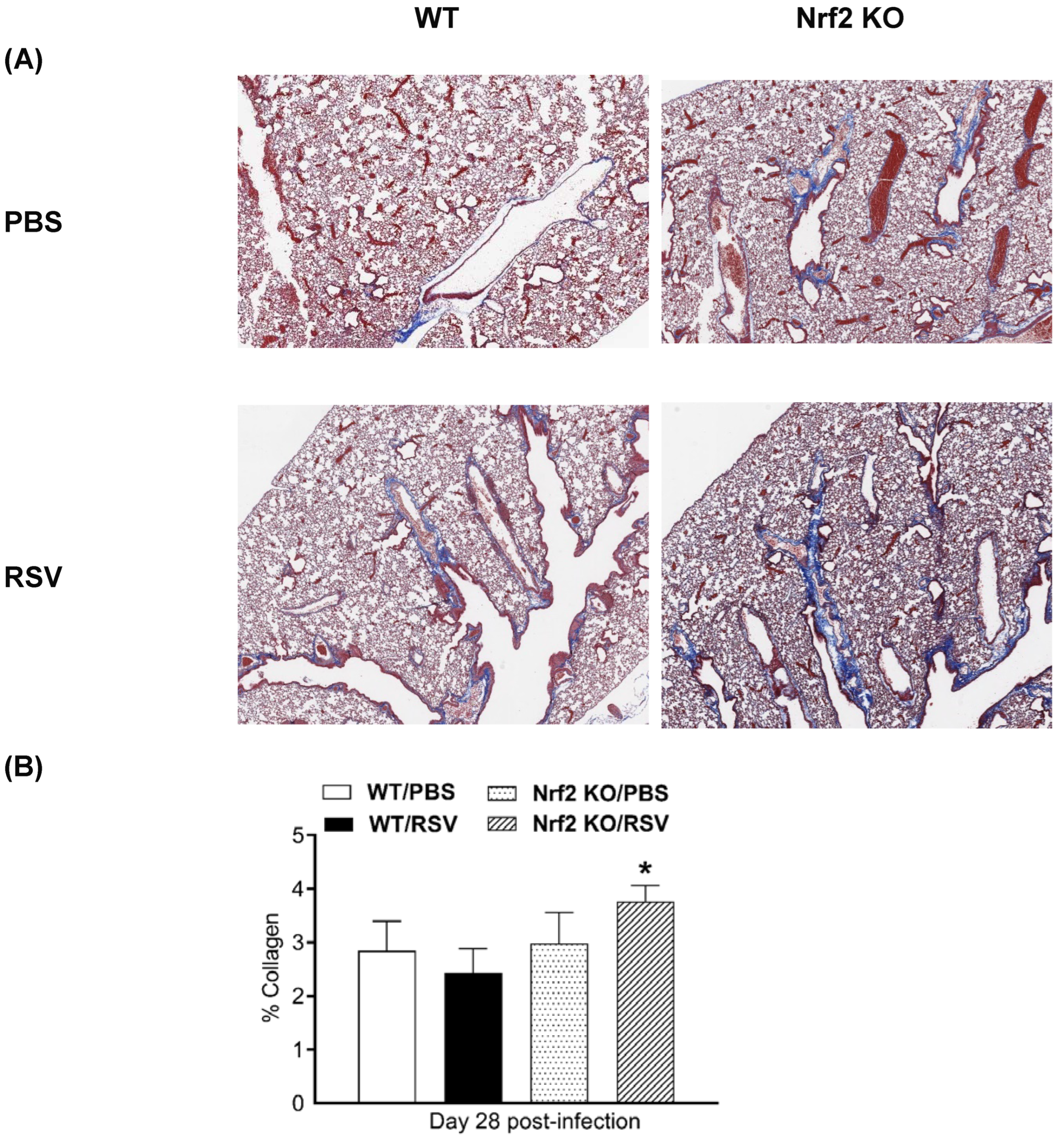
3.5. Histochemical Analysis of Collagen Deposition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 2010, 38, 5718–5734. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol 2007, 27, 6334–6349. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 2003, 35, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches - Pulmonary Disease/Asthma. Front Physiol 2021, 12, 727806. [Google Scholar] [CrossRef]
- Garofalo, R.P.; Kolli, D.; Casola, A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxid Redox Signal 2013, 18, 186–217. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kleeberger, S.R. Nrf2 protects against airway disorders. Toxicol. Appl. Pharmacol 2010, 244, 43–56. [Google Scholar] [CrossRef]
- Cho, H.Y.; Miller-DeGraff, L.; Perrow, L.A.; Gladwell, W.; Panduri, V.; Lih, F.B.; Kleeberger, S.R. Murine Neonatal Oxidant Lung Injury: NRF2-Dependent Predisposition to Adulthood Respiratory Viral Infection and Protection by Maternal Antioxidant. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Cho, H.Y.; Reddy, S.P.; Yamamoto, M.; Kleeberger, S.R. The transcription factor NRF2 protects against pulmonary fibrosis. Faseb j 2004, 18, 1258–1260. [Google Scholar] [CrossRef]
- Yan, B.; Ma, Z.; Shi, S.; Hu, Y.; Ma, T.; Rong, G.; Yang, J. Sulforaphane prevents bleomycin-induced pulmonary fibrosis in mice by inhibiting oxidative stress via nuclear factor erythroid 2-related factor-2 activation. Mol Med Rep 2017, 15, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.L.; Zhang, X.R.; Ma, Y.L.; Lu, Q.; Zhao, R.; Zhu, Y.Z.; Wang, Y.Y. The role of Nrf2/PIWIL2/purine metabolism axis in controlling radiation-induced lung fibrosis. Am J Cancer Res 2020, 10, 2752–2767. [Google Scholar] [PubMed]
- Schaedler, S.; Krause, J.; Himmelsbach, K.; Carvajal-Yepes, M.; Lieder, F.; Klingel, K.; Nassal, M.; Weiss, T.S.; Werner, S.; Hildt, E. Hepatitis B virus induces expression of antioxidant response element-regulated genes by activation of Nrf2. J. Biol. Chem 2010, 285, 41074–41086. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.; Olivarez, M.; Waris, G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J. Gen. Virol 2010, 91, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Smirnova, O.A.; Ivanova, O.N.; Masalova, O.V.; Kochetkov, S.N.; Isaguliants, M.G. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS. ONE 2011, 6, e24957. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koh, K.; Kim, Y.E.; Ahn, J.H.; Kim, S. Up-regulation of Nrf2 Expression by Human Cytomegalovirus Infection Protects Host Cells from Oxidative Stress. J. Gen. Virol 2013, 94, 1658–1668. [Google Scholar] [CrossRef]
- Gjyshi, O.; Bottero, V.; Veettil, M.V.; Dutta, S.; Singh, V.V.; Chikoti, L.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS. Pathog 2014, 10, e1004460. [Google Scholar] [CrossRef]
- Page, A.; Volchkova, V.A.; Reid, S.P.; Mateo, M.; Bagnaud-Baule, A.; Nemirov, K.; Shurtleff, A.C.; Lawrence, P.; Reynard, O.; Ottmann, M.; et al. Marburgvirus Hijacks Nrf2-Dependent Pathway by Targeting Nrf2-Negative Regulator Keap1. Cell Rep 2014, 6, 1026–1036. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Jantzi, P.D.; Esham, D.L.; Spratt, H.; Kurosky, A.; Casola, A.; Garofalo, R.P. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2011, 183, 1550–1560. [Google Scholar] [CrossRef]
- Qu, Y.; Haas de Mello, A.; Morris, D.R.; Jones-Hall, Y.L.; Ivanciuc, T.; Sattler, R.A.; Paessler, S.; Menachery, V.D.; Garofalo, R.P.; Casola, A. SARS-CoV-2 Inhibits NRF2-Mediated Antioxidant Responses in Airway Epithelial Cells and in the Lung of a Murine Model of Infection. Microbiol Spectr 2023, e0037823. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol 2009, 41, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Imani, F.; Miller-Degraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M.; Polack, F.P.; Kleeberger, S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit Care Med 2009, 179, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ivanciuc, T.; Sbrana, E.; Casola, A.; Garofalo, R.P. Protective Role of Nuclear Factor Erythroid 2-Related Factor 2 Against Respiratory Syncytial Virus and Human Metapneumovirus Infections. Front Immunol 2018, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Pazdrak, B.; Casola, A.; Saito, T.; Alam, R.; Crowe, S.E.; Mei, F.; Ogra, P.L.; Garofalo, R.P. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 1998, 72, 4756–4764. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Lu, R.; Chang, J.C.; Kan, Y.W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A 1996, 93, 13943–13948. [Google Scholar] [CrossRef] [PubMed]
- Ivanciuc, T.; Sbrana, E.; Ansar, M.; Bazhanov, N.; Szabo, C.; Casola, A.; Garofalo, R.P. Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection. Am J Respir Cell Mol Biol 2016, 55, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Increased Lung Catalase Activity Confers Protection Against Experimental RSV Infection. Sci Rep 2020, 10, 3653. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, L.F.; Kholodnykh, A.; Villarreal, P.; Tian, B.; Pal, R.; Freiberg, A.N.; Brasier, A.R.; Motamedi, M.; Vargas, G. Imaging of Murine Whole Lung Fibrosis by Large Scale 3D Microscopy aided by Tissue Optical Clearing. Sci. Rep 2018, 8, 13348. [Google Scholar] [CrossRef]
- Tian, B.; Patrikeev, I.; Ochoa, L.; Vargas, G.; Belanger, K.K.; Litvinov, J.; Boldogh, I.; Ameredes, B.T.; Motamedi, M.; Brasier, A.R. NF-κB Mediates Mesenchymal Transition, Remodeling, and Pulmonary Fibrosis in Response to Chronic Inflammation by Viral RNA Patterns. Am J Respir Cell Mol Biol 2017, 56, 506–520. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Z.; Litvinov, J.; Maroto, R.; Jamaluddin, M.; Rytting, E.; Patrikeev, I.; Ochoa, L.; Vargas, G.; Motamedi, M.; et al. Efficacy of Novel Highly Specific Bromodomain-Containing Protein 4 Inhibitors in Innate Inflammation-Driven Airway Remodeling. Am J Respir Cell Mol Biol 2019, 60, 68–83. [Google Scholar] [CrossRef]
- Bertram, C.A.; Klopfleisch, R. The Pathologist 2.0: An Update on Digital Pathology in Veterinary Medicine. Vet Pathol 2017, 54, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Riber-Hansen, R.; Vainer, B.; Steiniche, T. Digital image analysis: a review of reproducibility, stability and basic requirements for optimal results. Apmis 2012, 120, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y. Digital pathology in academia: Implementation and impact. Lab Anim (NY) 2021, 50, 229–231. [Google Scholar] [CrossRef]
- Singh, S.P.; Zhang, H.H.; Foley, J.F.; Hedrick, M.N.; Farber, J.M. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 2008, 180, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.N.; Prosser, D.M.; Forster, R.; Zhang, J.; Kuklin, N.A.; Abbondanzo, S.J.; Niu, X.D.; Chen, S.C.; Manfra, D.J.; Wiekowski, M.T.; et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 2000, 12, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kallal, L.E.; Schaller, M.A.; Lindell, D.M.; Lira, S.A.; Lukacs, N.W. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur. J. Immunol 2010, 40, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Imani, F.; Miller-Degraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus (RSV) disease. Am J Respir Crit Care Med 2009, 179, 138–150. [Google Scholar] [CrossRef]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Hong, C.; Casola, A.; Garofalo, R.P. Alveolar Macrophages Contribute to the Pathogenesis of hMPV Infection While Protecting Against RSV Infection. Am. J. Respir. Cell Mol. Biol 2014, 51, 502–515. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun 2016, 7, 11624. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun 2020, 11, 4938. [Google Scholar] [CrossRef]
- Bao, X.; Kolli, D.; Ren, J.; Liu, T.; Garofalo, R.P.; Casola, A. Human metapneumovirus glycoprotein G disrupts mitochondrial signaling in airway epithelial cells. PLoS ONE 2013, 8, e62568. [Google Scholar] [CrossRef]
- Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Increased Lung Catalase Activity Confers Protection Against Experimental RSV Infection. Sci. Rep 2020, 10, 3653. [Google Scholar] [CrossRef]
- Shi, X.; Shi, Z.; Huang, H.; Zhu, H.; Zhu, H.; Ju, D.; Zhou, P. PEGylated human catalase elicits potent therapeutic effects on H1N1 influenza-induced pneumonia in mice. Appl. Microbiol. Biotechnol 2013, 97, 10025–10033. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Komaravelli, N.; Mautemps, N.; Liu, T.; Garofalo, R.P.; Casola, A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am. J. Physiol Lung Cell Mol. Physiol 2012, 303, L991–1000. [Google Scholar] [CrossRef]
- Kim, H.J.; Barajas, B.; Wang, M.; Nel, A.E. Nrf2 activation by sulforaphane restores the age-related decrease of T(H)1 immunity: role of dendritic cells. J. Allergy Clin. Immunol 2008, 121, 1255–1261. [Google Scholar] [CrossRef]
- Kimura, D.; Saravia, J.; Jaligama, S.; McNamara, I.; Vu, L.D.; Sullivan, R.D.; Mancarella, S.; You, D.; Cormier, S.A. New mouse model of pulmonary hypertension induced by respiratory syncytial virus bronchiolitis. Am J Physiol Heart Circ Physiol 2018, 315, H581–h589. [Google Scholar] [CrossRef]
- Becnel, D.; You, D.; Erskin, J.; Dimina, D.M.; Cormier, S.A. A role for airway remodeling during respiratory syncytial virus infection. Respir Res 2005, 6, 122. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Zhang, Z. Respiratory syncytial virus infection accelerates lung fibrosis through the unfolded protein response in a bleomycin-induced pulmonary fibrosis animal model. Mol Med Rep 2017, 16, 310–316. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Z.; Litvinov, J.; Maroto, R.; Jamaluddin, M.; Rytting, E.; Patrikeev, I.; Ochoa, L.; Vargas, G.; Motamedi, M.; et al. Efficacy of Novel Highly Specific Bromodomain-Containing Protein 4 Inhibitors in Innate Inflammation-Driven Airway Remodeling. Am. J. Respir. Cell Mol. Biol 2018. [Google Scholar] [CrossRef]
- Walters, D.M.; Cho, H.Y.; Kleeberger, S.R. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid Redox Signal 2008, 10, 321–332. [Google Scholar] [CrossRef]
- Castro, S.M.; Guerrero-Plata, A.; Suarez-Real, G.; Adegboyega, P.A.; Colasurdo, G.N.; Khan, A.M.; Garofalo, R.P.; Casola, A. Antioxidant Treatment Ameliorates Respiratory Syncytial Virus-induced Disease and Lung Inflammation. Am. J. Respir. Crit Care Med 2006, 174, 1361–1369. [Google Scholar] [CrossRef]
- Casola, A.; Burger, N.; Liu, T.; Jamaluddin, M.; A. R., B.; Garofalo, R.P. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with Respiratory Syncytial Virus: role in viral-induced Interferon Regulatory Factor activation. J Biol Chem 2001, 276, 19715–19722. [Google Scholar] [CrossRef]
- Liu, T.; Castro, S.; Brasier, A.R.; Jamaluddin, M.; Garofalo, R.P.; Casola, A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J. Biol. Chem 2004, 279, 2461–2469. [Google Scholar] [CrossRef]
- Chambliss, J.M.; Ansar, M.; Kelley, J.P.; Spratt, H.; Garofalo, R.P.; Casola, A. A Polymorphism in the Catalase Gene Promoter Confers Protection against Severe RSV Bronchiolitis. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Morris, D.R.; Ansar, M.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Selective Blockade of TNFR1 Improves Clinical Disease and Bronchoconstriction in Experimental RSV Infection. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Ansar, M.; Qu, Y.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Lack of Type I Interferon Signaling Ameliorates Respiratory Syncytial Virus-Induced Lung Inflammation and Restores Antioxidant Defenses. Antioxidants (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Difranza, J.R.; Masaquel, A.; Barrett, A.M.; Colosia, A.D.; Mahadevia, P.J. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC. Pediatr 2012, 12, 81. [Google Scholar] [CrossRef]
| Genotype | Gene Symbol | Gene name | ||
|---|---|---|---|---|
| WT | Nrf2 KO | |||
| *FC | *FC | |||
| 5.69 | 5.68 | B2m | Beta-2 microglobulin | |
| 6.92 | 3.55 | Ccl1 | Chemokine (C-C motif) ligand 1 | |
| 2.17 | 2.70 | Ccl11 | Chemokine (C-C motif) ligand 11 | |
| 21.15 | 52.90 | Ccl12 | Chemokine (C-C motif) ligand 12 | |
| 2.77 | 2.65 | Ccl17 | Chemokine (C-C motif) ligand 17 | |
| 5.74 | 5.98 | Ccl19 | Chemokine (C-C motif) ligand 19 | |
| 193.79 | 664.12 | Ccl2 | Chemokine (C-C motif) ligand 2 | |
| 7.62 | 112.99 | Ccl20 | Chemokine (C-C motif) ligand 20 | |
| 5.84 | 1.84 | Ccl22 | Chemokine (C-C motif) ligand 22 | |
| 172.11 | 445.40 | Ccl3 | Chemokine (C-C motif) ligand 3 | |
| 282.90 | 977.64 | Ccl4 | Chemokine (C-C motif) ligand 4 | |
| 7.48 | 13.70 | Ccl5 | Chemokine (C-C motif) ligand 5 | |
| 97.70 | 311.62 | Ccl7 | Chemokine (C-C motif) ligand 7 | |
| 9.50 | 12.49 | Ccr1 | Chemokine (C-C motif) receptor 1 | |
| 2.20 | 1.75 | Ccr2 | Chemokine (C-C motif) receptor 2 | |
| 5.53 | 7.15 | Ccr5 | Chemokine (C-C motif) receptor 5 | |
| 11.93 | 11.42 | Csf1 | Colony stimulating factor 1 (macrophage) | |
| 15.95 | 14.83 | Csf2 | Colony stimulating factor 2 (macrophage) | |
| 166.25 | 384.91 | Csf3 | Colony stimulating factor 3 (macrophage) | |
| 21.35 | 86.81 | Cxcl1 | Chemokine (C-X-C motif) ligand 1 | |
| 1862.03 | 3375.38 | Cxcl10 | Chemokine (C-X-C motif) ligand 10 | |
| 16.79 | 28.27 | Cxcl13 | Chemokine (C-X-C motif) ligand 13 | |
| 1532.46 | 1648.33 | Cxcl9 | Chemokine (C-X-C motif) ligand 9 | |
| 6.07 | 7.29 | Cxcr2 | Chemokine (C-X-C motif) receptor 2 | |
| 2.65 | 3.23 | Fasl | Fas ligand (TNF superfamily, member 6) | |
| 10.66 | 12.69 | Ifng | Interferon gamma | |
| 6.69 | 8.00 | Il15 | Interleukin 15 | |
| 16.87 | 37.80 | Il1a | Interleukin 1 alpha | |
| 16.44 | 61.88 | Il1b | Interleukin 1 beta | |
| 43.84 | 151.66 | Il1rn | Interleukin 1 receptor antagonist | |
| 62.00 | 78.08 | Il27 | Interleukin 27 | |
| 2.72 | 2.16 | Il2rg | Interleukin 2 receptor, gamma chain | |
| 5.22 | 5.53 | Il10ra | Interleukin 10 receptor, alpha | |
| 1.84 | 1.78 | Il10rb | Interleukin 10 receptor, beta | |
| 3.59 | 2.86 | Lta | Lymphotoxin A | |
| 6.51 | 6.55 | Nampt | Nicotinamide phosphoribosyl transferase | |
| 8.75 | 33.94 | Osm | Oncostatin M | |
| 15.49 | 40.16 | Tnf | Tumor necrosis factor | |
| 2.27 |
1.95 |
Tnfrsf11b | Tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | |
| 2.97 | n.s. | Ccr8 | Chemokine (C-C motif) receptor 8 | |
| 3.21 | n.s | Il17f | Interleukin 17f | |
| 2.16 | n.s. | Il2rb | Interleukin 2 receptor, beta | |
| 3.15 |
n.s. |
Tnfsf10 | Tumor necrosis factor (ligand) superfamily, member 10 | |
| 3.08 | n.s. | Tnfsf4 | Tumor necrosis factor (ligand) superfamily, member 4 | |
| n.s. | 3.62 | Ccl8 | Chemokine (C-C motif) ligand 8 | |
| n.s. | 13.1 | Cxcl5 | Chemokine (C-X-C motif) ligand 5 | |
| n.s. | 2.5 | Il11 | Interleukin 11 | |
| n.s. | 7.88 | Il13 | Interleukin 13 | |
| Genotype | Gene Symbol | Gene name | ||
|---|---|---|---|---|
| WT | Nrf2 KO | |||
| *FC | *FC | |||
| -1.90 | -2.57 | Ccl6 | Chemokine (C-C motif) ligand 6 | |
| -2.37 | -7.01 | Ccr3 | Chemokine (C-C motif) receptor 3 | |
| -1.67 | -2.54 | Cxcl12 | Chemokine (C-X-C motif) ligand 12 | |
| -7.26 | -3.55 | Cxcl15 | Chemokine (C-X-C motif) ligand 15 | |
| -2.71 | -14.30 | IL5ra | Interleukin 5 receptor, alpha | |
| -3.75 | -4.38 | Il16 | Interleukin 16 | |
| -7.72 | -2.56 | Spp1 | Secreted phosphoprotein 1 | |
| -3.68 | n.s. | Ccr6 | Chemokine (C-C motif) receptor 6 | |
| -2.01 | n.s. | Ccr10 | Chemokine (C-C motif) receptor 10 | |
| -4.24 | n.s. | Il17b | Interleukin 17B | |
| -3.81 | n.s. | Tnfsf11 | Tumor necrosis factor (ligand) superfamily, member 11 | |
| -1.73 | n.s. | Il33 | Interleukin 33 | |
| n.s. | -3.14 | Cx3cl1 | Chemokine (C-X3-C motif) ligand 1 | |
| n.s. | -5.01 | Il5 | Interleukin 5 | |
| n.s. | -2.19 | IL4 | Interleukin 4 | |
| n.s. | -3 | Bmp2 | Bone morphogenetic protein 2 | |
| n.s. | -1.62 | Vegfa | Vascular endothelial growth factor A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





