Submitted:
04 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
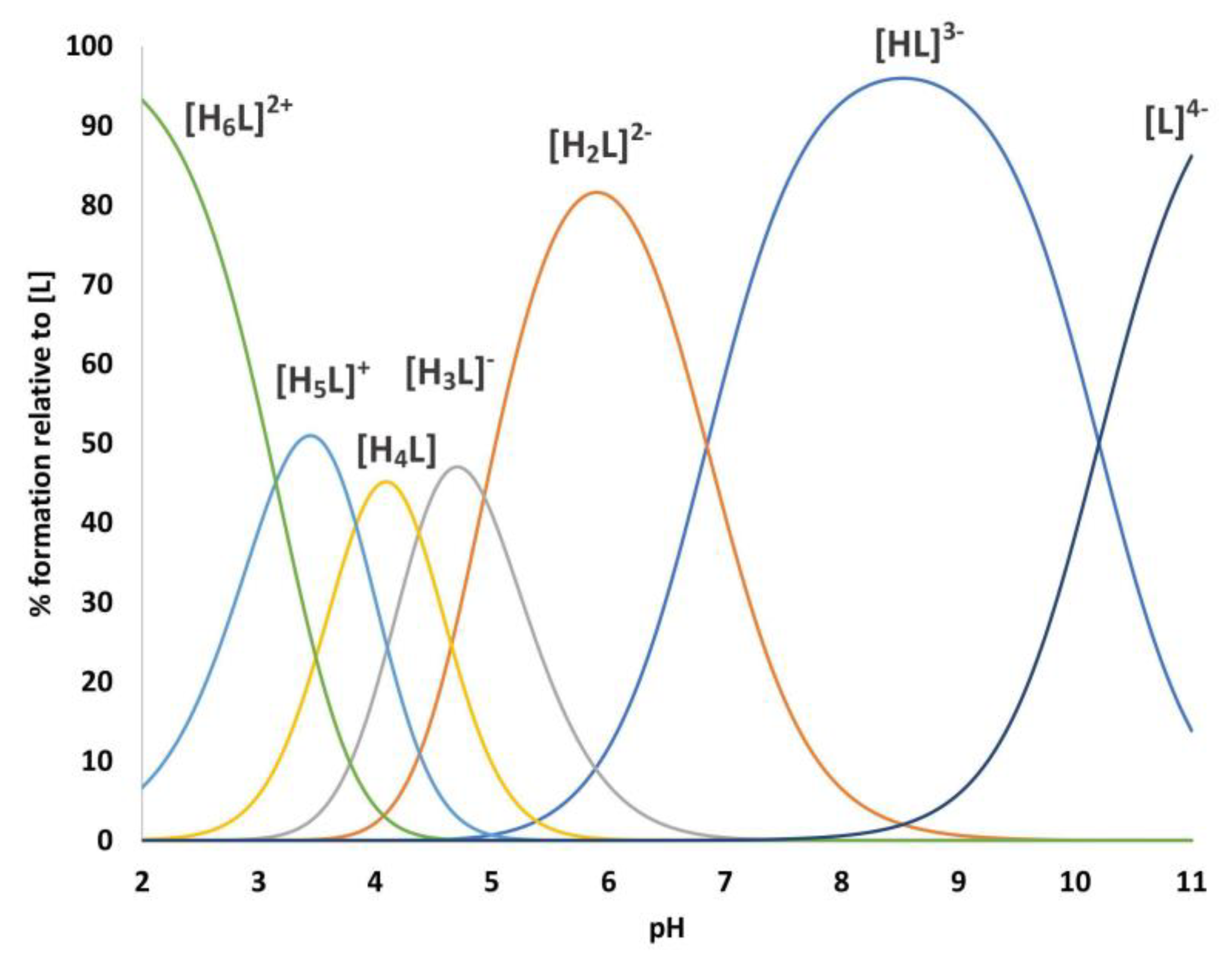
2.1. Protonation equilibria
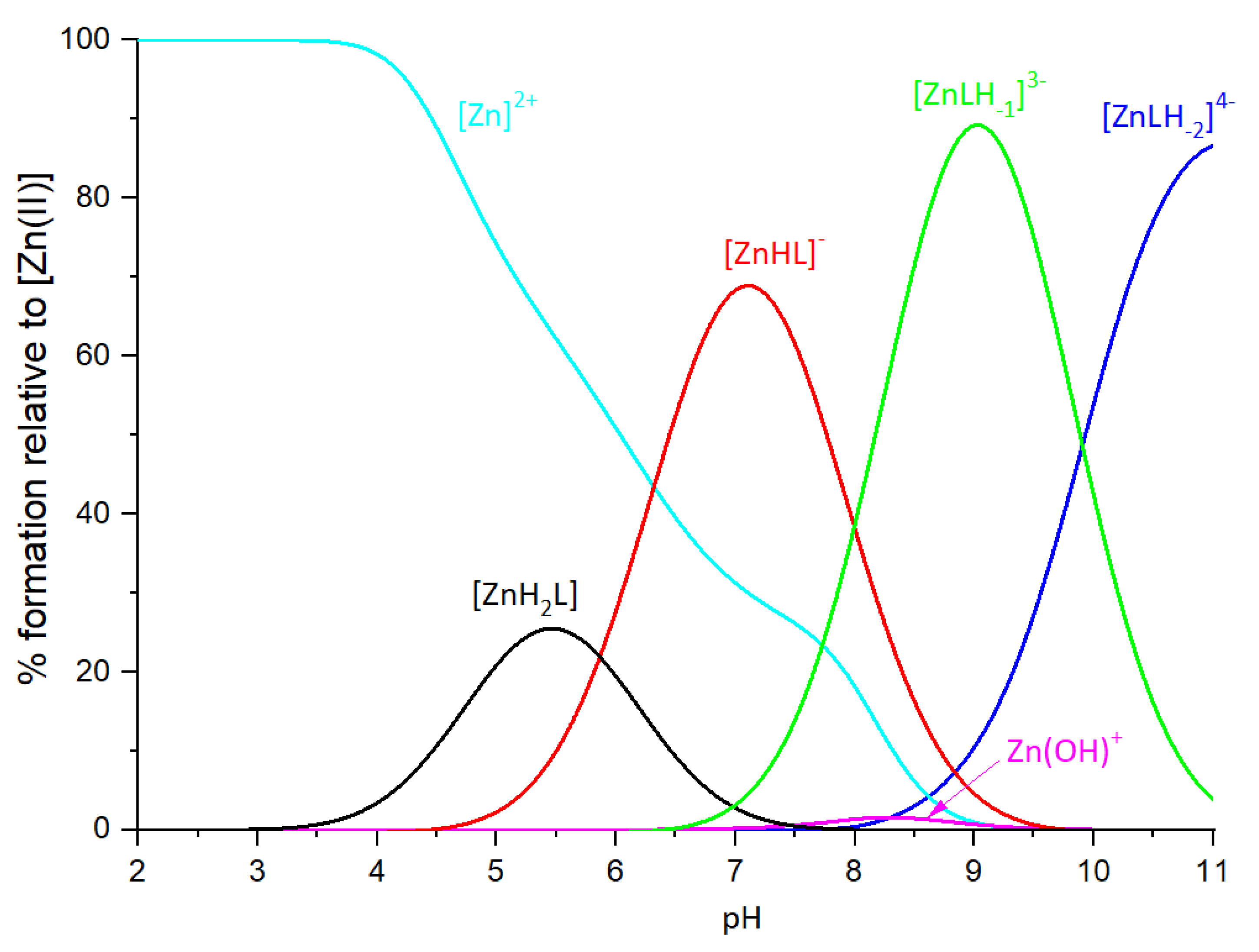
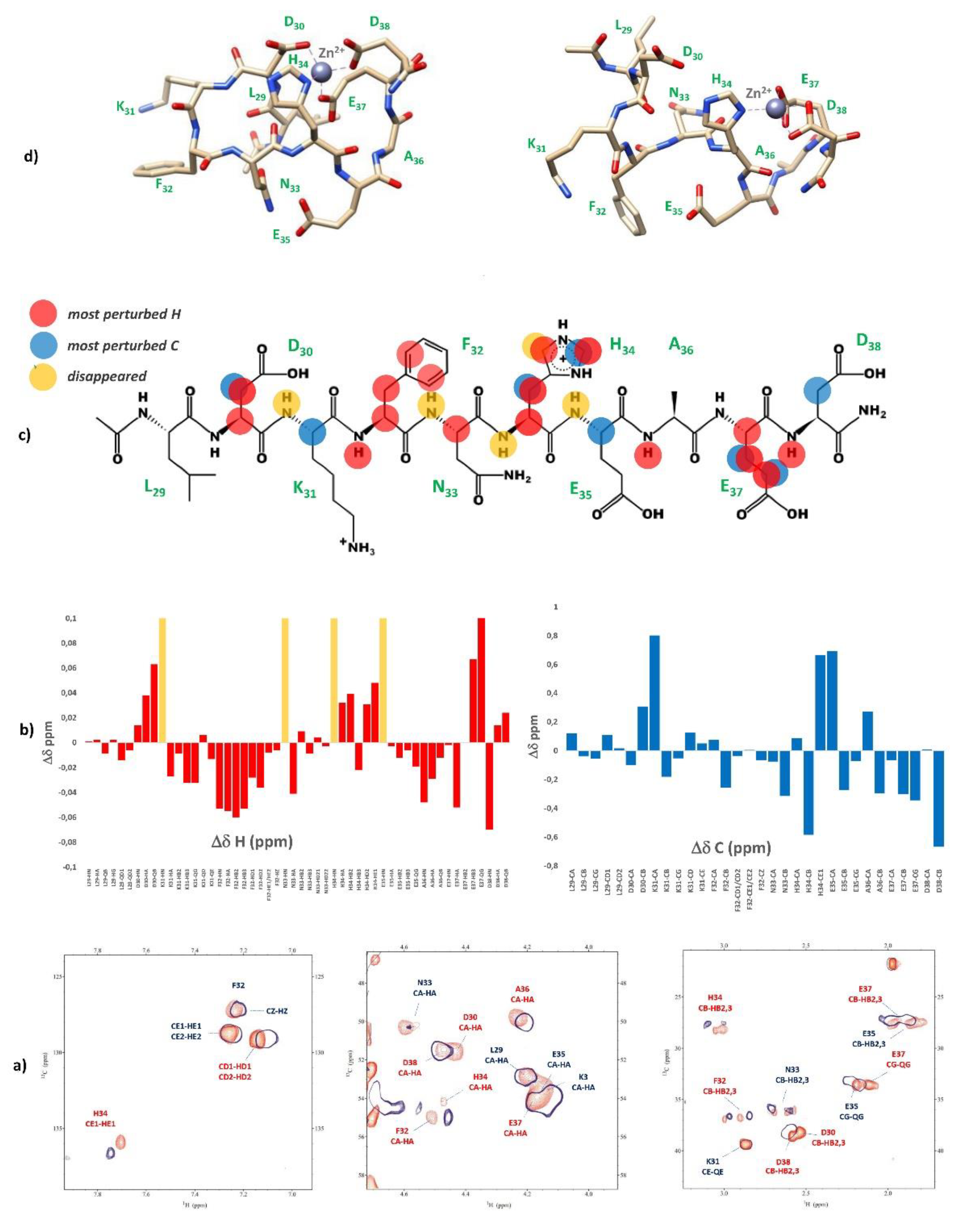
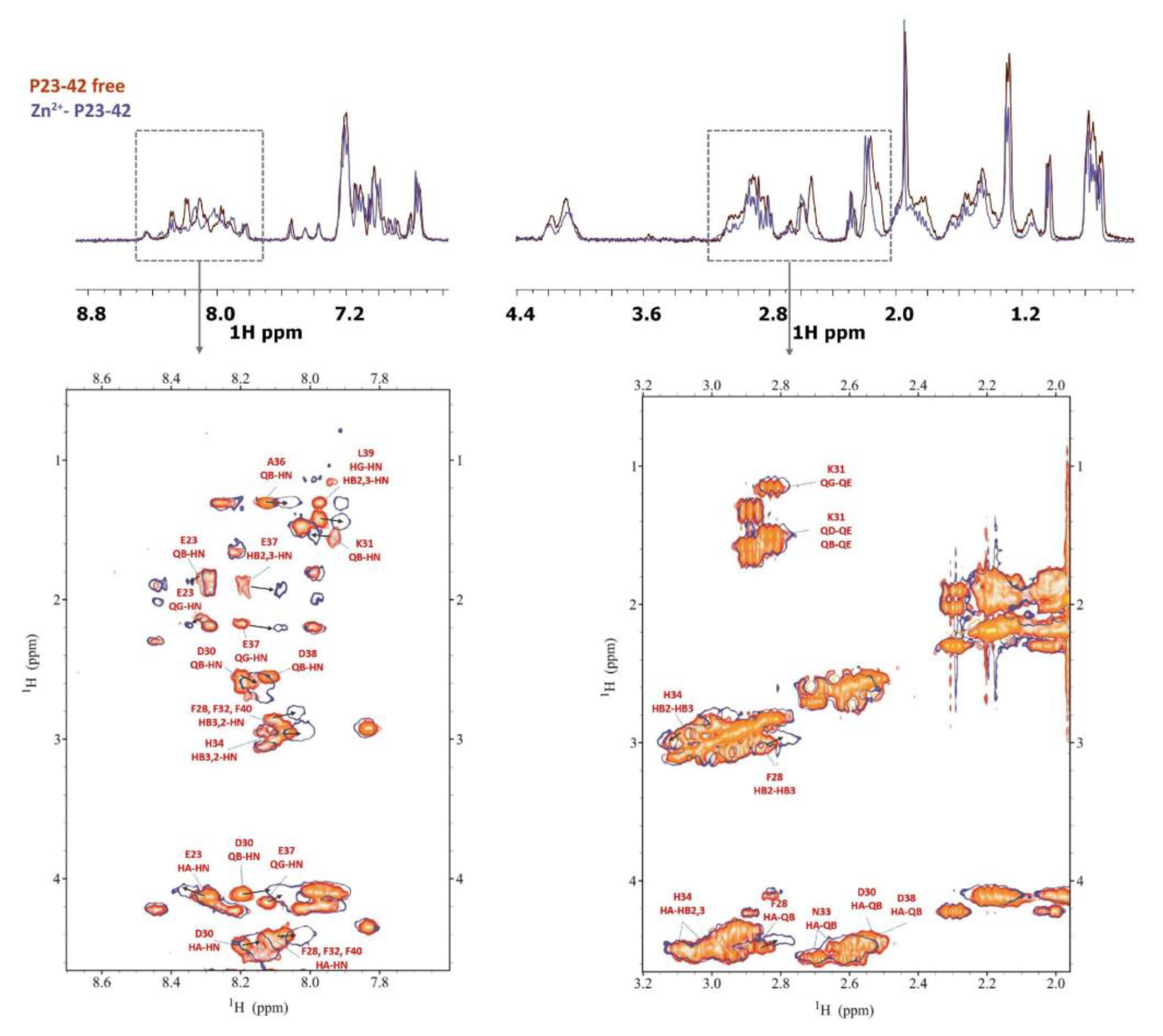
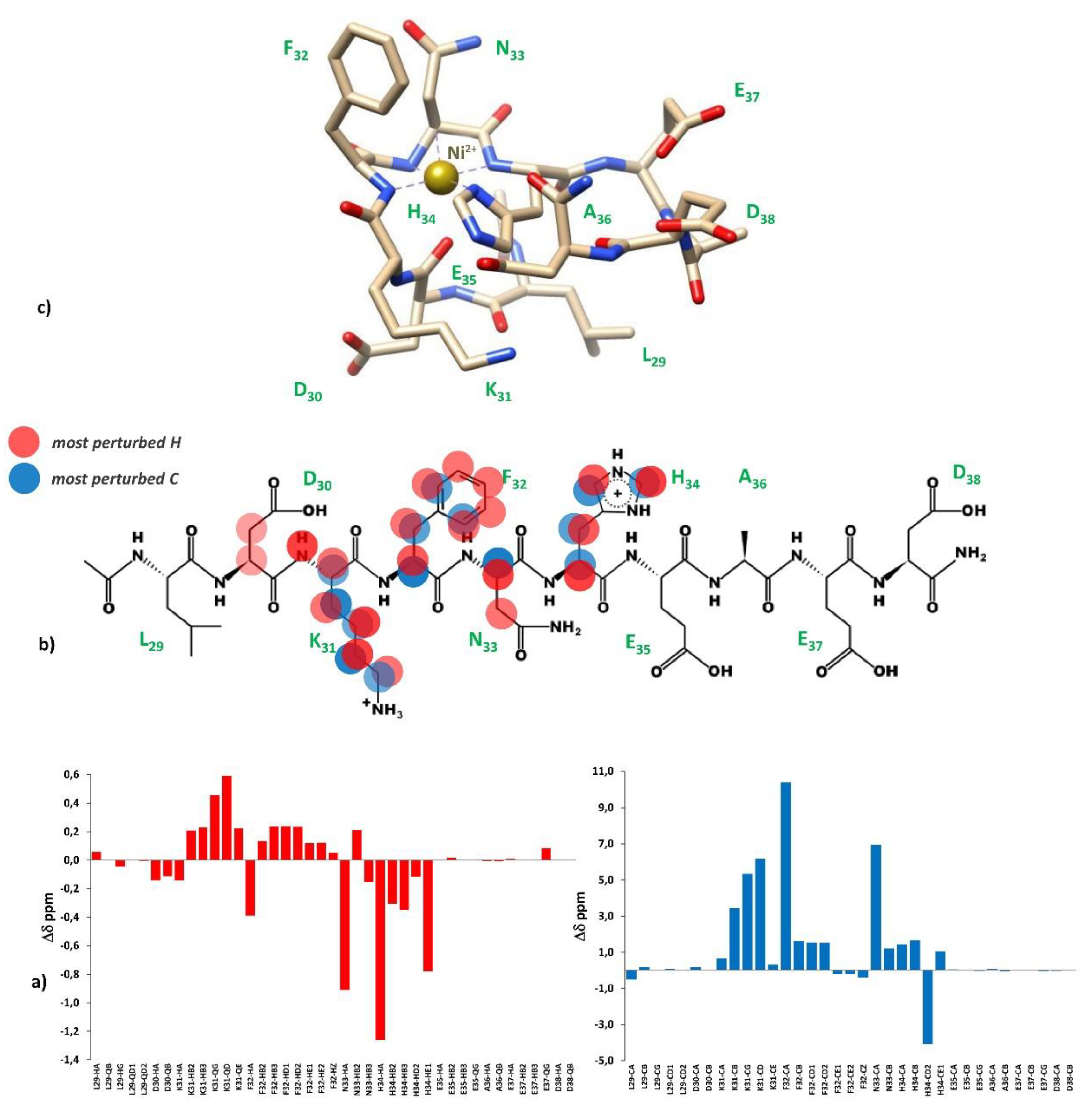
2.2. Zinc(II) complexes
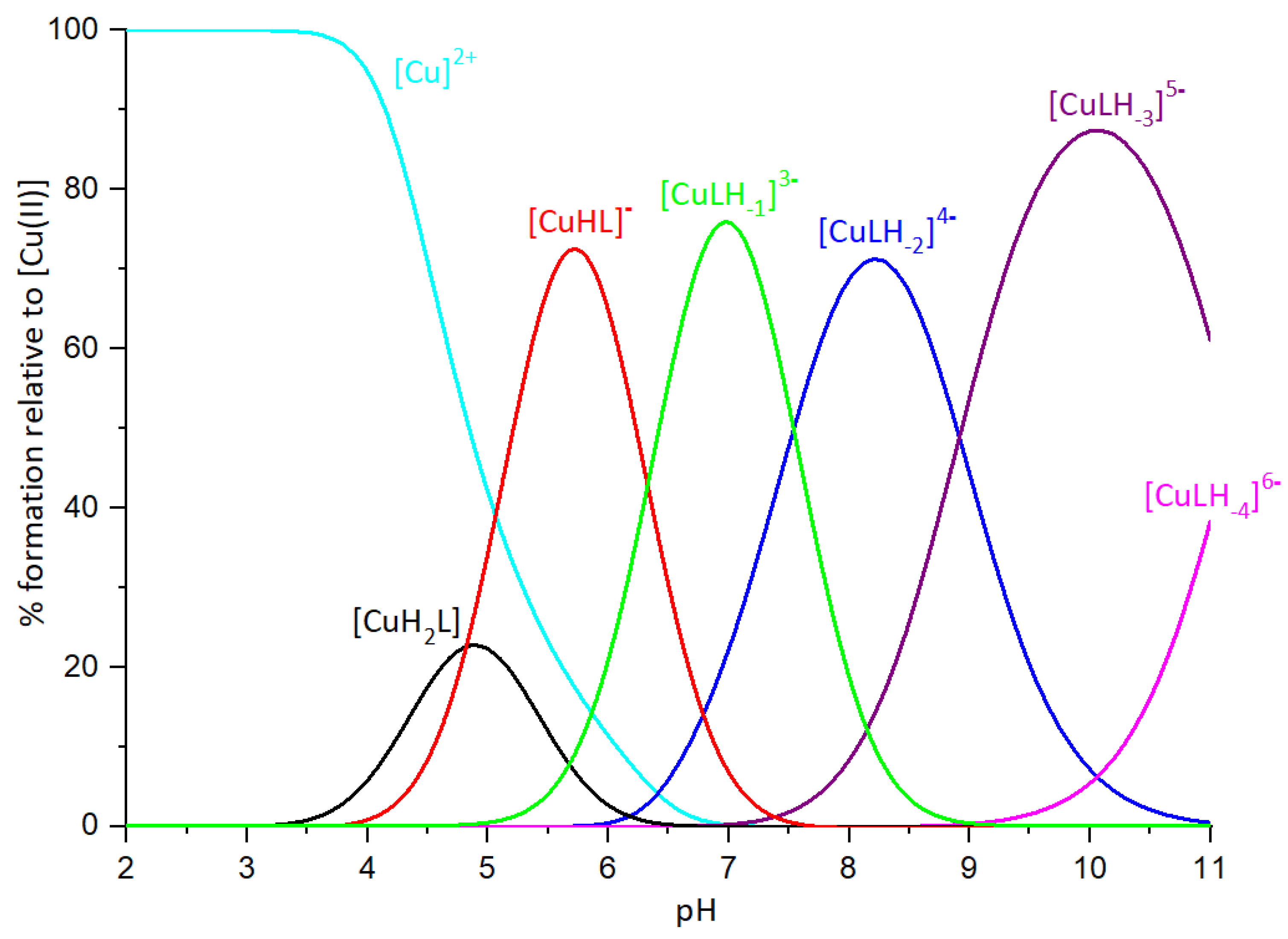
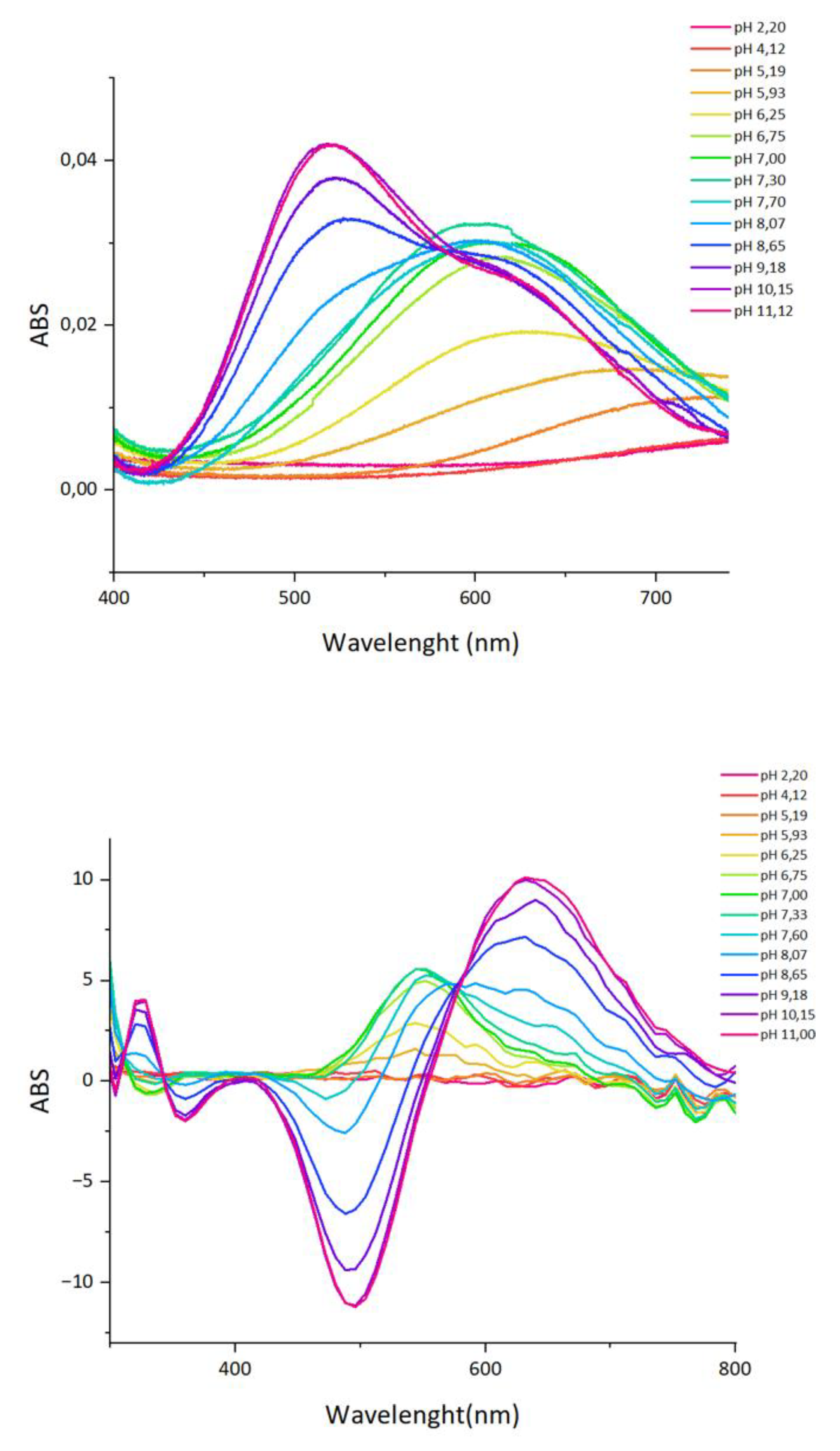
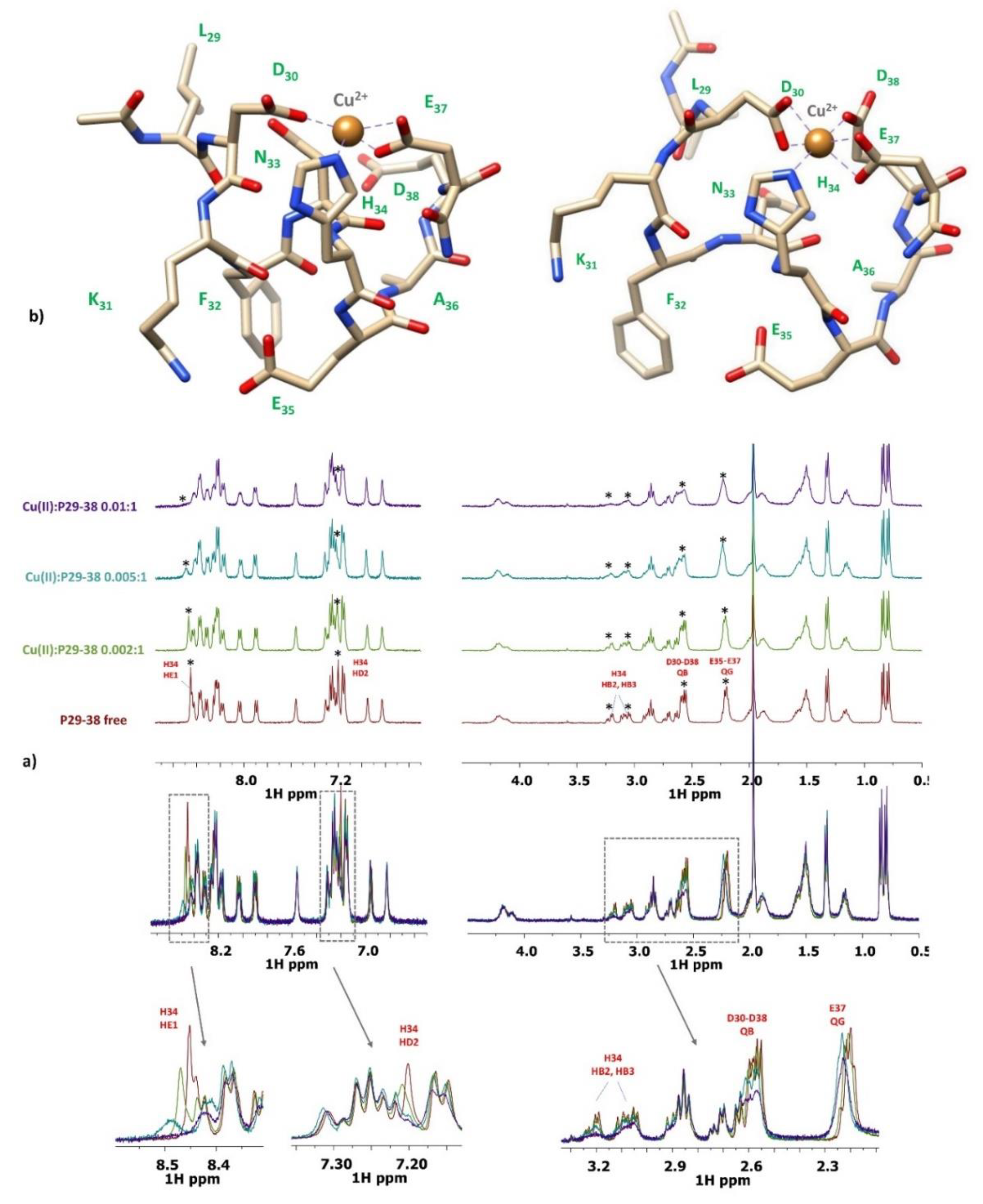
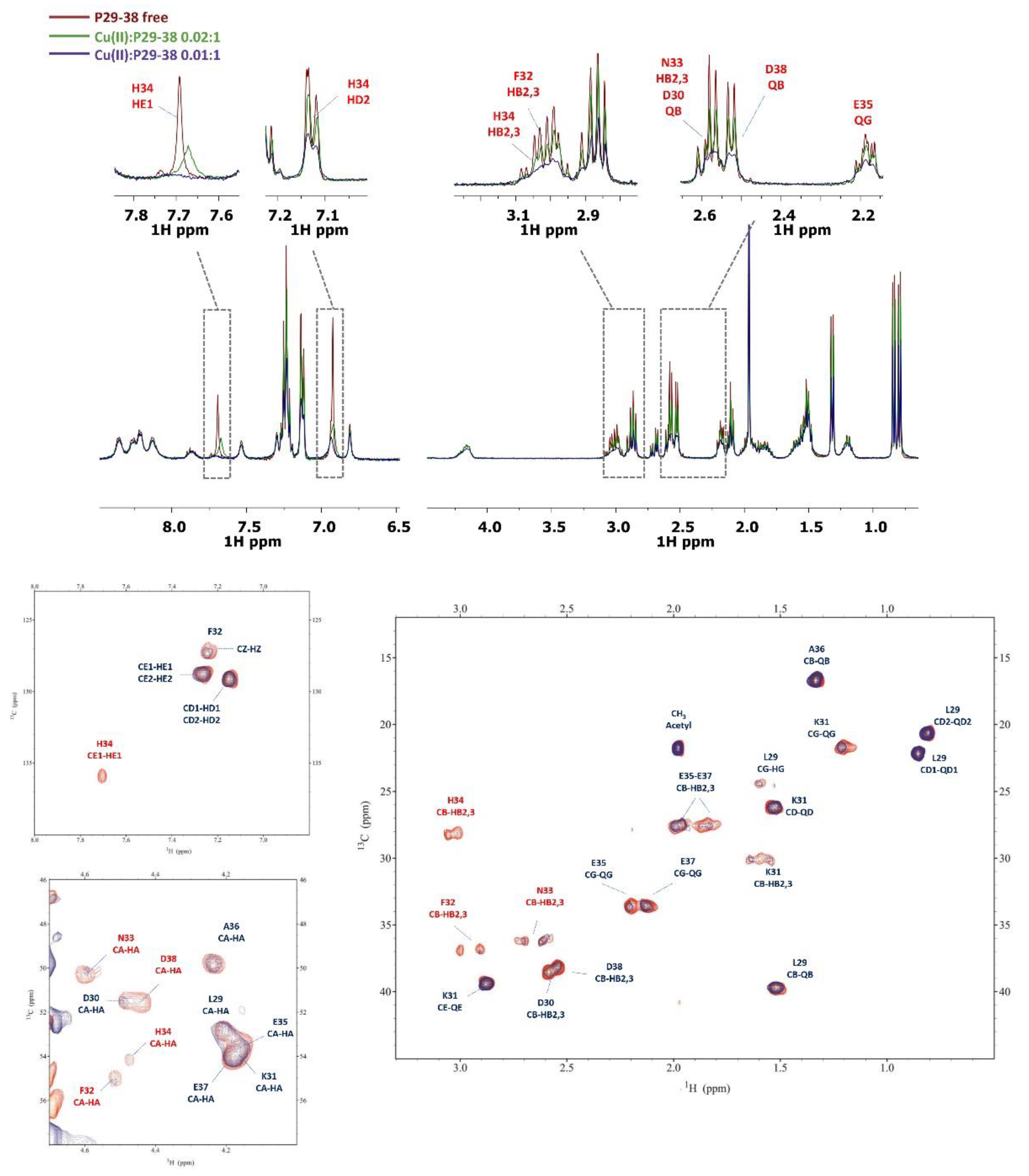
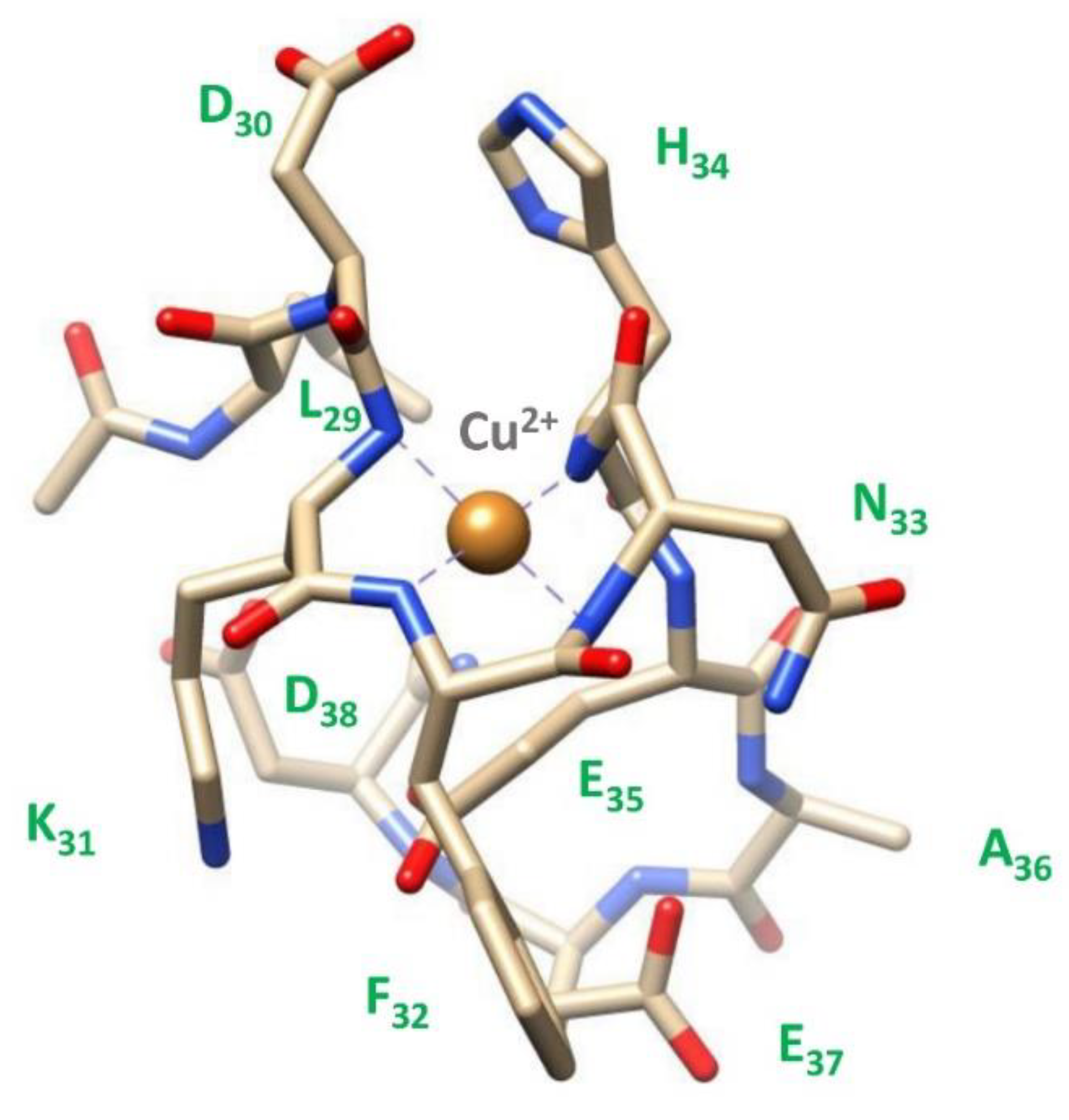
2.3. Copper(II) complexes
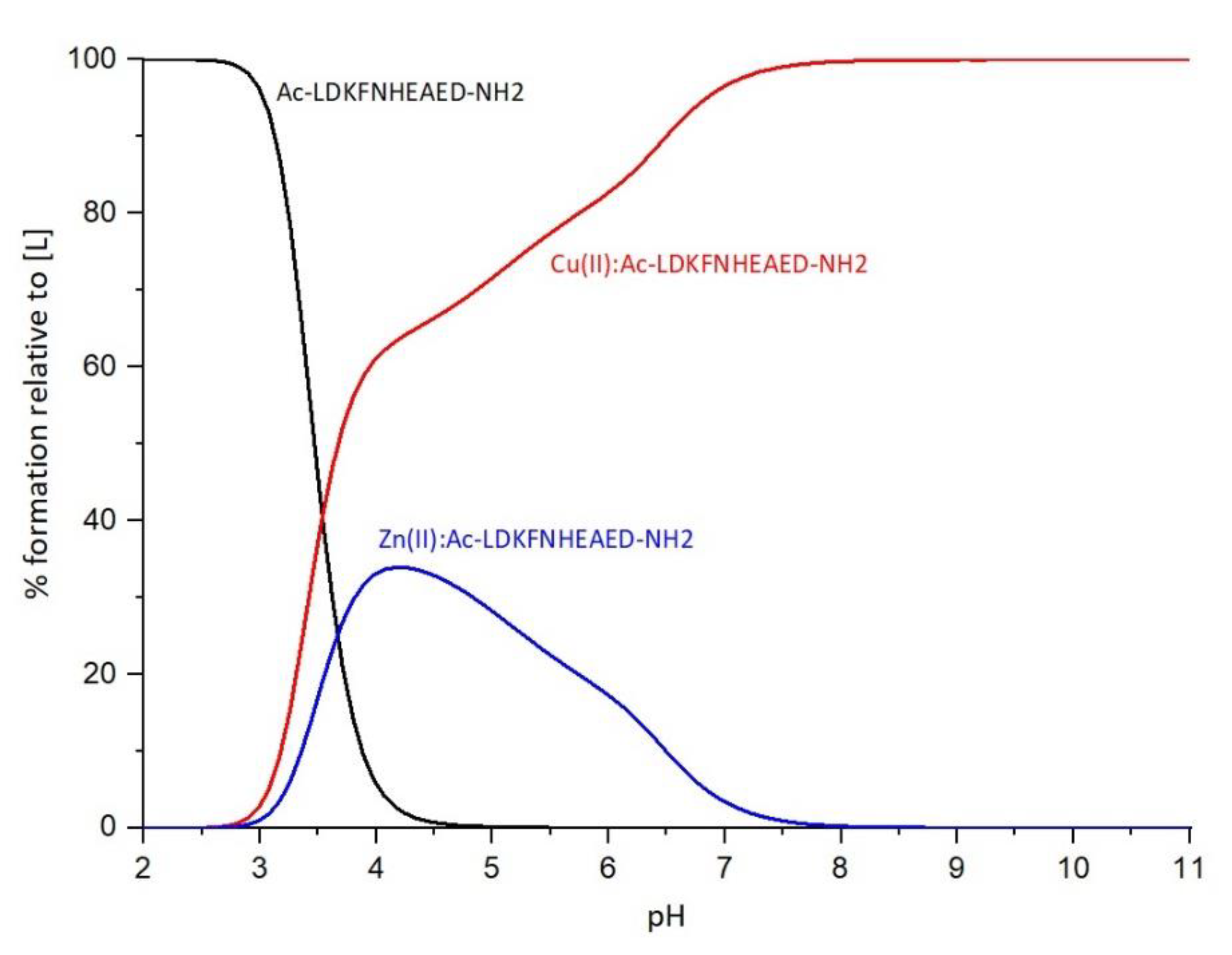
2.4. Competition diagrams
3. Materials and Methods
3.1. Peptide synthesis
3.2. Potentiometric measurements
3.3. UV-Vis and CD measurements
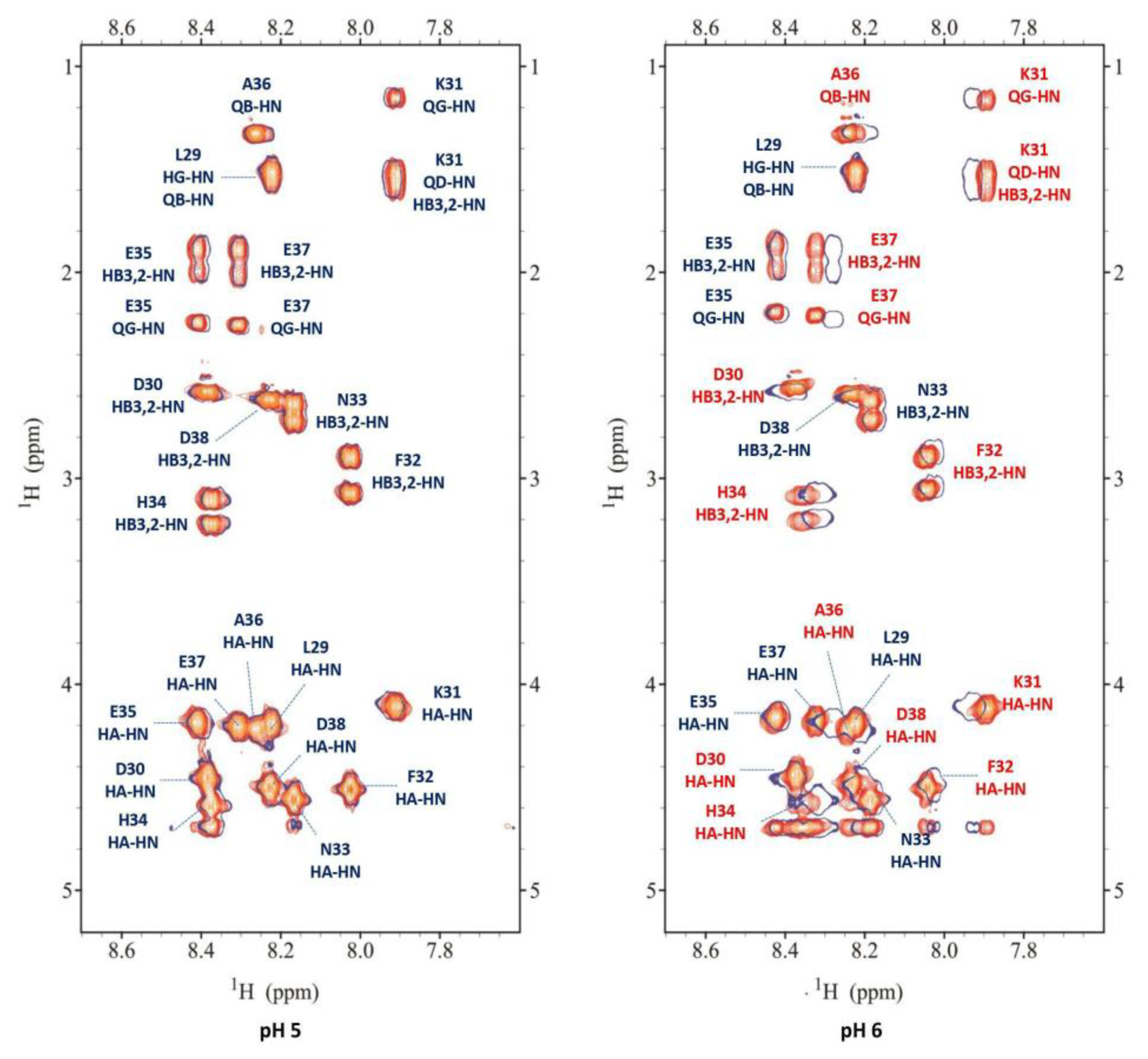
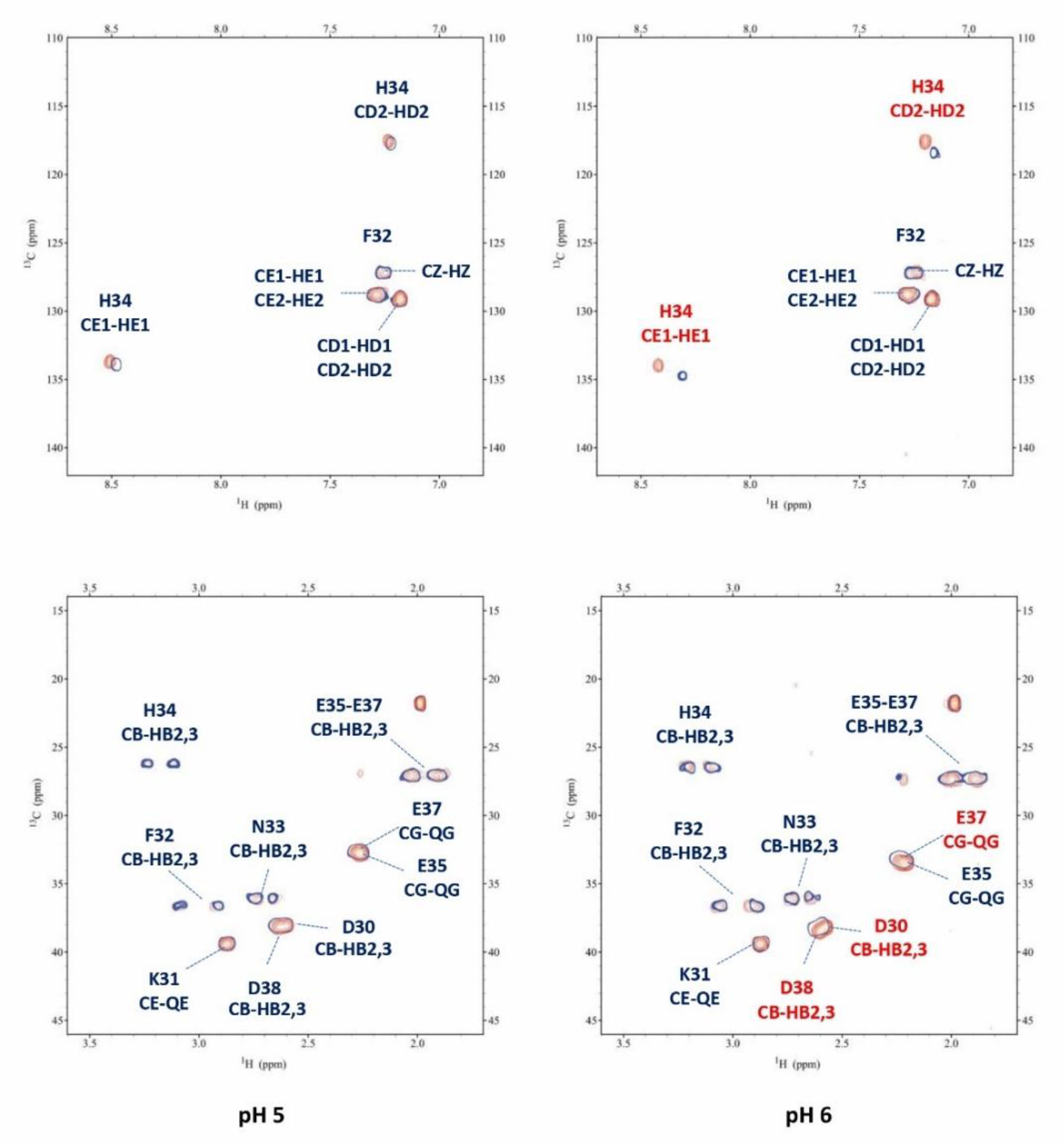
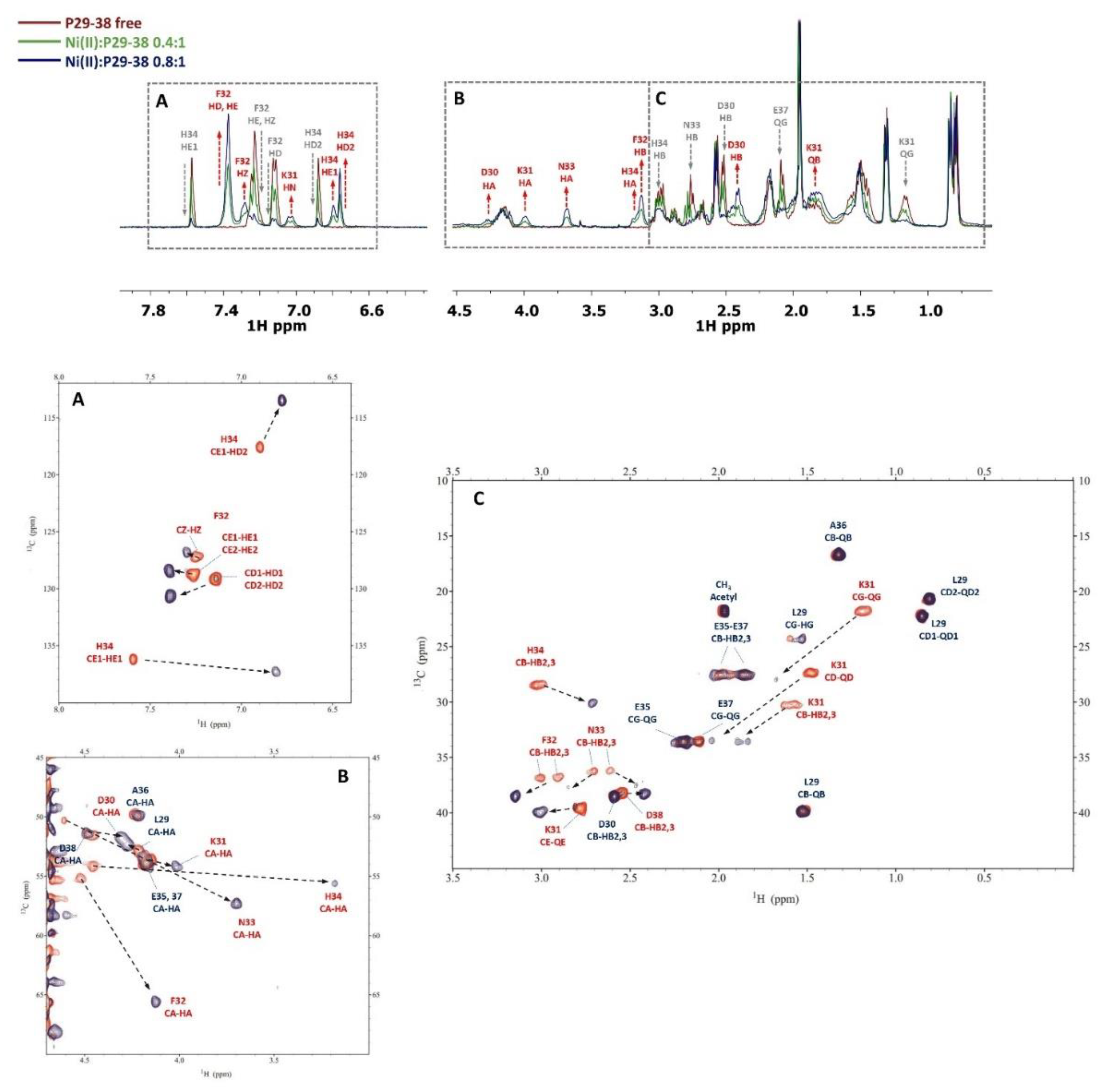
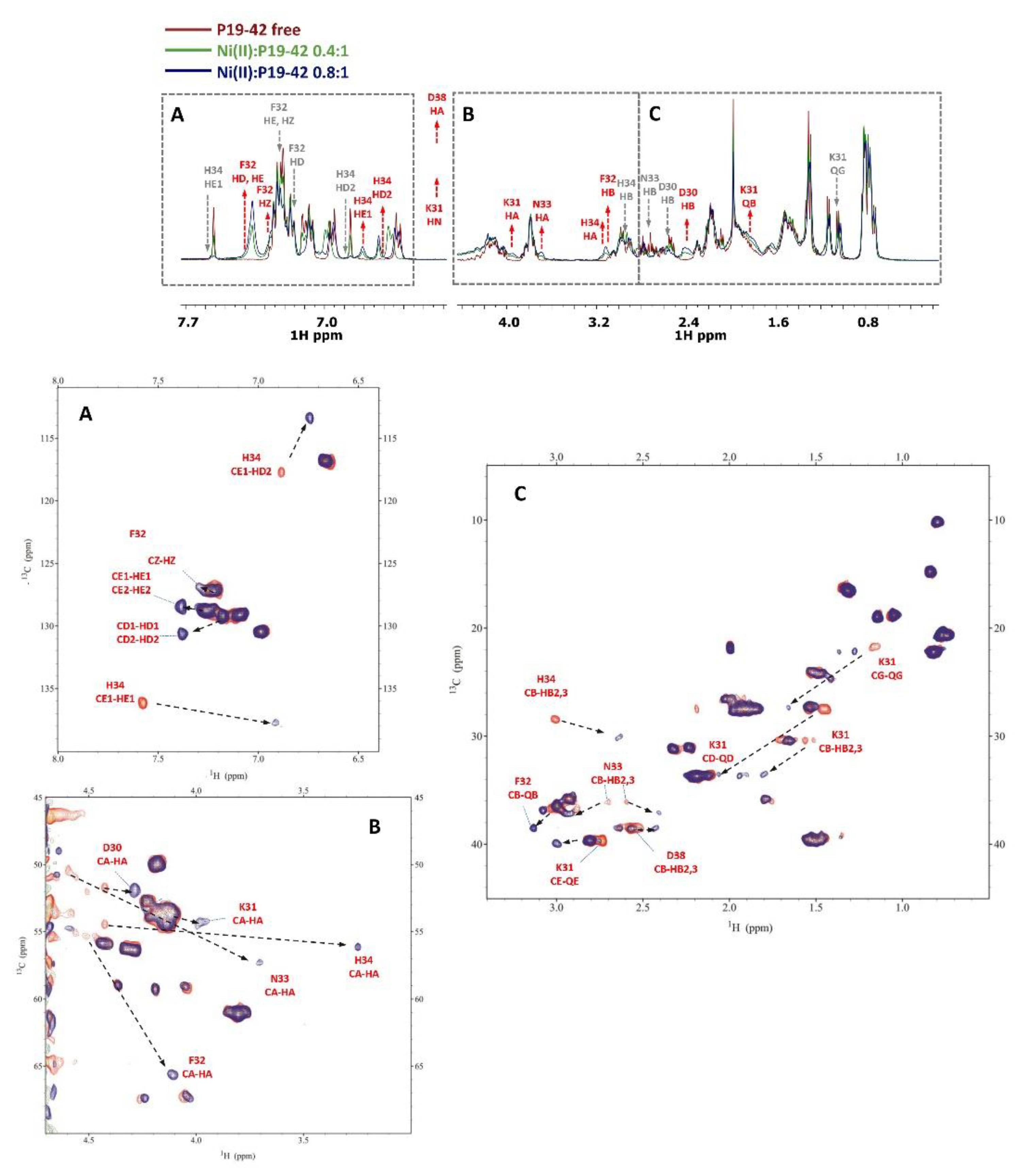
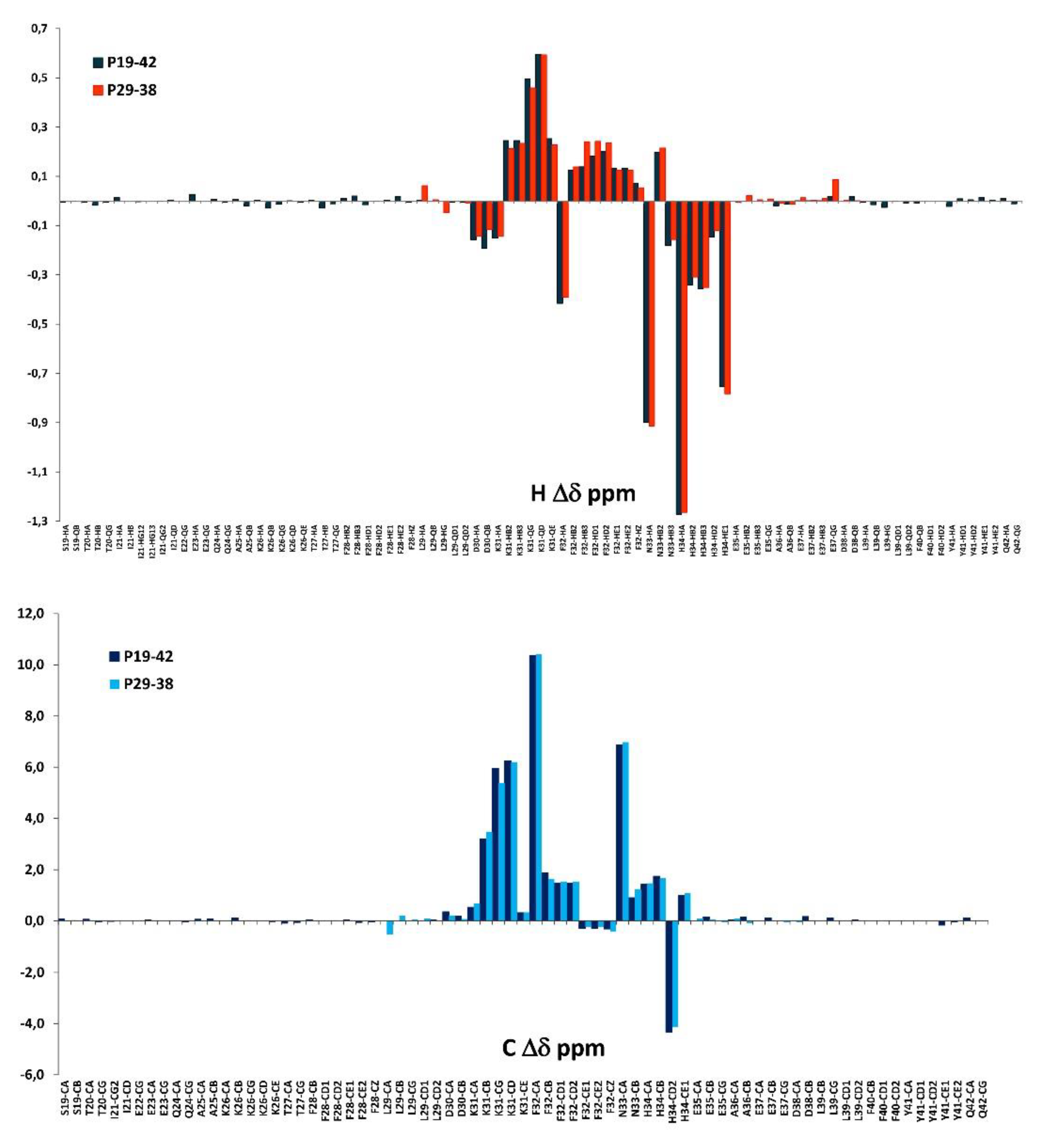
3.4. NMR measurements
3.5. Molecular dynamics measurements
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Cooper, M.E.; Haagmans, B.L.; Hooper, N.M.; Korstanje, R.; Osterhaus, A.D.; Timens, W.; Turner, A.J.; Navis, G.; van Goor, H. The emerging role of ACE2 in physiology and disease. J Pathol 2007, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Matera, M.G.; Cazzola, M.; Bianco, A. Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respir Med 2020, 168, 105996. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pohlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol Cell 2020, 78, 779–784 e775. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y.; et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun 2021, 12, 848. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int Rev Immunol 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
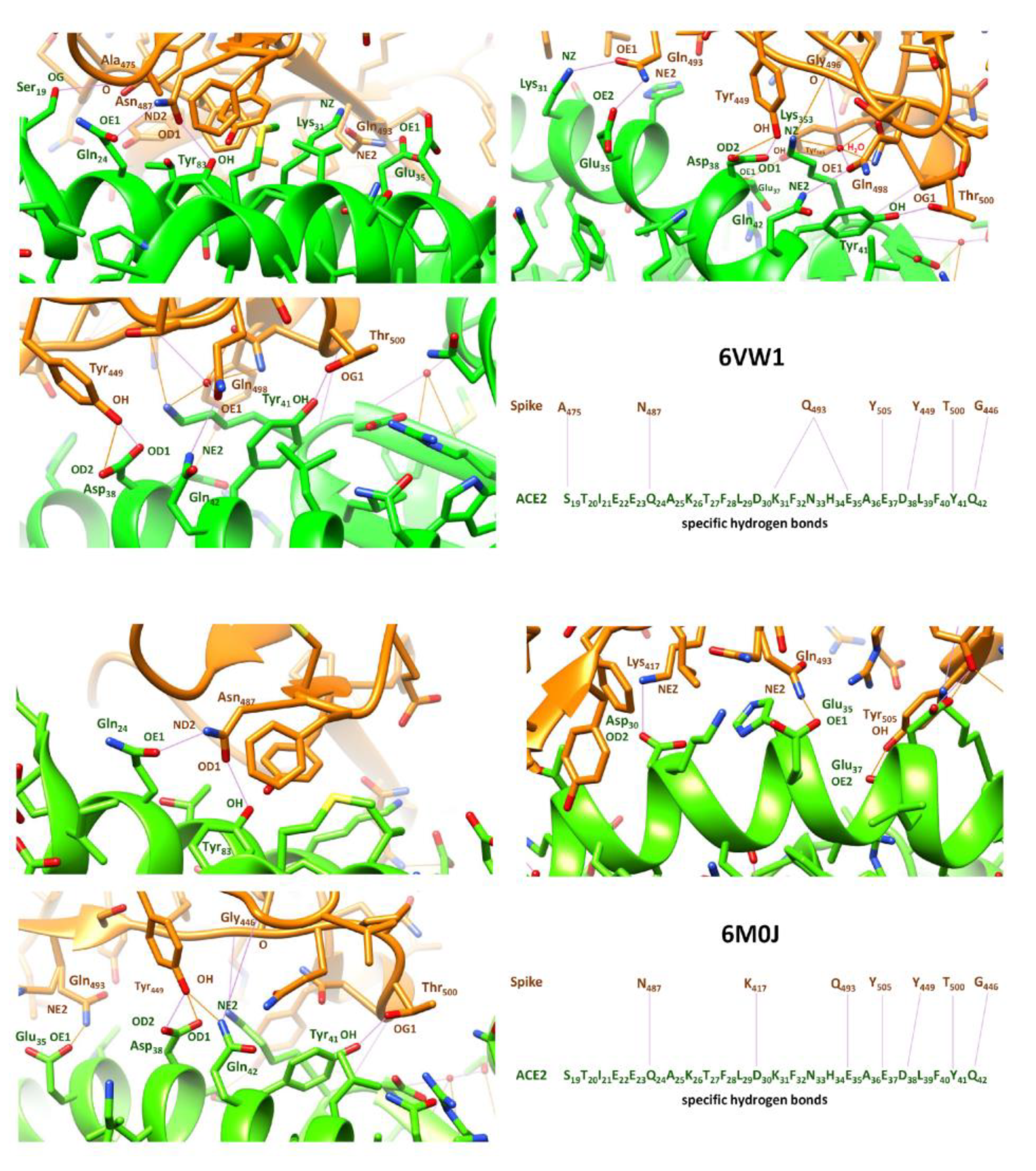
- Lim, H.; Baek, A.; Kim, J.; Kim, M.S.; Liu, J.; Nam, K.Y.; Yoon, J.; No, K.T. Hot spot profiles of SARS-CoV-2 and human ACE2 receptor protein protein interaction obtained by density functional tight binding fragment molecular orbital method. Sci Rep 2020, 10, 16862. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Sui, J.; Kuhn, J.H.; Moore, M.J.; Luo, S.; Wong, S.K.; Huang, I.C.; Xu, K.; Vasilieva, N.; et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 2005, 24, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Vijayan, R. Dynamics of the ACE2-SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms. Sci Rep 2020, 10, 14214. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol 2020, 11, 1712. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 2004, 279, 17996–18007. [Google Scholar] [CrossRef]
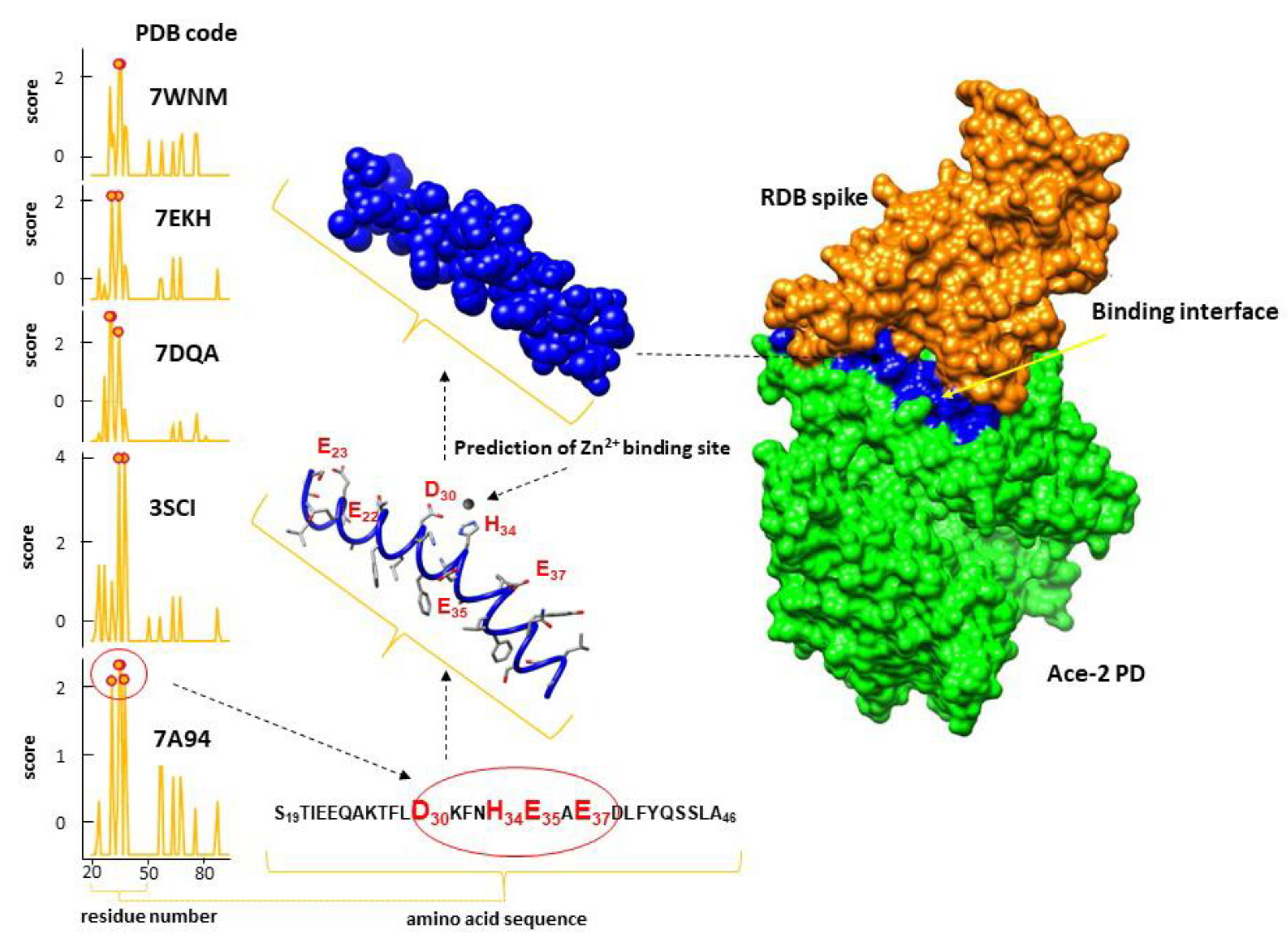
- Fatouros, P.R.; Roy, U.; Sur, S. Implications of SARS-CoV-2 spike protein interactions with Zn-bound form of ACE2: a computational structural study. Biometals 2023, 1–10. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, C.C.; Yu, C.S.; Liu, Y.Y.; Liu, J.J.; Wei, S.T.; Lin, Y.F. MIB2: metal ion-binding site prediction and modeling server. Bioinformatics 2022, 38, 4428–4429. [Google Scholar] [CrossRef]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Peng, G.; Wilken, M.; Geraghty, R.J.; Li, F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J Biol Chem 2012, 287, 8904–8911. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zheng, L.; Xu, J.; Wang, J.; Hu, C.; Lan, J.; Zhang, X.; Zhang, J.; Xu, K.; Cheng, H.; et al. Reduced graphene oxide membrane as supporting film for high-resolution cryo-EM. Biophysics Reports 2021, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Su, C.; Zhang, Y.; Bai, C.; Zheng, A.; Qiao, C.; Wang, Q.; Niu, S.; Chen, Q.; Zhang, Y.; et al. Molecular insights into receptor binding of recent emerging SARS-CoV-2 variants. Nat Commun 2021, 12, 6103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Wu, L.; Ma, R.; Han, P.; Huang, B.; Qiao, C.; Wang, Q.; Tan, W.; Gao, G.F.; Han, P. A binding-enhanced but enzymatic activity-eliminated human ACE2 efficiently neutralizes SARS-CoV-2 variants. Signal Transduct Target Ther 2022, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Gumienna-Kontecka, E.; Piras, F.; Ostrowska, M.; Piasta, K.; Krzywoszynska, K.; Medici, S.; Zoroddu, M.A. Exploring the Specificity of Rationally Designed Peptides Reconstituted from the Cell-Free Extract of Deinococcus radiodurans toward Mn(II) and Cu(II). Inorg Chem 2020, 59, 4661–4684. [Google Scholar] [CrossRef]
- Magri, A.; Tabbi, G.; Di Natale, G.; La Mendola, D.; Pietropaolo, A.; Zoroddu, M.A.; Peana, M.; Rizzarelli, E. Zinc Interactions with a Soluble Mutated Rat Amylin to Mimic Whole Human Amylin: An Experimental and Simulation Approach to Understand Stoichiometry, Speciation and Coordination of the Metal Complexes. Chemistry 2020, 26, 13072–13084. [Google Scholar] [CrossRef]
- Gampp, H.; Maeder, M.; Meyer, C.J.; Zuberbuhler, A.D. Calculation of equilibrium constants from multiwavelength spectroscopic data--II: SPECFIT: two user-friendly programs in basic and standard FORTRAN 77. Talanta 1985, 32, 257–264. [Google Scholar] [CrossRef]
- Prenesti, E.; Daniele, P.G.; Prencipe, M.; Ostacoli, G. Spectrum–structure correlation for visible absorption spectra of copper(II) complexes in aqueous solution. Polyhedron 1999, 18, 3233–3241. [Google Scholar] [CrossRef]
- Lesiow, M.K.; Pietrzyk, P.; Bienko, A.; Kowalik-Jankowska, T. Stability of Cu(ii) complexes with FomA protein fragments containing two His residues in the peptide chain. Metallomics 2019, 11, 1518–1531. [Google Scholar] [CrossRef]
- Magri, A.; Munzone, A.; Peana, M.; Medici, S.; Zoroddu, M.A.; Hansson, O.; Satriano, C.; Rizzarelli, E.; La Mendola, D. Coordination Environment of Cu(II) Ions Bound to N-Terminal Peptide Fragments of Angiogenin Protein. Int J Mol Sci 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Kowalik-Jankowska, T.; Medici, S.; Peana, M.; Kozlowski, H. Copper(II) binding to Cap43 protein fragments. Dalton Trans 2008, 6127–6134. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Kowalik-Jankowska, T.; Kozlowski, H.; Molinari, H.; Salnikow, K.; Broday, L.; Costa, M. Interaction of Ni(II) and Cu(II) with a metal binding sequence of histone H4: AKRHRK, a model of the H4 tail. Biochim Biophys Acta 2000, 1475, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Kadej, A.; Kuczer, M.; Czarniewska, E. Copper(II) complexes of the Neb-colloostatin analogues containing histidine residue structure stability biological activity. Polyhedron 2017, 134, 365–375. [Google Scholar] [CrossRef]
- Rowinska-Zyrek, M.; Wiech, A.; Wa Tly, J.; Wieczorek, R.; Witkowska, D.; Ozyhar, A.; Orlowski, M. Copper(II)-Binding Induces a Unique Polyproline Type II Helical Structure within the Ion-Binding Segment in the Intrinsically Disordered F-Domain of Ecdysteroid Receptor from Aedes aegypti. Inorg Chem 2019, 58, 11782–11792. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. The involvement of amino acid side chains in shielding the nickel coordination site: an NMR study. Molecules 2013, 18, 12396–12414. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Zdyb, K.; Medici, S.; Pelucelli, A.; Simula, G.; Gumienna-Kontecka, E.; Zoroddu, M.A. Ni(II) interaction with a peptide model of the human TLR4 ectodomain. J Trace Elem Med Biol 2017, 44, 151–160. [Google Scholar] [CrossRef]
- Jones, C.E.; Klewpatinond, M.; Abdelraheim, S.R.; Brown, D.R.; Viles, J.H. Probing copper2+ binding to the prion protein using diamagnetic nickel2+ and 1H NMR: the unstructured N terminus facilitates the coordination of six copper2+ ions at physiological concentrations. J Mol Biol 2005, 346, 1393–1407. [Google Scholar] [CrossRef]
- Peana, M.F.; Medici, S.; Ledda, A.; Nurchi, V.M.; Zoroddu, M.A. Interaction of Cu(II) and Ni(II) with Ypk9 protein fragment via NMR studies. ScientificWorldJournal 2014, 2014, 656201. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. Journal of the Chemical Society (Resumed) 1953, 3192–3210. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O'Halloran, T.V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Gran, G.; Dahlenborg, H.; Laurell, S.; Rottenberg, M. Determination of the equivalent point in potentiometric titrations. Acta chem. scand 1950, 4, 559–577. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. Superquad - a new computer program for determination of stability constants of complexes by potentiometric titration. Inorganica Chimica Acta 1983, 79, 219–220. [Google Scholar] [CrossRef]
- Brown, P.L.; Ekberg, C. Hydrolysis of metal ions. John Wiley & Sons, 2016. [Google Scholar]
- Mesmer, C.F.B.a.R.S. The Hydrolysis of Cations. John Wiley & Sons, New York, London, Sydney, Toronto 1976. 489 Seiten. Berichte der Bunsengesellschaft für physikalische Chemie 1977, 81, 245–246. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coordination Chemistry Reviews 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H.S.; Whewell, R.J. The use of electronic computing techniques in the calculation of stability constants. Journal of Inorganic and Nuclear Chemistry 1971, 33, 2051–2065. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Slusarenko, A.J.; Rink, L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. British Journal of Nutrition 2022, 127, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Wang, K.; Viveiros, A.; Kellner, M.J.; Penninger, J.M. Angiotensin-converting enzyme 2-at the heart of the COVID-19 pandemic. Cell 2023, 186, 906–922. [Google Scholar] [CrossRef] [PubMed]
| Ligand Species | Logβ | logK | Residue |
|---|---|---|---|
| [HL]3- | 10.21 (1) | 10.21 | Lys |
| [H2L]2- | 17.05 (2) | 6.84 | His |
| [H3L]- | 21.98 (2) | 4.93 | Glu |
| [H4L] | 26.36 (2) | 4.38 | Glu |
| [H5L]+ | 30.19 (2) | 3.83 | Asp |
| [H6L]2+ | 33.34 (2) | 3.15 | Asp |
| Species | logβ | logK | Deprotonation | Coordination |
|---|---|---|---|---|
| [ZnH2L] | 20.34 (8) | Asp, Asp, Glu, (Glu) | ||
| [ZnHL]- | 14.55 (6) | 5.79 | His | (Asp), Asp, Glu, His |
| [ZnLH-1]3- | -1.50 (5) | 8.025*2 | 2H2O | (Asp), Asp, Glu, His |
| [ZnLH-2]4- | -11.42 (6) | 9.92 | Lys | (Asp), Asp, Glu, His |
| Species | logβ | logK | Deprotonation | Coordination |
|---|---|---|---|---|
| [CuH2L] | 20.78(5) | Asp, Asp, Glu, Glu | ||
| [CuHL]- | 16.01(3) | 4.77 | His | Asp, Asp, (Glu), His |
| [CuLH-1]3- | 3.35(3) | 2*6.33 | amide, amide | His, 2Namide, (Asp or Glu) |
| [CuLH-2]4- | -4.17(4) | 7.52 | amide | His, 3N-amide |
| [CuLH-3]5- | -13.09(5) | 8.92 | amide | 4N-amide |
| [CuLH-4]6- | -24.29(5) | 11.20 | Lys | 4N-amide |
| Complex form | Coordination | λ [nm] | ε [M−1·cm−1] |
|---|---|---|---|
| [CuHL]- | 1N {NIm} | 720 | 50 |
| [CuLH-1]3- | 3N {NIm, 2N-} | 622 | 52 |
| [CuLH-2]4- | 4N {NIm, 3N-} | 608 | 94 |
| [CuLH-3]5- | 4N {4N-} | 520 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





