1. Introduction
The SARS-CoV-2 virus is highly infectious, and it was the causative agent of the outbreak of the COVID disease in 2019. The WHO declared it a global pandemic [
1]. More than 676 million cases have been reported worldwide, with over 6.88 million deaths since late 2019 [
2]. In the current situation, COVID-19 continues to endanger human welfare and impose unquestionable costs on healthcare. A reasonable strategy for lowering mortality brought on by viral lung penetration is to neutralize SARS-CoV-2 before it enters human cells [
3]. It has been accepted that vaccination-induced immunity is essential for controlling the spread of COVID-19. Because many recently discovered variations are immune-evading [
4], novel treatments that prevent viral cell entrance are required as a supplementary option.
The cryo-electron microscopy structure of the SARS-CoV-2 spike trimer has just been published in two separate investigations [
5,
6]. However, a closer look at one of the available spike structures indicated that the RBD was only partially modelled, specifically for the receptor-binding motif (RBM) that directly interacts with ACE2. The interaction with the N -terminal peptidase domain of ACE2 is specifically facilitated by the Betacoronavirus spike (S) glycoprotein S1 subunit C-terminal (BCOV_S1_CTD) [
7]. Therefore, sequence and structural analysis of BCOV_S1_CTD in the major variations was carried out for more information. In the site analysis of the sequence alignment, 89.11% conserved sites and 10.88% variable sites were identified. Based on this investigation, nearly 10.88% of sites played an important role in amino acid substitution. The spike glycoprotein (S protein) structure is likely to change as a result of these mutations [
8]. Receptor binding domain (RBD) was a primary target of efficient neutralizing antibodies, according to earlier investigations on SARS-CoV. But it’s necessary to consider whether these alterations impact the antigenicity of S proteins and their capacity to bind neutralizing antibodies. Suppose the S protein’s B-cell epitopes altered and could no longer bind to the neutralizing antibodies [
9]. As a result, the developed vaccines, based on prototype S protein, lose their effectiveness. Here, we share the immuno-bioinformatic resources from the IEDB and related resources that were used to predict the B cell epitopes in the BCOV_S1_CTD of S protein from the major variants and compare the changes in the likely epitope sites from dominant and extremely rare mutations of S protein. The Omicron variant and its sub-variant have been seen to have multiple mutations simultaneously [
10]. We observed that different S proteins’ BCOV_S1_CTD mutations might have varying effects on the proteins’ putative functional epitopes. Access to affordable and reliable vaccinations is now a significant problem with vaccine usage. The theory underlying the design of the three primary vaccine types (protein subunit, adenoviral vector, and mRNA) and the effectiveness of vaccinations against various SARS-CoV-2 variants are discussed in the sections.
Furthermore, the most optimistic possibility of stopping the pandemic is a successful rollout of COVID-19 immunizations. SARS-CoV-2 continues to evolve, posing a bigger risk to public health globally due to its quicker transmission and increased infectivity efficiency. The WHO categorizes them into variations of interest (VOI) and variants under monitoring (VUMs) to more accurately analyze the effects of various variants and promote preventative or medicinal countermeasures.
2. The SARS-CoV-2 Structure
The SARS-CoV-2 virus, a member of the b-CoV class of human coronaviruses, is an enclosed virus with a diameter ranging from 80 to 220 nm with positive single-stranded RNA within its shell [
11]. Four structural proteins, delicate lipid envelopes, and genomic RNA comprise the complete SARS-CoV-2 particle. The membrane protein (M), nucleocapsid protein (N), spike protein (S) and envelop protein (E) are the four structural proteins. In the replication process of viruses, the M protein is essential. Its presence makes viruses and host components possible to assemble on the cell membrane to create progeny viral particles [
12]. In viral transcription and assembly, the complex produced by the N protein and genomic RNA is crucial. The N-terminal, C-terminal, and disordered central regions of the N protein are known as the NTD, CTD, and RNA binding domains [
13].
Table 1 displays the coronavirus spike glycoprotein domains profile from SPIKE_SARS2. The SARS-CoV-2 E protein is a very small, fully functional membrane protein that participates in various viral life cycle processes, including pathogenicity and assembly. For SARS-CoV-2 to enter cells, the S protein, which is present as trimers on the viral membrane surface, is crucial [
14]. It comprises the S2 subunit, the spike protein’s most conserved structural component [
14]. On the surface of the viral membrane, the E protein and the M protein are sequentially organized. The N protein interacts with the viral RNA to create the virus particle’s core, while the S protein builds the virus particle.
Table 1.
The coronavirus spike glycoprotein domain profile from SPIKE_SARS2 (UniProt ID: P0DTC2 ).
Table 1.
The coronavirus spike glycoprotein domain profile from SPIKE_SARS2 (UniProt ID: P0DTC2 ).
| S.No. |
ScanProsite ID |
Name |
Start position |
End position |
Details |
| 1. |
PS51922 |
BCOV_S1_NTD |
9 |
303 |
Betacoronavirus spike (S) glycoprotein S1 subunit N-terminal (N.T.D.) domain |
| 2. |
PS51921 |
BCOV_S1_CTD |
334 |
527 |
Betacoronavirus spike (S) glycoprotein S1 subunit C-terminal (CTD) domain |
| 3. |
PS51923 |
COV_S2_HR1 |
896 |
1001 |
Coronavirus spike (S) glycoprotein S2 subunit heptad repeat 1 (HR1) region |
| 4. |
PS51924 |
COV_S2_HR2 |
1143 |
1225 |
Coronavirus spike (S) glycoprotein S2 subunit heptad repeat 2 (HR2) region |
2.1. SARS-CoV-2 S Protein Attached to ACE2
The homotrimeric spike glycoprotein on the envelope of coronaviruses, which consists of an S1 subunit and an S2 subunit in each spike monomer, binds to the cellular receptors. Such binding sets off a series of processes that result in viral and cell membranes fusing for cell penetration. The SARS-CoV spike protein’s interaction with the cell receptor ACE2 has previously been studied using cryo-electron microscopy. These studies have revealed that receptor binding causes the S1 and ACE2 to dissociate, which causes the S2 to transition from the metastable pre-fusion state to an even more stable post-fusion state necessary for membrane fusion [
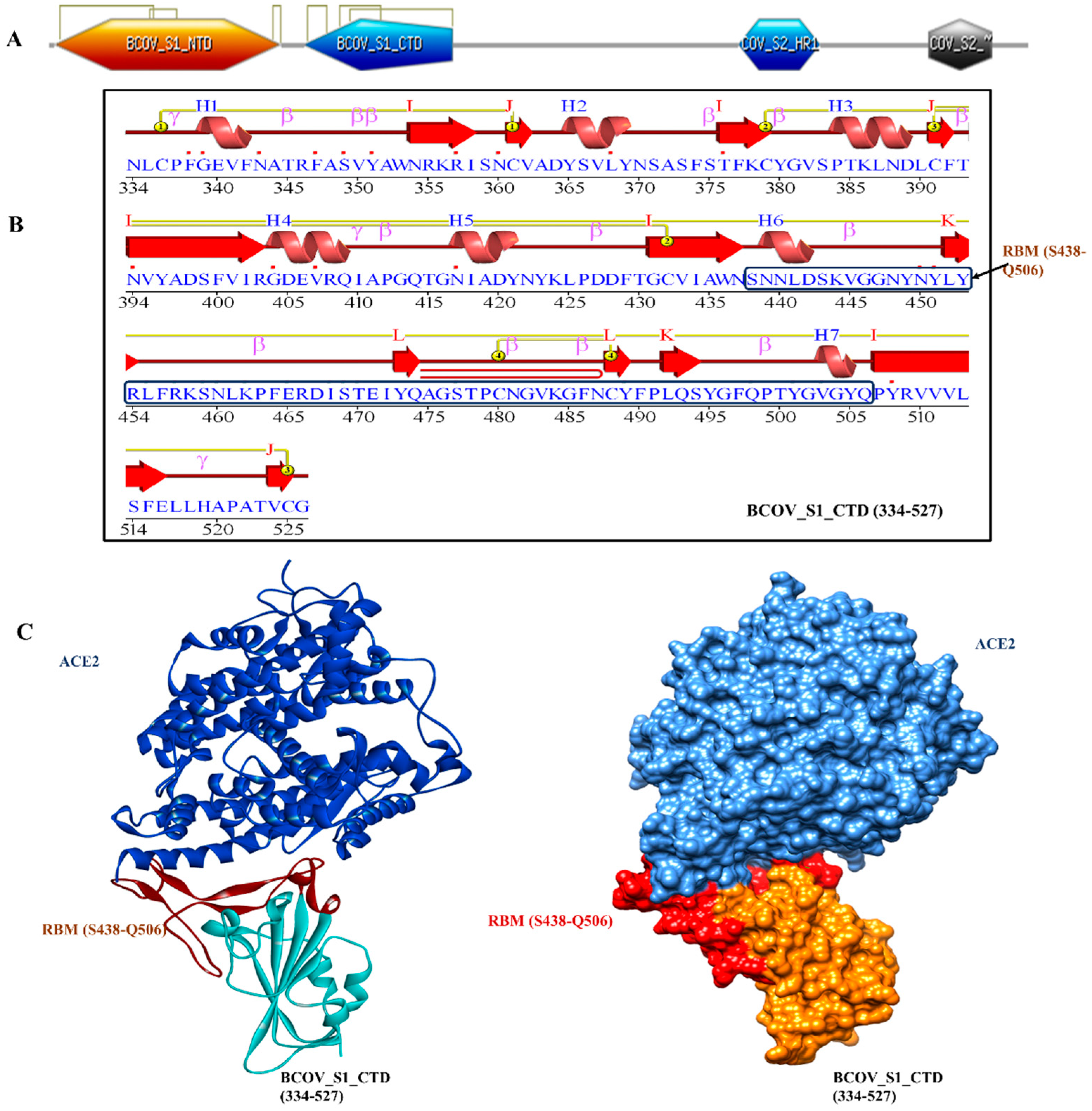
15]. In order to infect target cells, SARS-CoV has to first bind to the ACE2 receptor, which is a crucial first step. The X-ray crystallography was used to establish the structure of the BCOV_S1_CTD -ACE2 complex in order to better comprehend the interaction between the BCOV_S1_CTD and ACE2. Particularly, we observed an interaction between the N-terminal peptidase domain of ACE2 (residues Ser19-Asp615) and the C-terminal portion of the Betacoronavirus spike (S) glycoprotein S1 subunit (BCOV_S1_CTD) (residues 334-527) (
Figure 1A–C). The peptide substrate binding region is formed by the interaction of two lobes in the N-terminal peptidase domain of ACE2. The bottom side of the minor lobe of ACE2 is in contact with the expanded RBM in the BCOV_S1_CTD, which has a concave outer surface (residues S438–Q506) that can be incorporated into the N-terminal helix of ACE2 (
Figure 1C).
Figure 1.
The structure of BCOV_S1_CTD bound to ACE2. (A) Overall domain profile of the SARS-CoV-2 spike monomer. FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat , NTD: N-terminal domain and BCOV_S1_CTD. (B) Sequence and secondary structures of BCOV_S1_CTD and RBM sequence bound to ACE2 is shown in red. (C) The BCOV_S1_CTD core is shown in cyan and RBM in red.
Figure 1.
The structure of BCOV_S1_CTD bound to ACE2. (A) Overall domain profile of the SARS-CoV-2 spike monomer. FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat , NTD: N-terminal domain and BCOV_S1_CTD. (B) Sequence and secondary structures of BCOV_S1_CTD and RBM sequence bound to ACE2 is shown in red. (C) The BCOV_S1_CTD core is shown in cyan and RBM in red.
The VADAR analysis prediction of BCOV_S1_CTD revealed 6 % helix, 77 % beta, 53% coil, and 56% turns with mean H bond energy of−1.6 (SD=1.1) against the expected value of−2.0 (SD=0.8) in the protein [
16]. As measured by several servers, these parameters indicated that the model was efficient and structural stability was maintained, thereby validating the structure. The PDBsum tool was used to analyze the secondary structural elements more intensively. Here, the amino acid sequence of BCOV_S1_CTD is the secondary structure displaying 7 helices (H1–H7), while β-sheet motifs are composed of 13 β-strands, 26 betas, and 3 gamma turns (
Figure 1B).
3. Multiple Sequence and Structure Alignment of BCOV_S1_CTD
S protein sequence retrieval and BCOV_S1_CTD conserved domain region identification of all major variants were made using the UniProtKB database and PROSITE server. The FASTA protein sequences were used for multiple sequence alignment (MSA). CLUSTAL OMEGA-based sequence alignment was used to identify the conserved region and mutation among the sequences of major variants [
17]. The SALIGN web server was used to determine the best alignment procedure based on the inputs while allowing the user to override default parameter values [
18]. Multiple sequence alignments were guided by a dendrogram computed based on a matrix of all pairwise alignment scores. Further MEGA 7 tool was used to calculate conserved, variable, passim-informative and singleton sites [
17].
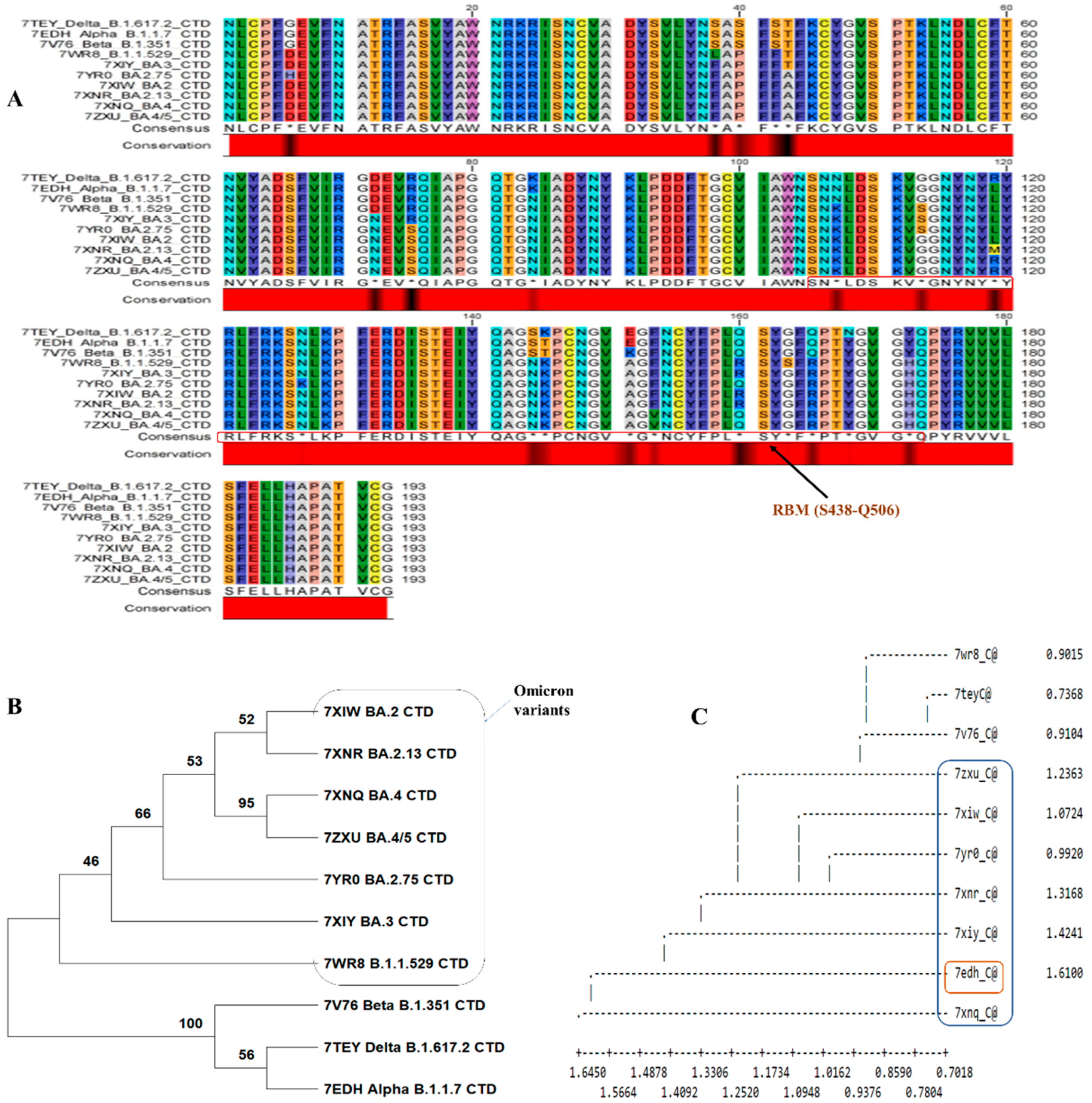
In the site analysis of the sequence alignment, 89.11% conserved sites, 10.88% variable sites, 9.3% passim-informative sites and 1.5% singleton sites were identified. Based on this investigation, nearly 10.88% of sites played an important role in amino acid substitution. The conserved and consensus sequence is shown in
Figure 2A.
Figure 2A shows a black region in the conserved region showing substitution within major variants. The phylogenetic tree was constructed based on alignment data, and it has been found that two major clusters were formed. The first cluster contains omicron variants and differs from the second cluster (
Figure 2B,C). The second cluster contains variants of Delta_B.1.617.2, Alpha_B.1.1.7, and Beta_B.1.351. When specific BCOV_S1_CTDs’ PDB data were used for structural classification, it showed that the Alpha_B.1.1.7 variant was deviating. The omicron variants evolved from the Delta_B.1.617.2 and Beta_B.1.351 variants (
Figure 2C).
Figure 2.
Analysis of the BCOV_S1_CTD sequence. (A) The MSA of BCOV_S1_CTD was performed using Crustal Omega, Sequence alignment showing the BCOV_S1_CTD in major variants of Covid-19. The critical residues for binding between SARS-CoV RBM and human ACE2 protein are indicated in red boxes. (B) Phylogenetic trees of the SARS-CoV-2-related lineage estimated from the entire BCOV_S1_CTD region. The results of 1,000 bootstrap repetlicates’ worth of branch supports are displayed. (C) Dendrogram showing the structural alignment of the BCOV_S1_CTD in major variants.
Figure 2.
Analysis of the BCOV_S1_CTD sequence. (A) The MSA of BCOV_S1_CTD was performed using Crustal Omega, Sequence alignment showing the BCOV_S1_CTD in major variants of Covid-19. The critical residues for binding between SARS-CoV RBM and human ACE2 protein are indicated in red boxes. (B) Phylogenetic trees of the SARS-CoV-2-related lineage estimated from the entire BCOV_S1_CTD region. The results of 1,000 bootstrap repetlicates’ worth of branch supports are displayed. (C) Dendrogram showing the structural alignment of the BCOV_S1_CTD in major variants.
4. B-Cell Epitopes Prediction in the BCOV_S1_CTD of S Protein
The S protein functions as a bridge to link to the receptors on the host cell, fusing the viral and host cellular membranes and ultimately allowing the virus to enter the host cell [
11]. The S protein is an I-type transmembrane glycoprotein that contains the transmembrane domain (TM), ectodomain, and CT domain. The ectodomain is made up of two subunits (S1 and S2): the N-terminal domain (NTD) and BCOV_S1_CTD are located in the S1 subunit, whereas the fusion peptide (FP) and heptad repeat (HR) domains 1 and 2 are located in the S2 subunit. While the S2 subunit completes the viral fusion and entrance task, the BCOV_S1_CTD is in command of attaching to the angiotensin-converting enzyme 2 (ACE2) receptor of host cells [
5]. Thus, the S protein is a critical target of the SARS-CoV-2 vaccination to prevent Covid-2019 and trigger viral growth and transmission. RBD has been identified as a primary target of efficient neutralizing antibodies in previous investigations on SARS-CoV.
The immuno-bioinformatic tools from the IEDB and related resources were used to predict the B cell epitopes in the BCOV_S1_CTD of S protein from the major variants of SARS-CoV-2 and compare the changes of the likely epitope sites from dominant and rare mutations of S protein. It was observed that different S protein mutations might have varying effects on those proteins’ putative effective epitopes.
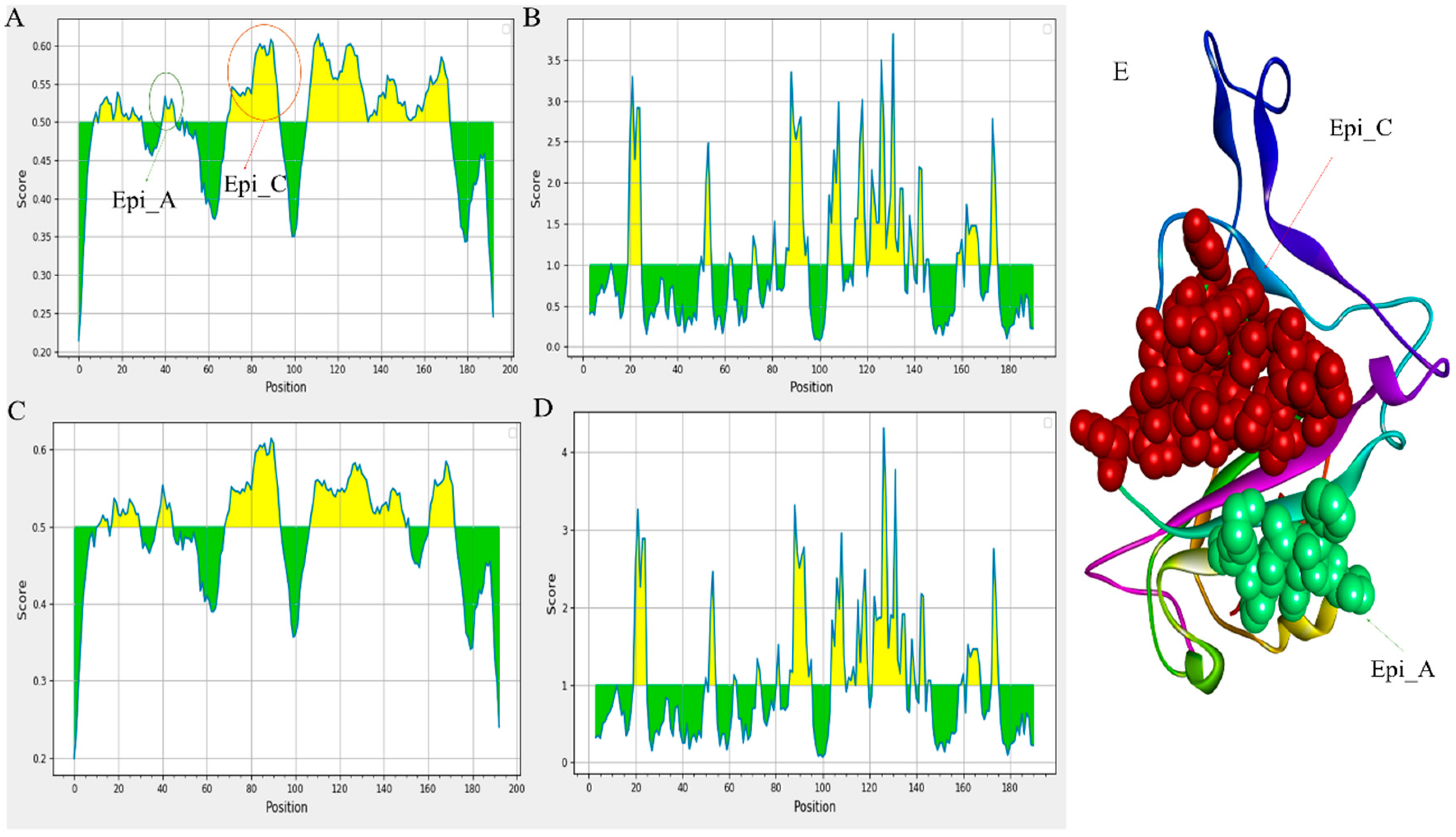
The defence against viral infection depends heavily on humoral immunity. The B cell epitopes of the S protein, typically present on the viral surface as unprocessed natural antigen molecules, are recognized by the B cell receptor (BCR) or neutralizing antibodies. We screened the S protein sequence using the BepiPred-2.0 prediction tool on the IEDB server to identify the probable linear B-cell epitopes. The distribution of the B-cell linear epitopes identified in major variants is depicted in
Figure 3 [
19,
20]. Most of the B-cell epitopes were found on the RBD domains of the S protein (BCOV_S1_CTD). Subsequently, using the Vaxijen 2.0 tool to analyze antigenicity and the Emini Surface Accessibility Prediction tool to analyze accessibility, the effective epitopes were identified (
Figure 3A–D) [
19].
Figure 3.
Prediction of B cell linear epitopes and accessibility analysis of BCOV_S1_CTD of S protein. (A)The distribution of all the predicted B-cell linear epitopes by BepiPred-2.0. The displayed possible epitope residues are those with scores above the cutoff (value is adjusted at 0.50) and are highlighted in yellow. Y-axes indicates residue scores and X-axes exhibits residue positions of the BCOV_S1_CTD of S protein (BA.2.13 variant). (B) The surface accessibility analyses using Emini surface accessibility scale. The residues with scores above the threshold (the default value is 1.00) are predicted to have good accessibility (BA.2.13 variant). Same for the (C) and (D) of the BCOV_S1_CTD of S protein (BA.2.75 variant). (E) Shows the B-cell linear epitope (Epi_C), which is mostly conserved across all the omicron variants and the B-cell linear epitope (Epi_A), which has the maximum antigenicity among all the predicted B-cell linear epitopes.
Figure 3.
Prediction of B cell linear epitopes and accessibility analysis of BCOV_S1_CTD of S protein. (A)The distribution of all the predicted B-cell linear epitopes by BepiPred-2.0. The displayed possible epitope residues are those with scores above the cutoff (value is adjusted at 0.50) and are highlighted in yellow. Y-axes indicates residue scores and X-axes exhibits residue positions of the BCOV_S1_CTD of S protein (BA.2.13 variant). (B) The surface accessibility analyses using Emini surface accessibility scale. The residues with scores above the threshold (the default value is 1.00) are predicted to have good accessibility (BA.2.13 variant). Same for the (C) and (D) of the BCOV_S1_CTD of S protein (BA.2.75 variant). (E) Shows the B-cell linear epitope (Epi_C), which is mostly conserved across all the omicron variants and the B-cell linear epitope (Epi_A), which has the maximum antigenicity among all the predicted B-cell linear epitopes.
In the accompanying
Table 2, we have predicted the linear B cell epitope for 10 variants. Additionally, we have analyzed the epitope alterations by comparing them with the epitopes from the prototype BCOV_S1_CTD (
Table 2). Initially, 2 effective epitopes have been identified in the Delta_B.1.617.2 variant out of 5 expected epitopes. In contrast, of the five predicted epitopes for the BA.2.13 variant, four were proven to be effective (
Table 2). In
Table 2, possible B-cell linear epitopes are given along with their positions, sequences, average scores, and antigenicity. This study has revealed that the BA.2.13 variant has four RBD domain epitopes: PFFAFK (40-25), IRGNEVSQIAPGQTGNIADYNYKLPD (69-94), KLDSKVGGNYNYMYRLFRKSNLKPFERD (107-134) and STEIYQAGNKPCNGVAGFNCYFPLRSYGFRPTYGVGHQ (136-173), which have more significant antigenicity and accessibility. Mutations in B-cell epitopes for the neutralizing antibody may result from mutations in the BCOV_S1_CTD of the S protein, affecting its structure and function.
Figure 3A,E depict the B-cell linear epitope (Epi_C), which is largely conserved across all omicron variants. Between all the anticipated B-cell linear epitopes, Epi_A has the highest antigenicity (
Figure 3E). The study also reveals that Epi_C of omicron variants has greater antigenicity compared to that of other variants; this might happen due to some mutations: D72N, R75S and K84N in identified BCOV_S1_CTD.
We have compared the predicted epitopes of the major mutants, examined the correlation between epitope changes caused by different mutations, and assessed the impact of mutation on B cell epitopes in order to examine the effects of the aforementioned common mutations of the BCOV_S1_CTD [
21]. The B-cell linear epitopes of the Delta_B.1.617.2, Alpha_B.1.1.7, and Beta_B.1.351 variants are almost identical (
Table 2) due to a rare mutation in the BCOV_S1_CTD of its S protein, which is strongly confirmed by the MSA analysis of these variants (
Figure 2). A mutation in the BCOV_S1_CTD of the S protein, however, has caused differences in the B-cell linear epitopes in every Omicron variant (B.1.1.529 to BA.4). On the size or position of B-cell epitopes, it was found that although certain alterations had little or no impact, some modifications had a considerable impact. The most significant finding is that the most common mutation only slightly changes the accessibility and antigenicity of the B-cell epitopes on the S protein.
Table 2.
BipiPred linear epitopes 2.0 prediction programme on the IEDB server was used to predict the B cell epitope in the BCOV_S1_CTD of S protein. Along with their start and end position, average score and antigenicity score with Vaxijen 2.0 tool.
Table 2.
BipiPred linear epitopes 2.0 prediction programme on the IEDB server was used to predict the B cell epitope in the BCOV_S1_CTD of S protein. Along with their start and end position, average score and antigenicity score with Vaxijen 2.0 tool.
| Proteins/ CTD-Domain |
Average Score |
Position |
Sequences |
Antigenicity |
| Delta_B.1.617.2 |
0.503 |
11-30 |
ATRFASVYAWNRKRISNCVA |
0.2689 (NA) |
| 39-45 |
ASFSTFK |
0.0865 (NA) |
| 69-94 |
IRGDEVRQIAPGQTGKIADYNYKLPD |
0.9322 (A) |
| 107-152 |
NLDSKVGGNYNYRYRLFRKSNLKPFER
DISTEIYQAGSKPCNGVEG |
0.3435 (NA) |
| 161-173 |
SYGFQPTNGVGYQ |
0.7632 (A) |
| Alpha_B.1.1.7 |
0.503 |
11-30 |
ATRFASVYAWNRKRISNCVA |
0.2689 (NA) |
| 39-45 |
ASFSTFK |
0.0865 (NA) |
| 69-94 |
IRGDEVRQIAPGQTGKIADYNYKLPD |
0.9322 (A) |
| 107-152 |
NLDSKVGGNYNYRYRLFRKSNLKPFER
DISTEIYQAGSKPCNGVEG |
0.3435 (NA) |
| 161-173 |
SYGFQPTNGVGYQ |
0.7632 (A) |
| Beta_B.1.351 |
0.501 |
11-30 |
ATRFASVYAWNRKRISNCVA |
0.2689 (NA) |
| 39-45 |
ASFSTFK |
0.0865 (NA) |
| 69-94 |
IRGDEVRQIAPGQTGKIADYNYKLPD |
0.9322 (A) |
| 107-152 |
NLDSKVGGNYNYRYRLFRKSNLKPFER
DISTEIYQAGSKPCNGVEG |
0.3435 (NA) |
| 161-173 |
SYGFQPTNGVGYQ |
0.7632 (A) |
| B.1.1.529 |
0.501 |
8-30 |
VFNATRFASVYAWNRKRISNCVA |
0.2656 (NA) |
| 38-45 |
LAPFFTFK |
1.0698 (A) |
| 69-94 |
IRGDEVRQIAPGQTGNIADYNYKLPD |
0.9322 (A) |
| 108-134 |
LDSKVSGNYNYLYRLFRKSNLKPFERD |
0.3225 (NA) |
| 137-173 |
TEIYQAGNKPCNGVAGFNCYFPLRSYSFRPTYGVGHQ |
0.5562 (A) |
| BA.2 |
0.502 |
8-30 |
VFNATRFASVYAWNRKRISNCVA |
0.2656 (NA) |
| 39-46 |
APFFAFKC |
1.2004 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
1.0563 (A) |
| 108-152 |
LDSKVGGNYNYLYRLFRKSNLKPFER
DISTEIYQAGNKPCNGVAG |
0.2073 (NA) |
| 156-173 |
YFPLRSYGFRPTYGVGHQ |
0.4765 (A) |
| BA.2.13 |
0.499 |
11-30 |
ATRFASVYAWNRKRISNCVA |
0.2689 (NA) |
| 40-45 |
PFFAFK |
1.9601 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
1.0563 (A) |
| 107-134 |
KLDSKVGGNYNYMYRLFRKSNLKPFERD |
0.4904 (A) |
| 136-173 |
STEIYQAGNKPCNGVAGFNCYFPLRSYGFRPTYGVGHQ |
0.4726 (A) |
| BA.2.75 |
0.494 |
12-16 |
TRFAS |
0 (NA) |
| 18-30 |
YAWNRKRISNCVA |
0.3936 (NA) |
| 38-45 |
FAPFFAFK |
1.1148 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
1.0563 (A) |
| 108-150 |
LDSKVSGNYNYLYRLFRKSKLKPFER
DISTEIYQAGNKPCNGV |
0.0655 (NA) |
| 162-173 |
YGFRPTYGVGHQ |
0.7884 (A) |
| BA.3 |
0.497 |
11-16 |
ATRFAS |
-0.151 (NA) |
| 18-30 |
YAWNRKRISNCVA |
0.3936 (NA) |
| 38-45 |
FAPFFAFK |
1.1148 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
1.0563 (A) |
| 108-150 |
LDSKVSGNYNYLYRLFRKSKLKPFER
DISTEIYQAGNKPCNGV |
0.0655 (NA) |
| 162-173 |
YGFRPTYGVGHQ |
0.7884 (A) |
| BA.4 |
0.498 |
11-16 |
ATRFAS |
-0.151 (NA) |
| 18-30 |
YAWNRKRISNCVA |
0.3936 (NA) |
| 39-45 |
APFFAFK |
1.2513 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
0.9322 (A) |
| 108-150 |
LDSKVSGNYNYLYRLFRKSKLKPFER
DISTEIYQAGNKPCNGV |
0.0655 (NA) |
| 162-173 |
YGFRPTYGVGHQ |
0.7884 (A) |
| BA.4/5 |
0.498 |
11-16 |
ATRFAS |
-0.151 (NA) |
| 18-30 |
YAWNRKRISNCVA |
0.3936 (NA) |
| 39-45 |
APFFAFK |
1.2513 (A) |
| 69-94 |
IRGNEVSQIAPGQTGNIADYNYKLPD |
0.9322 (A) |
| 108-150 |
LDSKVSGNYNYLYRLFRKSKLKPFER
DISTEIYQAGNKPCNGV |
0.0655 (NA) |
| 162-173 |
YGFRPTYGVGHQ |
0.7884 (A) |
5. Novel COVID-19 Therapeutic Strategies
The COVID-19 vaccine was developed after extensive studies to shield individuals from SARS-CoV-2 infection. While 199 vaccines are still in the preclinical development phases, at least 183 potential vaccines have started clinical testing [
22]. Nucleic acids, Protein subunits, virus-like particles, live attenuated and inactivated viruses, and replicating and non-replicating viral vectors have all been used in vaccine development strategies [
23]. The vaccines that the World Health Organisation (WHO) has approved were created using a range of techniques and have varied levels of efficacy. Given that the S protein of SARS-CoV-2 is essential for receptor binding. Full-length S protein or key components, like its receptor binding domain (RBD), have been employed as the major target antigen for nucleic vaccine candidates [
24]. However, newly developing SARS-CoV-2 strains have modified these antigens. Twelve vaccine candidates are undergoing clinical testing and have been given the go-ahead by several national regulatory organizations. The bulk (32%) of the recommended vaccine types are protein-based vaccines, with 21 potential vaccines now in Phase III and one in Phase IV. The European Medicines Agency (EMA) approved the first protein-based vaccine NVX-CoV2373 from Novavax with an effectiveness of 89.7%. In order to prevent SARS-CoV-2, this drug was approved in December 2021 [
25]. The second-largest type of vaccine in development, which accounts for around 24% is RNA-based vaccines [
22]. The first of these vaccines to be approved by the WHO for use in an emergency to control COVID-19 was Pfzer-BioNTech’s BNT162b2 [
26]. Surprisingly, 66 days after the SARS-CoV-2 sequence was made public, Moderna’s mRNA-1273 commenced its first US clinical trial (
Table 3). These two products—the first RNA vaccines approved for clinical use—have sufficiently demonstrated RNA-based immunizations’ advantages and potential applications [
27]. For the COVID-19 vaccine competition, 222 vaccine candidates were developed by teams from 79 countries, and more than 67% of them have begun Phase II trials. After Protein subunit (32%), and RNA (24%) vaccines, inactivated viruses (13%), non-replicating viral vectors (13%), and DNA (9%) were found as the most effective vaccine candidates studied in clinical studies [
22]. Since the pandemic’s start, a lot has been learnt about the various vaccine kinds, their efficacy, and their safety. Today, a major issue with vaccine use is equitable access to effective immunizations. In the following sections, we review the theory behind and design of the three main vaccine types (protein subunit, adenoviral vector, and mRNA) and the efficacy of vaccines against various SARS-CoV-2 variants (
Table 3).
5.1. Comparative Effectiveness of Vaccines against COVID 19
5.1.1. Vaccines with the Protein Subunit
Immune responses to one or more isolated viral proteins rather than the whole virus are elicited by protein subunit vaccines. Since the vaccine contains no live organisms, the risk of pathogenicity is completely reduced, and the vaccinations can even be administered to patients with impaired immune systems [
28]. Subunit vaccines are also a proven technique with a long application history, and the products are comparatively stable during storage and transit. However, protein subunit vaccinations frequently have a limited capacity to induce an immune response and may need adjuvants and numerous doses to achieve protective immune responses [
29]. Recombinant protein creation and production need a lengthy and intricate procedure. Several protein subunit vaccines authorized for clinical use are the human papillomavirus, hepatitis B, and influenza vaccines [
30,
31,
32]. SARS-CoV-2 protein subunit vaccines frequently target full-length S proteins or their antigenic components, such as the S1 subunit and RBD [
33]. While 17 protein subunit vaccines against SARS-CoV-2 have been granted emergency use permits, 55 candidates are presently undergoing clinical testing. One of these is NVX-CoV2373, which has been approved in 37 nations and is regarded as one of the finest protein subunit vaccines for SARS-CoV-2. A two-dose course of the NVX-CoV2373 vaccine enabled good protection against the B.1.1.7 variant and offered 89.7% protection against SARS-CoV-2 infection, according to phase III clinical studies [
34].
5.1.2. Vector-Based Adenovirus the COVID-19 Vaccine
The majority of viral vectors utilized for SARS-CoV-2 vaccinations are, by far, adenoviruses. These non-enveloped icosahedral viruses containing DNA were first discovered in the 1950s [
35] and feature a capsid with a diameter of around 90 nm. Adenoviral vectors effectively transfer the desired genes into the host cells, which is accompanied by host immunological responses that prevent vector transduction and transgenic expression. Adenoviral vectors constitute an excellent vaccine platform because of their high immunogenicity and transitory gene expression, which eliminates the need for additional adjuvants. Since adenoviral vector-based vaccines are easier to manufacture and develop more quickly than protein or subunit vaccines, they were identified as options for vaccine platforms after the SARS-CoV-2 genome sequence was determined in January 2020. So far, four adenoviral vector-based vaccines have been given the go-ahead by various regional organizations. Human adenovirus 5 (Ad5) was once the most common viral vector utilized to create vaccines. Ad5-nCoV, the CanSino Ad5 vector-based COVID-19 vaccine, was created and approved in China [
36]. Ad5-nCoV is 57.5% successful at treating symptomatic COVID-19 infection with a single dose [
37].
The AstraZeneca ChAdOx1 nCoV-19 vaccine (AZD1222; brand name, Vaxzevria) was first given conditional authorization by the European Medicines Agency (EMA). In non-human primates exposed to SARS-CoV-2, the AZD1222 vaccine efficiently decreased lung damage after a single dose (
Table 3) [
38]. According to two Phase III trials [
39,
40], the overall immunization efficacy in those who got two standard doses was estimated to be about 70%. It is significant to note that AZD1222-induced antibodies might encourage complement deposition, antibody-dependent NK cell activation, and neutrophil/monocyte phagocytosis [
41], all of which may help contain SARSCoV-2 infection. Similarly, the Janssen COVID-19 vaccine (Ad26.COV2.S) was initially authorized by the US Food and Drug Administration (FDA). Beginning in July 2020, phase I/II trials have demonstrated good immunogenicity and tolerance [
42]. According to Phase III research, the Janssen COVID-19 vaccine is 66.9% effective for COVID-19 and provides higher protection (76.7%) over severe-to-critical symptoms 14 days following vaccination [
43]. In a second instance, the Gamaleya Research Institute used the heterologous prime-boost strategy of Ad26 and Ad5 (each encoding the full-length S protein) to create the Russian Sputnik V vaccine. Interim analysis of Phase III clinical research in Russia demonstrated 91.6% efficacy against COVID-19 [
44].
5.1.3. Vaccines Based on mRNA
The scientific community was forced to develop vaccines swiftly while maintaining their safety and efficacy due to the development of the COVID-19 pandemic. Due to the straightforward, trustworthy, and adaptable technological method utilized to create new candidate vaccines, mRNA-based vaccines are undoubtedly the greatest option for fast development. The COVID-19 vaccines were developed and tested using this technology, which has so far outperformed the more time-consuming conventional methods of vaccine creation. Additionally, mRNA vaccines have a high level of efficacy due to their quick uptake and expression, which is supported by the formulation’s non-infectious and non-integrating characteristics. The ability to produce mRNA vaccines at a low cost may be their most significant benefit [
45].
5.2. Therapeutic Antibodies with High Efficacy
Finding therapeutic antibodies for the Omicron variation is a significant difficulty for researchers since these antibodies’ efficiency in neutralizing the Omicron variant is compromised [
46,
47]. As a result, various researchers have attempted to examine the efficiency of therapeutic antibodies against the Omicron form over time. The Omicron antibodies such as Bamlanivimab (LY-CoV555), Tixagevimab (COV2-2196), Imdevimab (REGN10987), Casirivimab (REGN10933), and Sotrovimab precursors (S309) have all been evaluated by Takashita et al. They also tested a wide range of monoclonal antibody combinations like Tixagevimab and Cilgavimab, Imdevimab and Casirivimab, and Etesevimab and Bamlanivimab. Additionally, it was discovered that these monoclonal antibody mixtures could neutralize both the wild strain and the Delta and Alpha strains. Combining Bamlanivimab and Etesevimab indicated a concurrently diminished neutralizing activity against the Gamma variant. These combinations can no longer neutralize the Beta and Omicron versions [
48]. They also discovered that the combination of Casirivimab and Imdevimab has demonstrated activity against the Gamma and Beta strains. The Omicron one was still unaffected by this combo. On the other hand, the Cilgavimab-tixagevimab combination was shown to have significant neutralization power against the Beta, Gamma, and Omicron ones [
48]. However, it was established that the Omicron pseudotype was unresponsive to many monoclonal antibodies [
49]. The therapeutic antibodies against Omicron have been identified with the aid of several in silico investigations. Shah and Woo have proposed that Sotrovimab (GSK, S203 mAb) and Evusheld (AstraZeneca mAbs) may be used in conjunction to successfully suppress the Omicron variant in this region [
50].
Additionally, researchers have tried to understand the relationship between the Omicron spike protein and the neutralizing antibodies (nAB). It could help us understand the special interaction mechanisms that these antibodies have. Recent investigations have revealed that ZCB11 may be a possible antibody for an Omicron. Zhou et al. have described the connection between ZCB11 and the spike protein of the Omicron version (PDB id: 7XH8). In their study, ZCB11 was shown to target the viral RBD domain as well as the spike protein of the Omicron SARS-CoV-2 variants [
51].
Table 3.
Different types of vaccine in clinical trials against major variants of COVID-19. Available online:
https://clinicaltrials.gov.
Table 3.
Different types of vaccine in clinical trials against major variants of COVID-19. Available online:
https://clinicaltrials.gov.
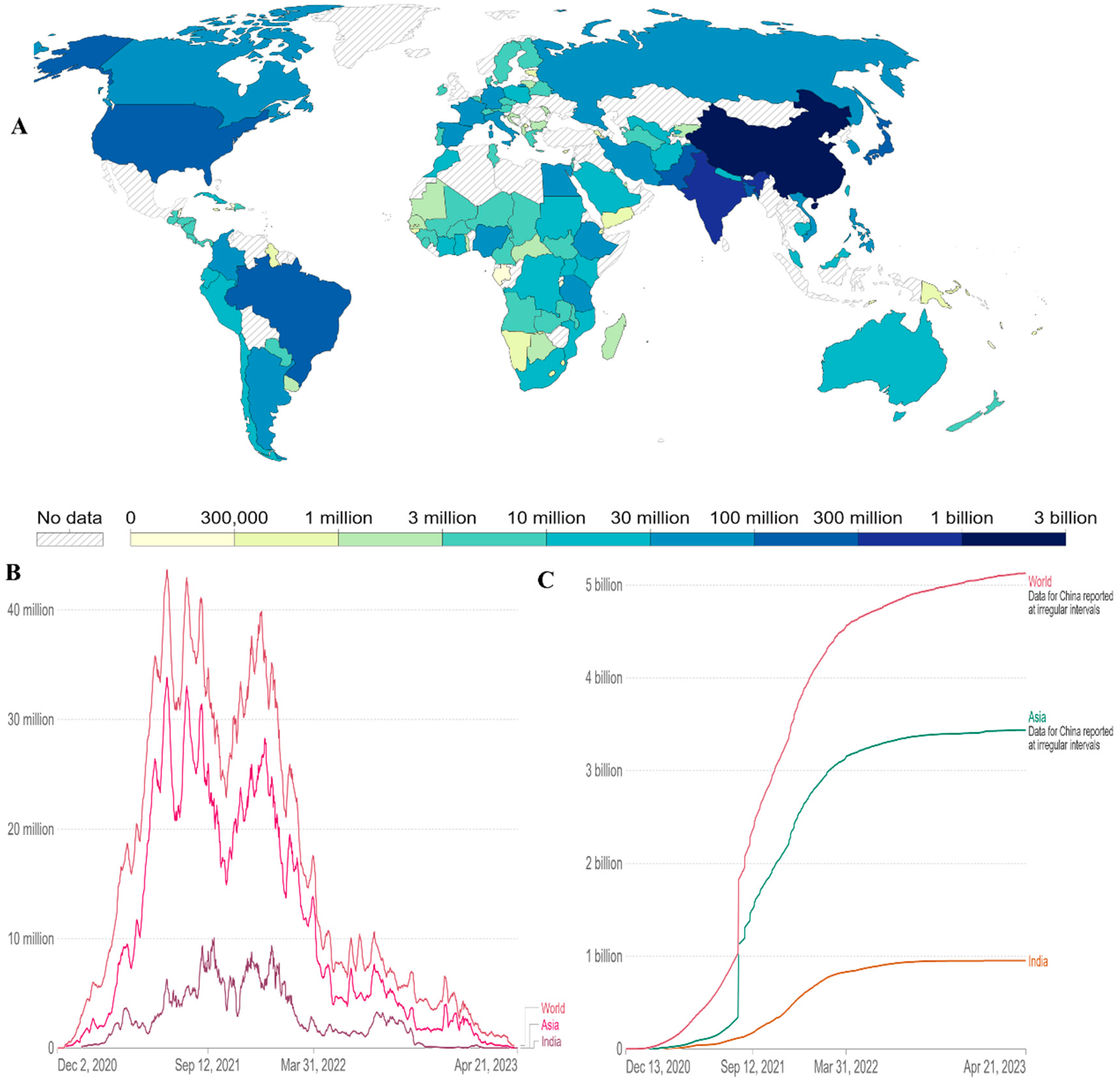
6. Global Vaccine Coverage
As of April 21 2023, there have been 6.88 million confirmed deaths and 676 million confirmed cases of infection with SARS-CoV-2, which causes COVID-19 [
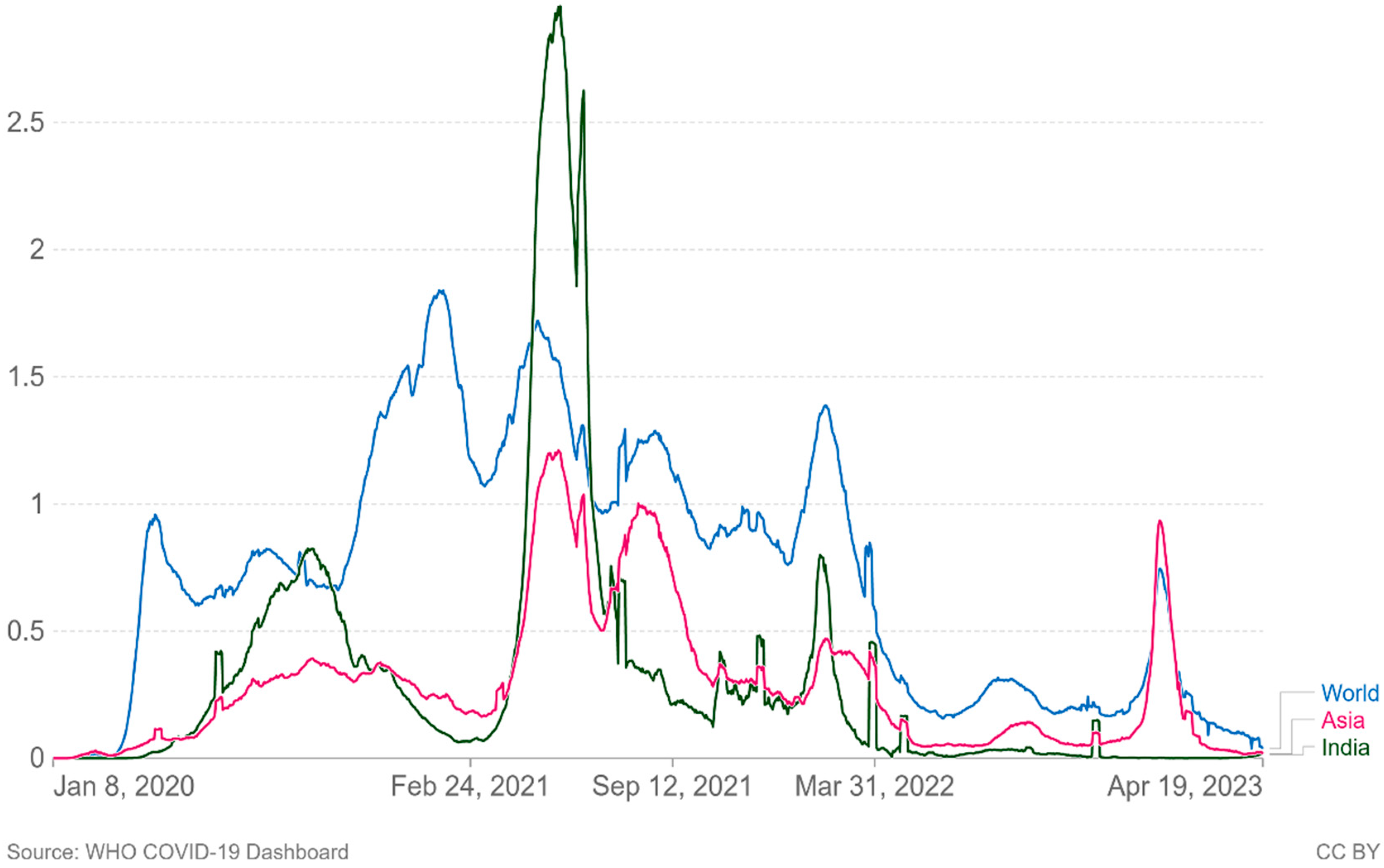
2]. In Asia and the rest of the world, new confirmed Covid-19 fatalities per million people (7-day rolling average) are increasing again (
Figure 4.). Since the beginning of the pandemic, virus transmission and mortality have been decreased through a variety of strategies, including preventative actions taken by individuals, such as social withdrawal, the wearing of face masks, prohibiting public gatherings of large numbers of people, placing travel restrictions on affected areas [
62]. Governments are looking to vaccination as a key component of responding to the pandemic following the successful development, assessment, and production of several vaccines.
Figure 4.
Cumulative number of new confirmed Covid-19 deaths per million people (7 day rolling average). Due to varying protocols and challenges in the attribution of the Couse of the death, the number of confirmed deaths may not accurately represent the true number of deaths by Covid-19.
Figure 4.
Cumulative number of new confirmed Covid-19 deaths per million people (7 day rolling average). Due to varying protocols and challenges in the attribution of the Couse of the death, the number of confirmed deaths may not accurately represent the true number of deaths by Covid-19.
We need timely cross-national statistics to comprehend the scope and pace of the vaccination implementation. A freely available worldwide dataset on given vaccines is available in the World in Data COVID-19 vaccination dataset (
https://ourworldindata.org/covid-vaccinations ). It has been frequently updated and covers the whole period beginning on December 2, 2020, when the initial vaccination data were released [
63]. The total number of COVID-19 vaccinations administered in each country, broken down into first and second doses, is tracked by this dataset when official statistics are available. Daily vaccination rates and population-adjusted figures are also derived. Users may compare rollout rates between nations, understand the scope and pace of vaccine rollouts relative to population, and assess the priorities for countries with one-dose and two-dose schedules.
The first reports of COVID-19 vaccines outside of clinical trials were reported on December 13, 2020, in the UK. The global vaccination time series since then are shown in this dataset. 13.37 billion doses had been administered globally as of April 21 2023. The total number of people (world, Asia and India) who completed the covid 19 vaccination protocol (all doses) is 5 billion worldwide. Particularly in Asia, it is more than 3 billion (
Figure 5A,C). Daily covid-19 vaccine doses administered (7-day rolling average, all doses, including boosters, are counted individually) have less in India than in the World (
Figure 5B) data till April 21 2023. At least one dose of an approved vaccine has been administered to more than 70% of the world’s population. A single day sees the administration of more than 270,000 doses of vaccination [
63]. In low-income nations, more than 29% of people have taken at least one dosage. This has drawn attention to important vaccination inequalities throughout the world.
Figure 5.
(A) Cumulative number of people who completed the covid 19 initial vaccination protocol administered by country till 21 April 2023.
(B) Daily covid-19 vaccine doses administered (7 day rolling average, all doses, including boosters are counted individually).
(C) Comparative analysis of number of people (world, Asia and India) the covid 19 vaccination protocol (all doses) administered by country till 21 April 2023. Data available online:
https://ourworldindata.org/coronavirus .
Figure 5.
(A) Cumulative number of people who completed the covid 19 initial vaccination protocol administered by country till 21 April 2023.
(B) Daily covid-19 vaccine doses administered (7 day rolling average, all doses, including boosters are counted individually).
(C) Comparative analysis of number of people (world, Asia and India) the covid 19 vaccination protocol (all doses) administered by country till 21 April 2023. Data available online:
https://ourworldindata.org/coronavirus .
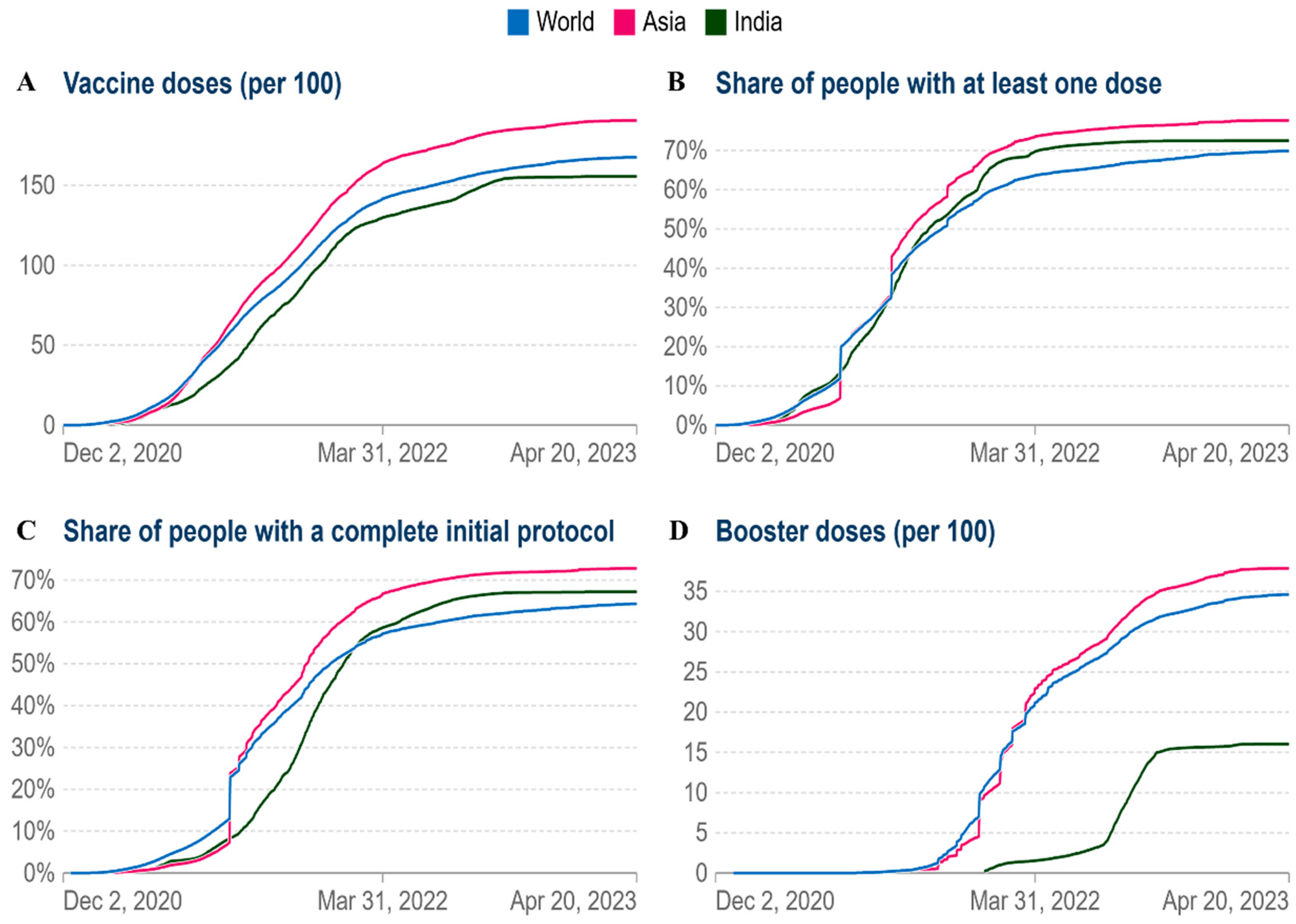
As of April 21 2023, India had 155.70 doses administered per 100 people, 167.6 doses administered globally, and Asia had 190.7 doses administered per 100 people (
Figure 6A). Currently, vaccination coverage in low-income nations has significantly grown. By April 21, 2023, at least one dosage had been administered to more than 80% of Asia (
Figure 6B). In Asia and worldwide, the cumulative percentage of people who have completed their initial immunization protocol has increased to 70% and 65%, respectively (
Figure 6C). As of April 21 2023, the cumulative number of booster doses administered per 100 people ranges from 40 per 100 in the case of Asia, 35 per 100 in the world and 16 per 100 in the case of India (
Figure 6D). According to the aforementioned facts, vaccination rates have significantly grown worldwide.
Figure 6.
Comparative analysis of vaccine doses administered per 100 people in world, Asia and India (A) The cumulative total vaccine doses administered per 100 people over time (B) Share of people with at least one dose. (C) People with a full initial vaccination protocol. (D) Booster doses administered per 100 people.
Figure 6.
Comparative analysis of vaccine doses administered per 100 people in world, Asia and India (A) The cumulative total vaccine doses administered per 100 people over time (B) Share of people with at least one dose. (C) People with a full initial vaccination protocol. (D) Booster doses administered per 100 people.
7. Emerging SARS-CoV-2 variants
SARS-CoV-2 continues to evolve, posing a significant risk to world health due to its increased infectivity and rapid transmission. The WHO categorizes them into variations of interest (VOI) and variants under monitoring (VUMs) in order to more accurately analyze the effects of various variants and promote preventative or medicinal countermeasures
https://www.who.int/activities/tracking-SARS-CoV-2-variants [
64]. There are currently one VOIs: XBB.1.5
Table 4A; and seven VUMs: BA.2.75, CH.1.1, BQ.1, XBB, XBB.1.16, XBB.1.9.1 and XBF
Table 4B. The appearance of Omicron has now prompted more focus and caution. The S protein is the region where most of the mutations in Omicron are located [
10], and there appears to be a propensity to accumulate mutations that facilitate immunological escape [
65,
66,
67]. According to a model, Omicron is around ten times as infectious as the original virus or twice as infectious as the Delta version. As Shown in
Figure 2, the following crucial mutation sites in the SARS-CoV-2 genome govern its virulence and ability to spread, which presents new opportunities for creating medications to treat the major emerging variants.
| A |
Pango lineage |
Nextstrain clade |
Genetic features |
Earliest documented samples |
Date of designation and risk assessments |
| |
XBB.1.5 |
23A |
Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e. BJ1 and BM.1.1.1, with a breakpoint in S1.
XBB.1 + S: F486P (similar Spike genetic profile as XBB.1.9.1)
|
05-01-2022 |
11-Jan-2023
XBB.1.5 Rapid Risk Assessment, January 11 2023
XBB.1.5 Updated Risk Assessment, February 24 2023 |
| |
|
|
|
|
|
| B |
Pango lineage |
Nextstrain clade |
Genetic features |
Earliest documented samples |
Date of designation and risk assessments |
| |
BA.2.75 |
22D |
BA.2 + S: K147E, S: W152R, S: F157L, S: I210V, S:G257S, S:D339H, S:G446S, S:N460K, S:Q493R reversion |
31-12-2021 |
06-Jul-2022 |
| |
CH.1.1 |
22D |
BA.2.75 + S: L452R, S: F486S |
27-07-2022 |
08-Feb-2023 |
| |
BQ.1 |
22E |
BA.5 + S: R346T, S:K444T, S:N460K |
07-02-2022 |
21-Sep-2022 |
| |
X.B.B. |
22F |
BA.2+ S:V83A, S:Y144-, S:H146Q, S:Q183E, S:V213E, S:G252V, S:G339H, S:R346T, S:L368I, S:V445P, S:G446S, S:N460K, S:F486S, S:F490S |
13-08-2022 |
12-Oct-2022 |
| |
XBB.1.16 |
Not assigned |
Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e., BJ1 and BM.1.1.1
XBB.1 + S: E180V, S: K478R and S: F486P |
23-01-2023 |
22-03-2023 |
| |
XBB.1.9.1 |
Not assigned |
Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e. BJ1 and BM.1.1.1
XBB.1 + S:F486P (similar Spike genetic profile as XBB.1.5) |
05-12-2022 |
30-03-2022 |
| |
X.B.F. |
Not assigned |
Recombinant of BA.5.2.3 and CJ.1 (BA.2.75.3 sublineage)
BA.5 + S:K147E, S:W152R, S:F157L, S:I210V, S:G257S, S:G339H, S:R346T, S:G446S, S:N460K, S:F486P, S:F490S |
27-07-2022 |
08-Feb-2023 |
8. Conclusion and Future Perspectives
The binding of the SARS-CoV-2 S protein to the ACE2 receptor is an essential step for the virus to invade the human body. The BCOV_S1_CTD of the S protein is the region in which the majority of the mutation sites are currently being investigated [
14,
68]. The present SARS-CoV-2 genome site mutations are all an outcome of drug screening and natural selection, which shows how the virus has adapted to the therapies. However, earlier drugs continue to be therapeutically effective against SARS-CoV-2 variants [
1] Despite several changes that made the virus more contagious [
1]. Currently, the majority of COVID-19 vaccines were developed to target S proteins in order to stimulate the production of neutralizing antibodies [
69] and the majority of vaccines undergoing phase 3 clinical trials are based on the early S protein [
70]. However, the SARS-CoV-2 virus can change and develop due to its fast and extensive worldwide distribution. This analysis revealed that almost 10.88% of sites were crucial in amino acid replacement. The S protein’s mutation in BCOV_S1_CTD may impact the B-cell epitopes and result in vaccination failure. Therefore, in this investigation, we used immuno-informatics methods to identify probable B-cell epitopes in BCOV_S1_CTD of the main variations to investigate the effect of mutations on the antigenicity of BCOV_S1_CTD. Most of the B-cell epitopes were found on the S protein’s BCOV_S1_CTD. This study reveals that Epi_C is the most potent and conserved epitope among the epitopes of the omicron variants. This would make it easier to employ SARS-CoV-2 prototype vaccines widely, even in locations with a high prevalence of the virus and an abundance of mutant strains [
71]. Within a year, scientists developed several COVID-19 vaccines that are incredibly effective. The challenge now is whether vaccines can be distributed rapidly and equally worldwide to match the speed at which they were produced. One of the best protein subunit vaccines for SARS-CoV-2, NVX-CoV2373 has received approval from 37 countries. Phase III clinical studies showed that an effective two-dose course of the NVX-CoV2373 vaccine gave 89.7% protection against SARS-CoV-2 infection and afforded significant protection against the B.1.1.7 variant. The above data shows that vaccine coverage has enormously increased throughout the world.
Efforts are being made over time to develop next-generation and mutation-proof vaccines [
47,
72]. The Omicron variant and its sub-variant have been shown to have many mutations. Hybrid immunity has recently been found to considerably increase immune defence against SARS-CoV-2 and other VOCs [
47]. Therefore, we must explore the possibility of hybrid immunity for protection against Omicron. Overall, the successful control of the current pandemic might rely on the continuous devotion of researchers, vaccine manufacturers, national regulators and the whole public health system.
Author Contributions
Study concept and design, AG, and RPS; manuscript writing, AG, APS, and VKS, manuscript editing, AG, APS, and RPS; All the authors have read and approved the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Amit Gupta (09/013(0912)/2019-EMR-I) and Ashish P. Singh (NTA Ref. No. 191620014505) are thankful to the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for the Senior Research Fellowship (SRF) and University Grants Commission (UGC), New Delhi, India, respectively, for the financial assistance in the form Senior Research Fellowship (SRF). The incentive grant received from IoE (Scheme no. 6031), Banaras Hindu University, Varanasi, India, to Rajeshwar P. Sinha is highly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses, 2023, 15, 167. [Google Scholar] [CrossRef]
- COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (Johns Hopkins University, accessed April 21 2023); https://arcg.is/0fHmTX. 21 April.
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, C.; Ye, C.; Ruan, Z.; Liang, Y.; Li, Y.; Wu, J.; Luo, Z. Advances in the development of therapeutic strategies against COVID-19 and perspectives in the drug design for emerging SARS-CoV-2 variants. Comput. Struct. Biotechnol. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Platt, D.; Parida, L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 2020, 98, 495. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature, 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet, 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Malik, Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of Novel Lichen Compounds as Inhibitors of SARS-CoV-2 Mpro: Ligand-Based Design, Molecular Dynamics, and ADMET Analyses. Biotechnol. Appl. Biochem. 2022, 194, 6386–6406. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (Johns Hopkins University, accessed April 21 2023); https://arcg.is/0fHmTX. Viruses, 2014, 6, 2991–3018.
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; Wang, X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLOS pathog. 2018, 14, e1007236. [Google Scholar] [CrossRef]
- Gupta, A.; Sahu, N.; Singh, V.K.; Sinha, R.P. Evolutionary aspects of mutation in functional motif and post-translational modifications in SARS-CoV-2 3CLpro (Mpro): an in-silico study. J. protein proteomics, 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Ji, L.; Wu, X.; Xu, D.; Cao, Z.; Han, J. Bioinformatics analysis of the epitope regions for norovirus capsid protein. B.M.C. Bioinform. 2013, 14, 1–6. [Google Scholar] [CrossRef]
- Braberg, H.; Webb, B.M.; Tjioe, E.; Pieper, U.; Sali, A.; Madhusudhan, M.S. SALIGN: a web server for alignment of multiple protein sequences and structures. Bioinformatics, 2012, 28, 2072–2073. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hoque, M.N.; Islam, M.R.; Akter, S.; Alam, A.R.U.; Siddique, M.A.; Saha, O.; Rahaman, M.M.; Sultana, M.; Crandall, K.A.; Hossain, M.A. Epitope-based chimeric peptide vaccine design against S, M and E proteins of SARS-CoV-2, the etiologic agent of COVID-19 pandemic: an in silico approach. PeerJ. 2020, 8, e9572. [Google Scholar] [CrossRef]
- Sun, P. , Ju, H., Liu, Z., Ning, Q., Zhang, J., Zhao, X., Huang, Y., Ma, Z. and Li, Y., Bioinformatics resources and tools for conformational B-cell epitope prediction. Comput. Math. Methods. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; Team, N.I.C. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms, 2021, 9, 1542. [Google Scholar] [CrossRef]
- WHO. March 30, 2023. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. 30 March.
- Savina, K.; Sreekumar, R.; Soonu, V.K.; Variyar, E.J. Various vaccine platforms in the field of COVID-19. Beni-Suef univ. j. basic appl. sci. 2022, 11, 35. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, H.; Gu, J.; Li, H.; Zheng, L.; Zou, Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines, 2020, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. first approval of the protein-based adjuvanted nuvaxovid (NVX-CoV2373) novavax vaccine for SARS-CoV-2 could increase vaccine uptake and provide immune protection from viral variants. Med. Sci. Monit. 2022, 28, e936523–1. [Google Scholar] [CrossRef] [PubMed]
- WHO. December 31, 2020. WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. 31 December.
- Ruffell, D. The future in an RNA molecule: from mRNA vaccines to therapeutics–An interview with Drew Weissman. FEBS Lett. 2021, 595, 2305. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Nygren, P.A.K.; Sta˚hl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; S. Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [CrossRef]
- Schillie, S.; Harris, A.; Link-Gelles, R.; Romero, J.; Ward, J.; Nelson, N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. Morbidity and Mortality Weekly Report, 2018, 67, 455. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, B.J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef]
- Schiller, J.; Lowy, D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine, 2018, 36, 4768–4773. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, EP; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; Goodman, A.L. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183.
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; Liu, R. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Guerrero, M.L.; Navarro, S.R.M.; Sued, O. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomized, double-blinded, placebo-controlled phase 3 trial. Lancet, 2022, 399, 237–248. [Google Scholar] [PubMed]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, JR; Avanzato, VA; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; Feldmann, F. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature, 2020, 586, 578–582.
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; McEvoy, C. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; Bibi, S. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet, 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; Verheul, M.K. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; Berghmans, P.J. Interim results of a phase 1–2a trial of Ad26. COV2. S Covid-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, PA; Truyers, C.; Fennema, H.; Spiessens, B.; Offergeld, K. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201.
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia. Lancet, 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D; Whitehead, K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Mallik, B. Omicron (B. 1.1. 529)-A new heavily mutated variant: Mapped location and probable properties of its mutations with an emphasis on S-glycoprotein. Int. J. Biol. Macromol. 2022, 219, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Chatterjee, S.; Mallik, B.; Sharma, A.R.; Chakraborty, C. Therapeutic role of neutralizing antibody for the treatment against SARS-CoV-2 and its emerging variants: a clinical and preclinical perspective. Vaccines, 2022, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; Saito, M. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Zhou, H.; Dcosta, B.M.; Samanovic, M.I.; Chivukula, V.; Herati, RS; Hubbard, S.R.; Mulligan, M.J.; Landau, N.R. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. EBioMedicine, 2022, 78, 103944.
- Shah, M.; Woo, H.G. Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front. Immunol. 2021, 12, 830527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, R.; Tang, B.; Chan, J.F.; Luo, M.; Peng, Q.; Yuan, S.; Liu, H.; Mok, B.W.; Chen, B.; et al. A broadly neutralizing antibody protects Syrian hamsters against SARS-CoV-2 Omicron challenge. Nat. Commun. 2022, 13, 3589. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04368728 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04470427 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04516746 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04505722 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04456595 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05383560 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04977479 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05077254 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05518487 (accessed on April 21 2023).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05308576 (accessed on April 21 2023).
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; Tatlow, H. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; Roser, M. Coronavirus pandemic (COVID-19). Our World in Data. 2020. https://ourworldindata.org/coronavirus. /: https.
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines, 2021, 9, 1195. [Google Scholar] [CrossRef]
- Science Brief: Omicron (B.1.1.529) Variant, CDC COVID-19 Science Briefs, Atlanta (GA), 2020 (https://www.ncbi.nlm.nih.gov/books/NBK575856/).
- Thakur, V.; Ratho, RK OMICRON (B. 1.1. 529): a new SARS-CoV-2 variant of concern mounting worldwide fear. J. Med. Virol. 2022, 94, 1821–1824. [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science, 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Shafqat, A.; Kashir, J.; Alkattan, K. Effect of SARS-CoV-2 mutations on the efficacy of antibody therapy and response to vaccines. Vaccines, 2021, 9, 914. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses, 2020, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: an Overview. 531 Curr. Trop. Med. Rep. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Abu-Raddad, L.J.; Andrews, N.; Davies, M.A.; Higdon, M.M.; Orenstein, W.A.; Patel, M.K. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine 2022, 40, 3516–3527. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Monteiro, V.S.; Hahn, A.M.; Grubaugh, N.D.; Lucas, C.; Chen, S. Bivalent mRNA vaccine booster induces robust antibody immunity against Omicron lineages BA.2, BA.2.12.1, BA.2.75 and BA.5. Cell Discov 2022, 8, 108. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).