1. Introduction
In recent decades, organotin compounds have attracted the attention of researchers in various fields such as the fundamental field (synthesis, structure, hybridization) [
1,
2,
3,
4,
5], as well as in the field of applications (agriculture, chemical industry, pharmacology) [
6,
7,
8,
9].
Organometallic chemistry plays a very important role in exploring the properties of metal ions for the design of new drugs [
10,
11]. This area has been stimulated by the success of various platinum-based complexes. Thus, cis-platinum or carboplatin is still considered to be the best-selling anticancer drug in the world, thanks to its reduced toxicity compared to other organometallic compounds [
10,
11,
12]. However, the use of these platinum complexes is severely limited by their side effects. This has prompted chemists to employ different strategies to develop new anti-cancer agents based on the other metals. The use of organometallic compounds as drugs is very common these days because they offer potential advantages over organic-based drugs [
13].
New organotin (IV) carboxylate complexes are being synthesized with the aim of obtaining new anti-cancer agents with more effective activity than cis-platinum or other clinically approved drugs [
13]. In addition to the advantages of high activity over the platinum compound, tin complexes are much cheaper. Thus, reduction of doses and enhancement of effects will be achieved [
13,
14,
15,
16].
During the last decades, organotin compounds are widely used in many industrial and agricultural applications. The most important applications are those related to the catalytic activity of organic reactions [
6], as well as their use as biocides due to their antifungal properties [
7,
8]. For some time, organotin compounds have been studied for their antitumor activity [
9]. Today, a number of interesting biological applications have been found for organotin complexes with amino acids [
17,
18], thiols [
19], o-phenanthroline, bipyridine, histidine, azomethines and carboxylates [
13,
20,
21,
22,
23], which are effective against various tumors.
Organotins are compounds containing at least one covalent tin-carbon bond (Sn-C), they are represented by the general formula RnSnX4-n, with n between 1 and 4. Organotin compounds are classified as mono-, di- , tri- and tetraorganotins, which are represented by RSnX3, R2SnX2, R3SnX and R4Sn respectively. With R can be an alkyl, or aryl group, and X can be an anionic species (halide, oxide, hydroxide, carboxylates or thiolates). The carbon-tin (C-Sn) bond is weaker than the carbon-carbon (C-C) or silicon-carbon (Si-C) bond, it is relatively non-polar, but it is stable in the presence of air and humidity, moreover the stability increases by the presence of anionic groups linked to the tin atom [
1,
2,
3,
4,
5].
In the literature, we noted studies concerning the synthesis of biomimetic oxidation catalysts to reproduce catecholase activity [
24,
25,
26,
27,
28,
29,
30]. Most of the results described use catalysts with the aim of mimicking the metallic active site environment of the catecholase enzyme and also understanding the catalytic properties for activating molecular oxygen.
Several studies have been carried out in the field of catecholase activity of catechol derivatives in o-quinone [
26,
31,
32,
33,
34,
35].
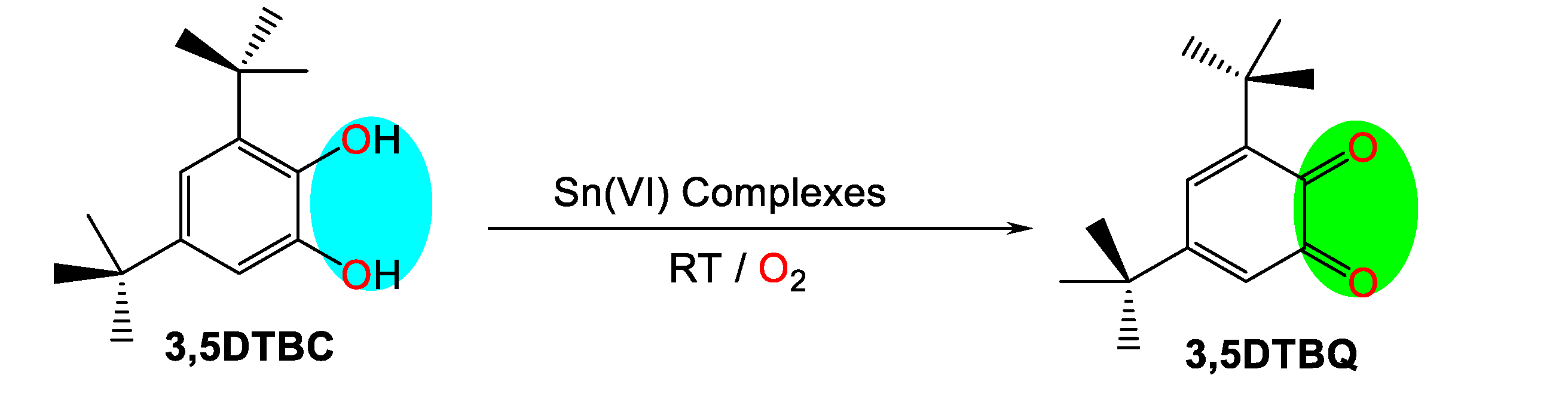
In this work, we studied the catalytic activity of certain organotin derivatives in the oxidation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-benzoquinone.
2. Methods and Materials
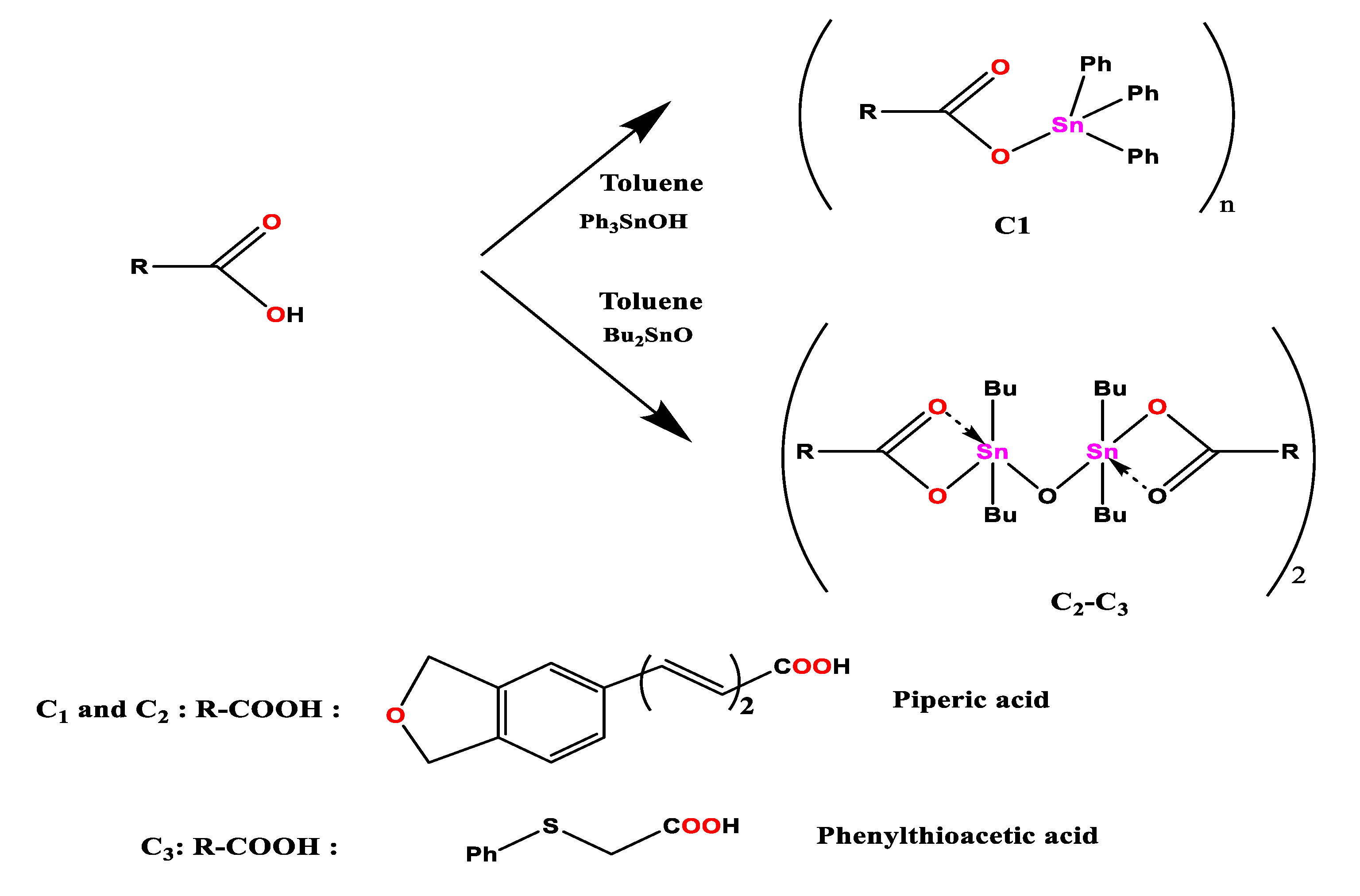
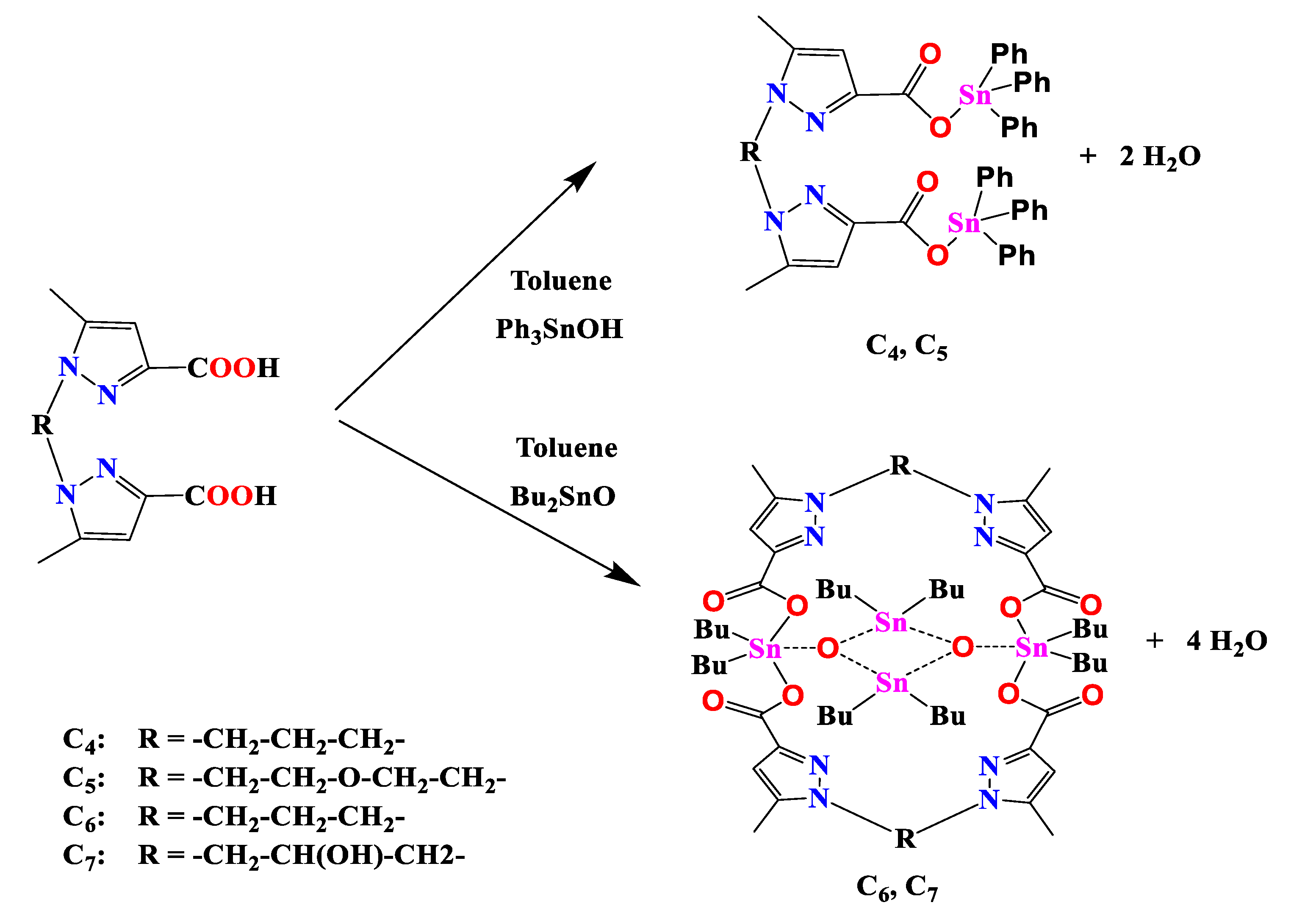
2.1. Synthesis of Organotin (IV) Carboxylates C1-C7
The elaboration of the target compounds
C1-C7 was performed as showed in the
Scheme 1 [
36,
37,
38].
2.2. Synthesis of Complex (C1):
A mixture of triphenyltin hydroxide (1 mmol) and piperic acid
L (1 mmol) was heated under reflux in toluene (50 mL) for 10h in a Dean-Stark apparatus for azeotropic removal of the water formed in the reaction (
Scheme 1). After cooling to room temperature, the solution was filtered. Yellow crystals, suitable for X-ray analysis, formed upon slow evaporation of the solvent after one week. (Yield = 75%): mp =143-145°C;
1H NMR (300 MHz, DMSO-d
6) δ: 5.90 (s, 2H, O-CH
2-O); 6.00 (d, 1H, CH-COO); 6,69 (d, 1H, C-CH=CH), 6,71 (d, 1H, C-CH=CH-C), 6.81 ( dd, 2H, C-CH=CH-CH), 6.91 (S, 1H, C-CH=C), 7.6 (dd, 1H, CH=CH-COO); 7.38-7.69 (m, 15H, SnPh
3),
13C NMR (DMSO-d
6) δ:101.54 (O-CH
2-O); 120.67 (CH-COO); 122.90 (C-CH=CH), 108.53 (C-CH=CH-C), 139.92 (C-CH=CH-CH), 124.80 (C-CH=CH-CH), 105.9 (C-CH=C), 146.00 (CH=CH-COO), 173.58 (COO), Sn-phenyl skeleton: 138.28 (C
ipso); 137.06 (C
ortho); 128.86 (C
meta); 130.13 (C
para). IR (cm
-1): 1630 ν
as(COO); 1420 ν
s(COO); 560 ν(Sn–C); 465 ν(Sn–O).
2.3. Synthesis of Complex (C2):
The complex
C2 was synthesized in a similar way to that of
C1, Dibutyltin oxide n-Bu
2SnO (1 mmol) and pipiric acid (1 mmol) in toluene (50 mL) were refluxed for 8h under azeotropic removal of H
2O by a Dean–Stark trap (
Scheme 1). After cooling down to the room temperature, the solution was filtered. The filtrate was gradually removed by evaporation under vacuum until solid product was obtained. The solid was then dissolved in minimum amount of ethanol and dichloromethane. Yellow crystals, suitable for X-ray analysis, formed up on slow evaporation of the solvent after one week. (Yield = 68%): mp =126-128°C;
1H NMR (300 MHz, DMSO-d
6) δ: 0.84 (t, 24 H,
J= 8 Hz, CH
3); 1.18–1.32 (m, 48H, CH
2CH
2CH
2); 5.92 (s, 8H, O-CH
2-O); 7.87 (d, 4H, CH-COO); 5.84 (d, 4H, C-CH=CH); 7.86 (d, 4H, C-CH=CH-C); 6.68 ( dd, 8H, C-CH=CH-CH); 6.94 (S, 4H, C-CH=C); 7.24 (dd, 4H, CH=CH-COO),
13C NMR (DMSO-d
6) δ: 101.37 (O-CH
2-O); 122.60 (CH-COO); 124.09 (C-CH=CH); 108.71 (C-CH=CH-C); 138.97 (C-CH=CH-CH); 124.09 (C-CH=CH-CH); 105.91 (C-CH=C); 143.83 (CH=CH-COO); 174.60 (COO);14.03 (CH
2–CH
3); 26.91, 27.07, 27.47, 28.3, 28.44 (CH
2–CH
2–CH
2). IR (cm
-1): 1638 ν
as(COO); 1425 ν
s(COO); 563 ν(Sn–C); 467 ν(Sn–O).
2.4. Synthesis of Complex (C3):
The synthesis of the title compound was carried out in an identical manner as described for 1 by using di-n-butyltin oxide (1mmol) and phenyl thioacetic acid (1mmol) (Scheme 3). After work-up, the solid was recrystallized from ethanol and dichloromethane, which up on slow evaporation afforded colorless crystals. (Yield = 65%): mp =142-144°C; 1H NMR (300 MHz, DMSO-d6) δ: 1.33 (t, 24 H, J= 8 Hz, CH3); 1.11–1.30 (m, 48H, CH2CH2CH2); 7.87 (d, 4H, CH-COO); 5.84 (m, 12H, C-CH=CH-CH=CH, Ph); 13C NMR (DMSO-d6) δ: 37.62 (S-CH2-COO); 125.86 (=CH-CH=CH-CH); 127.74 (=CH-CH=CH-CH); 128.94 (CH=C-S); 136.51 (CH=C-S); 174.74 (COO);14.03 (-CH2–CH3); 26.91, 27.07, 27.47, 28.3, 28.44 (CH2–CH2–CH2). IR (cm-1): 1638 νas(COO); 1425 νs(COO); 563 ν(Sn–C); 467 ν(Sn–O).
2.5. Synthesis of Complex (C4):
A mixture of triphenyltin hydroxide (2 mmol) and L (1 mmol) was heated under reflux in toluene (50 mL) for 10h in a Dean-Stark apparatus for azeotropic removal of the water formed in the reaction. After cooling to room temperature, the solution was filtered. Suitable colorless crystals were obtained by a slow evaporation of solvent (Yield = 68%): mp = 199-200°C; 1H NMR (300 MHz, DMSO-d6) δ: 2.13 (m,2H, -CH2-CH2-N); 2.24 (s, 6H, CH3-Pz); 3.88 (t, 4H, -CH2-N); 6.65 (s, 2H, HPz); 7.63-7.81 (m, 30 H, SnPh). 13C NMR (75 MHz, DMSO-d6) δ: 10.83(CH3-Pz); 58.5 (-CH2-N); 29.5 (CH2-CH2-N); 108.45 (CH-Pz); 142.20 (CH3–C=C); 143.69 (C-C=O); 166.08 (C=O), Sn-phenyl skeleton: 128.86 (Cmeta); 136.98 (Cipso); 136.75 (Cortho); 130.3 (Cpara). IR(cm-1): 1650 νas(COO); 1400 νs(COO); 530 ν(Sn–C); 460 ν(Sn–O).
2.6. Synthesis of Complex (C5):
The complex C5 was synthesized in a similar way to that of C4. Suitable colorless crystals were obtained by a slow evaporation of solvent. (Yield = 65%): mp = 206-207°C; 1H NMR(300 MHz, DMSO-d6) δ: 2.10 (s, 6H, CH3-Pz); 3.6 (t, 4H, -CH2-N); 4.03 (t, 4H, -O-CH2-CH2-N); 6.25 (s, 2H, H-Pz); 7.19-7.93 (m, 30H, SnPh).13C NMR (75 MHz, DMSO-d6) : 10.59 (CH3-Pz); 47.78(-CH2-N); 69.45 (-O-CH2-CH2-N); 108.55 (CH-Pz); 144.53 (CH3–C=C); 143.97 (C-C=O);168.17(C=O), Sn-phenyl skeleton: 138.38 (Cipso); 136.47 (Cortho); 128.71 (Cmeta); 130.13 (Cpara). IR (cm-1): 1650 νas(COO); 1400 νs(COO); 530 ν(Sn–C); 460 ν(Sn–O).
2.7. Synthesis of Macrocyclic Complex (C6):
A mixture of di-n-butyltin oxide (2 mmol) and L1 (1 mmol) was heated under reflux in toluene (50 mL) for 10 h in a Dean-Stark apparatus for azeotropic removal of the water formed in the reaction. After cooling to room temperature, the solution was filtered. Suitable colorless crystals were obtained by slow evaporation of the solvent. Yield = 67%. mp = 104-105 °C. 1H NMR (300 MHz, DMSO-d6) δ: 0.62-0.81 (t, 24 H, J= 8 Hz, CH3); 1.29–1.60 (m, 48H, CH2CH2CH2); 2.25 (s, 12H, CH3–Pz); 2.41 (m, 4H,–CH2–CH2–N); 4.10 (t, 8H, J= 6 Hz,–CH2–N); 6.53 (s, 4H, HPz). 13C NMR (75 MHz, DMSO-d6) δ: 11.27 (CH3–Pz); 14.03 (CH2–CH3); 26.06 (CH2–CH2–N); 26.91, 27.07, 27.47, 28.3, 28.44 (CH2–CH2–CH2); 46.43 (–CH2–N); 108.42 (CH–Pz); 138.85 (CH3–C=C); 145.30 (C–C=O); 166.28 (C=O). 119Sn NMR (150 MHz, CDCl3): -29.2, -183.4. IR (cm-1): 1630 νas(COO); 1420 νs(COO); 560 ν(Sn–C); 465 ν(Sn–O).
2.8. Synthesis of Macrocyclic Complex (C7)
The complex C7 was synthesized in a similar way to C6. Di-n-butyltin oxide (2 mmol) and L (1 mmol)]. Suitable colorless crystals were obtained by slow evaporation of the solvent. Yield = 62%. mp = 97-98 °C. 1H NMR (250 MHz, DMSO-d6) δ: 0.6–1.60 (m, 72H, CH2CH2CH2CH3); 1.75 (s, 8H, H2O); 2.27 (s, 12 H, CH3Pz); 3.95-4.24 (m, 8H, CH2-CH(HO)-CH2); 4.73 (m, 2H, HO-CH-CH2-Pz); 5.57 (d, 2H, J= 6Hz, HO-CH-CH2); 6.45 (s, 4H, HPz). 13C NMR (75 MHz, DMSO-d6) δ: 11.37 (CH3Pz); 13.62, 13.64 (CH2–CH3); 25.85, 26.74, 26.95, 27.45 (CH2–CH2–CH2); 52.45 (CH2-CH(HO)-CH2); 56.48 (CH2-CH(HO)-CH2); 108.42 (CH-Pz); 141.63 (CH3-C=C); 144.55 (C-C=O); 165.92 (C=O). 119Sn NMR (150 MHz, DMSO-d6): -31.7, -184.8. IR (cm-1): 1630 νas(COO); 1420 νs(COO); 560 ν(Sn–C); 465 ν(Sn–O).
2.9. Physical Measurements
The device used is a UV-visible spectrophotometer of the PyeUnicam UV 300 type with double beams. This will allow the monitoring of the formation of 3,5-di-tert-butyl-o-benzoquinone as a function of time at room temperature. The characteristic band of this compound is 400 nm. The oxidation reaction of 3,5-di-tert-butylcatechol is given in
Scheme 1.
Scheme 1.
Oxydation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-benzoquinone.
Scheme 1.
Oxydation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-benzoquinone.
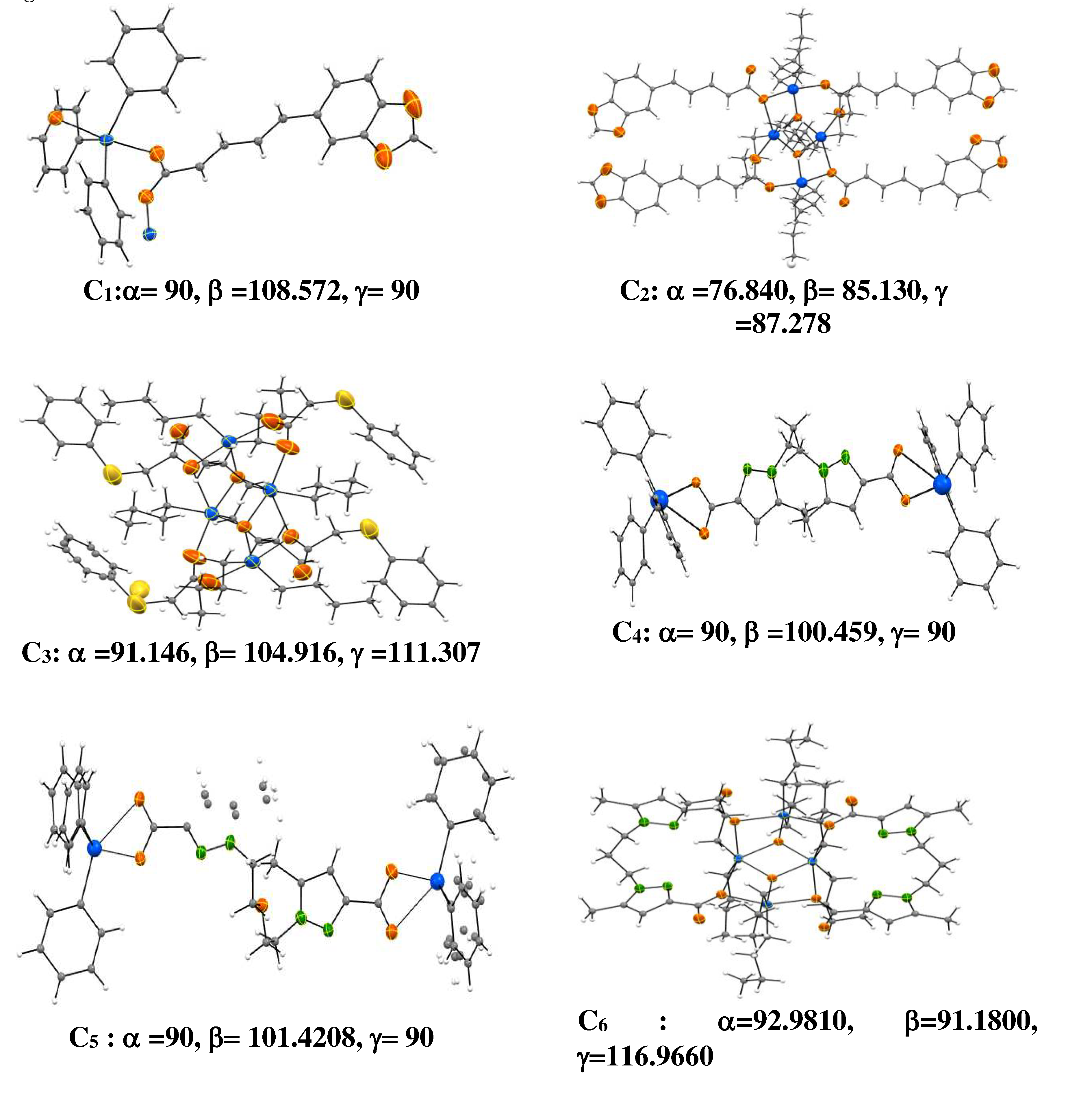
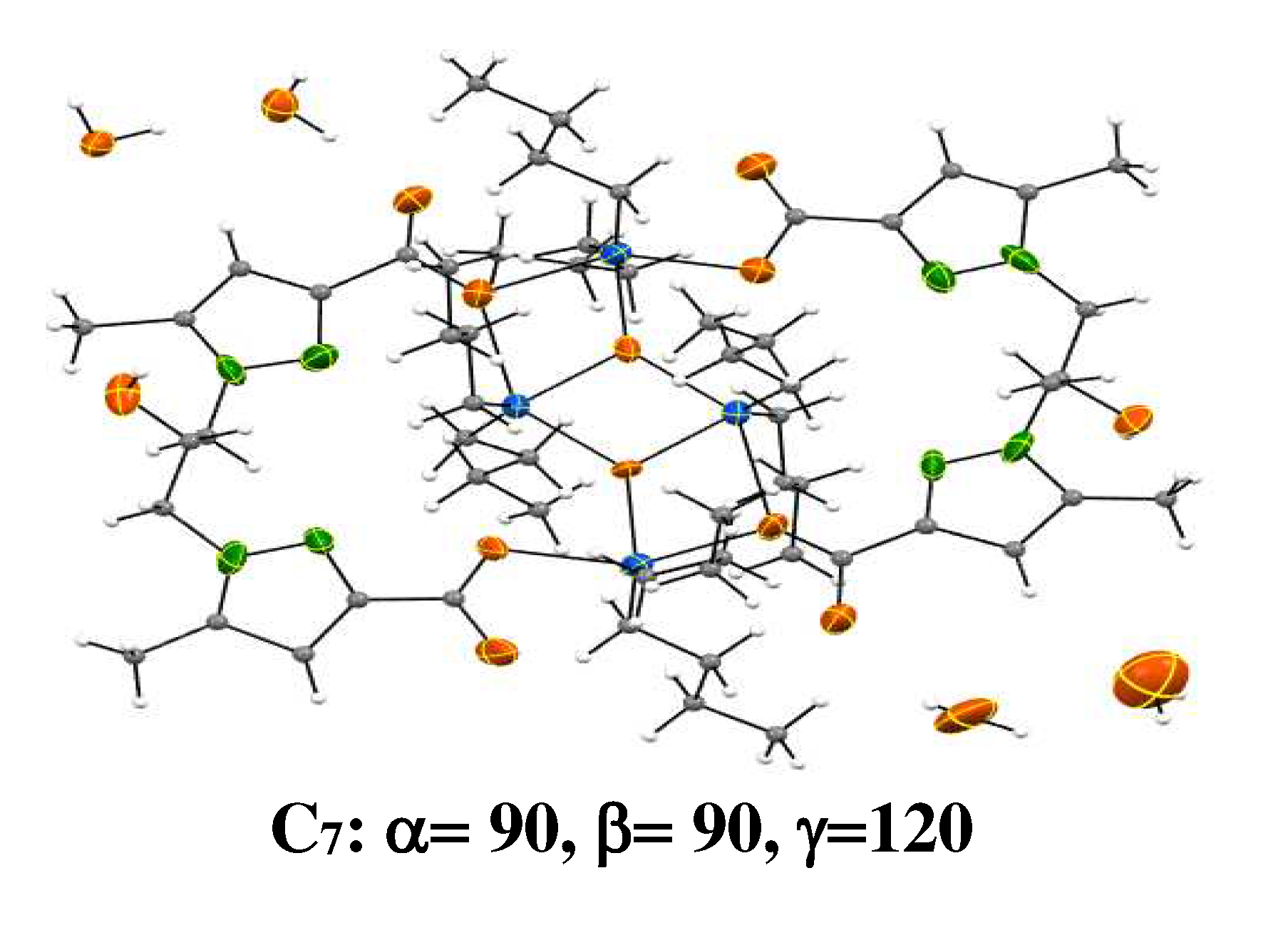
2.10. Organotin Complexes
The tridimensional structures of organotin complexes that tested in this study are schemed in
Figure 1.
2.11. Oxidation of Catechol in the Presence of Organotin Complexes
In an quartz tank, add 2 mL of a 3,5-di-tert-butylcatechol solution (10-1 mol. L-1) to 0.3 mL of the organotin complex (2.10-3mol.L-1). The evolution of the absorbance of 3,5-di-tert-butyl-o-benzoquinone is monitored for 1 hour. The manipulation was carried out in DMSO.
3. Results and Discussion
3.1. Catecholase Studies
In this work, we have carried out the study of the oxidation activity of the 3, 5-di-tert-butylcatechol to 3, 5-di-tert-butyl-o-benzoquinone by organotin complexes in the solvent dimethyl sulfoxide (DMSO).
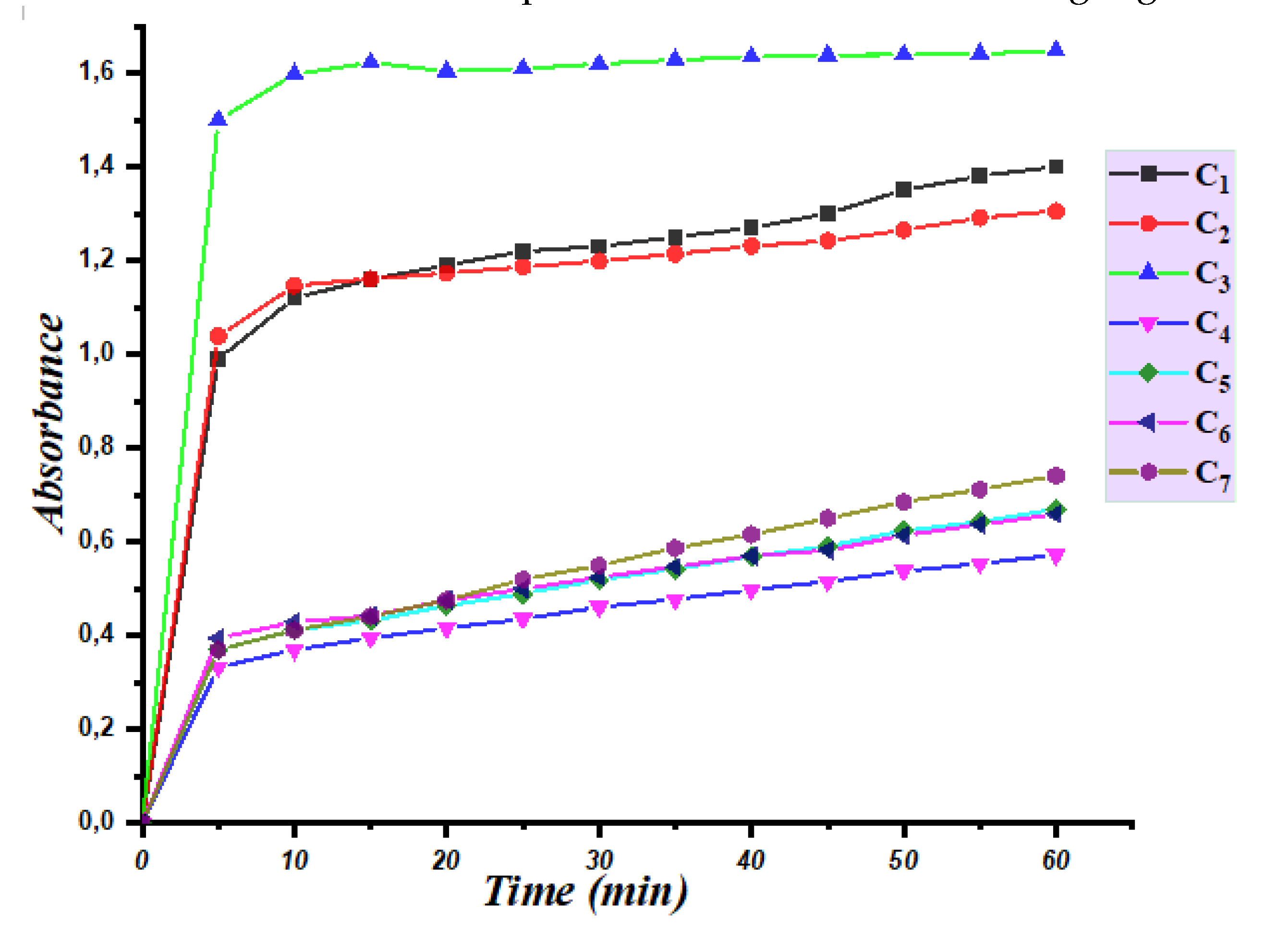
The catalytic activity of tin C1-C7 complexes has been studied in DMSO. The evolution of the absorbance as a function of time for these complexes is shown in the following Figure 2:
Figure 1.
3D structures of tested Sn(VI) complexes [
36,
37,
38].
Figure 1.
3D structures of tested Sn(VI) complexes [
36,
37,
38].
The calculation of the oxidation rates of 3,5-di-tert-butylcatechol in the presence of organotin complexes (
C1-C7), led us to the results gathered in
Table 1.
The data represents the catalytic activity of twelve different organotin complexes in the oxidation of 3,5-di-tert-butylcatechol, a commonly used substrate in biochemical assays. The measured values are given in µmol. L-1. min-1, which reflects the rate of reaction of the complexes in the presence of the substrate. The results show that the catalytic activity of the different organotin complexes varies significantly, with values ranging from 5.9 µmol. L-1. min-1 for C4 to 7.83 µmol. L-1. min-1 for C1. This suggests that the structural and chemical properties of the organotin complexes play a critical role in their catalytic activity. Interestingly, the data reveals that the top-performing complexes, C1 and C2, exhibit catalytic a5ctivities of 7.83 µmol. L-1. min-1 and 7.72 µmol. L-1. min-1, respectively, which are significantly higher than the other complexes tested. This suggests that these two compounds possess unique structural features that contribute to their high catalytic activity. Conversely, some of the organotin complexes, such as complexes (C4 and C6), show relatively low catalytic activity with values of 5.9 µmol. L-1. min-1. These results suggest that the design of organotin complexes with optimized structural and chemical properties is crucial to achieving high catalytic activity. Overall, the data provides valuable insights into the catalytic activity of organotin complexes and highlights the potential for designing novel catalysts with enhanced activity and selectivity. This knowledge can be utilized to develop more efficient and cost-effective catalysts for various applications in industry and biomedicine.
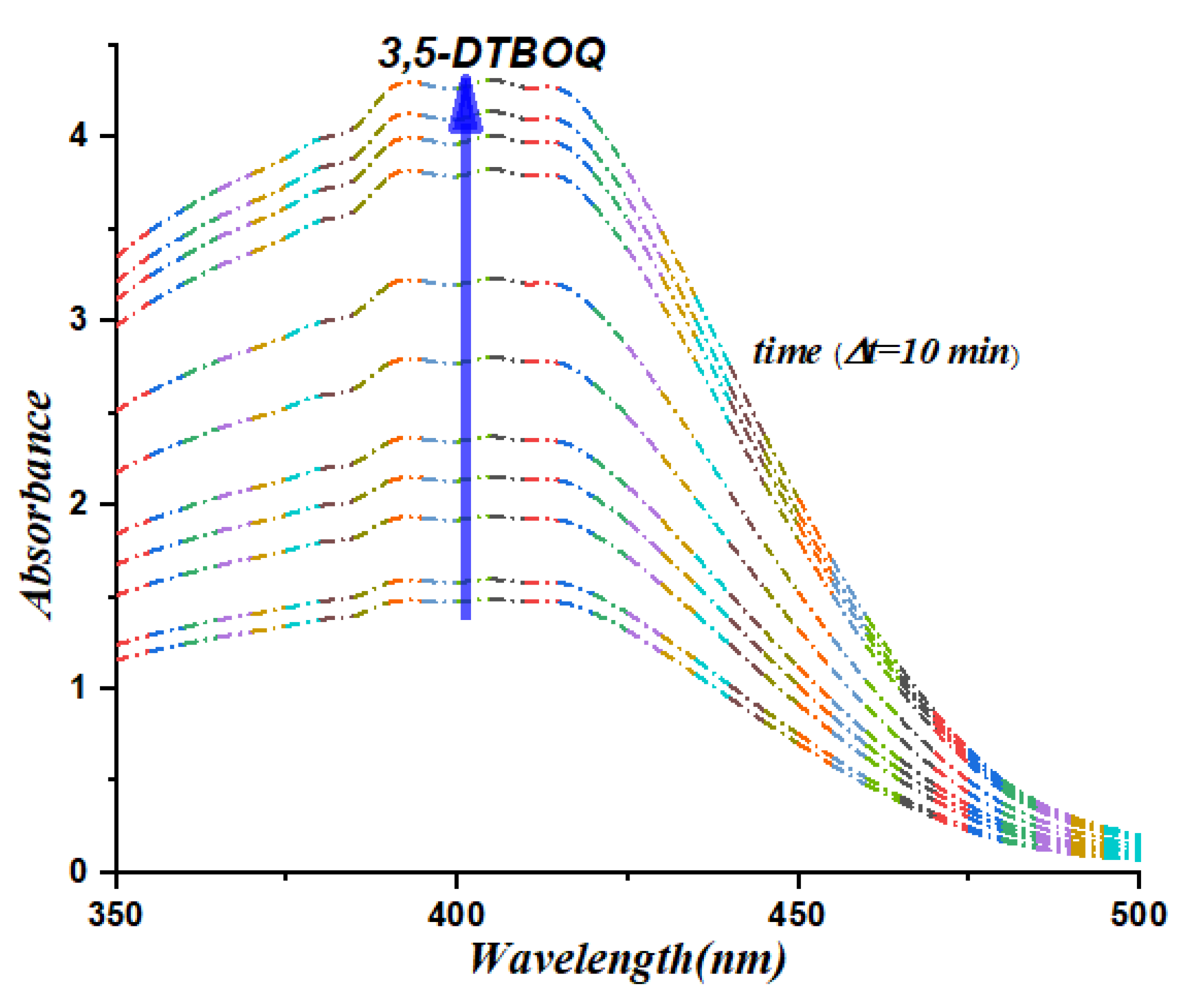
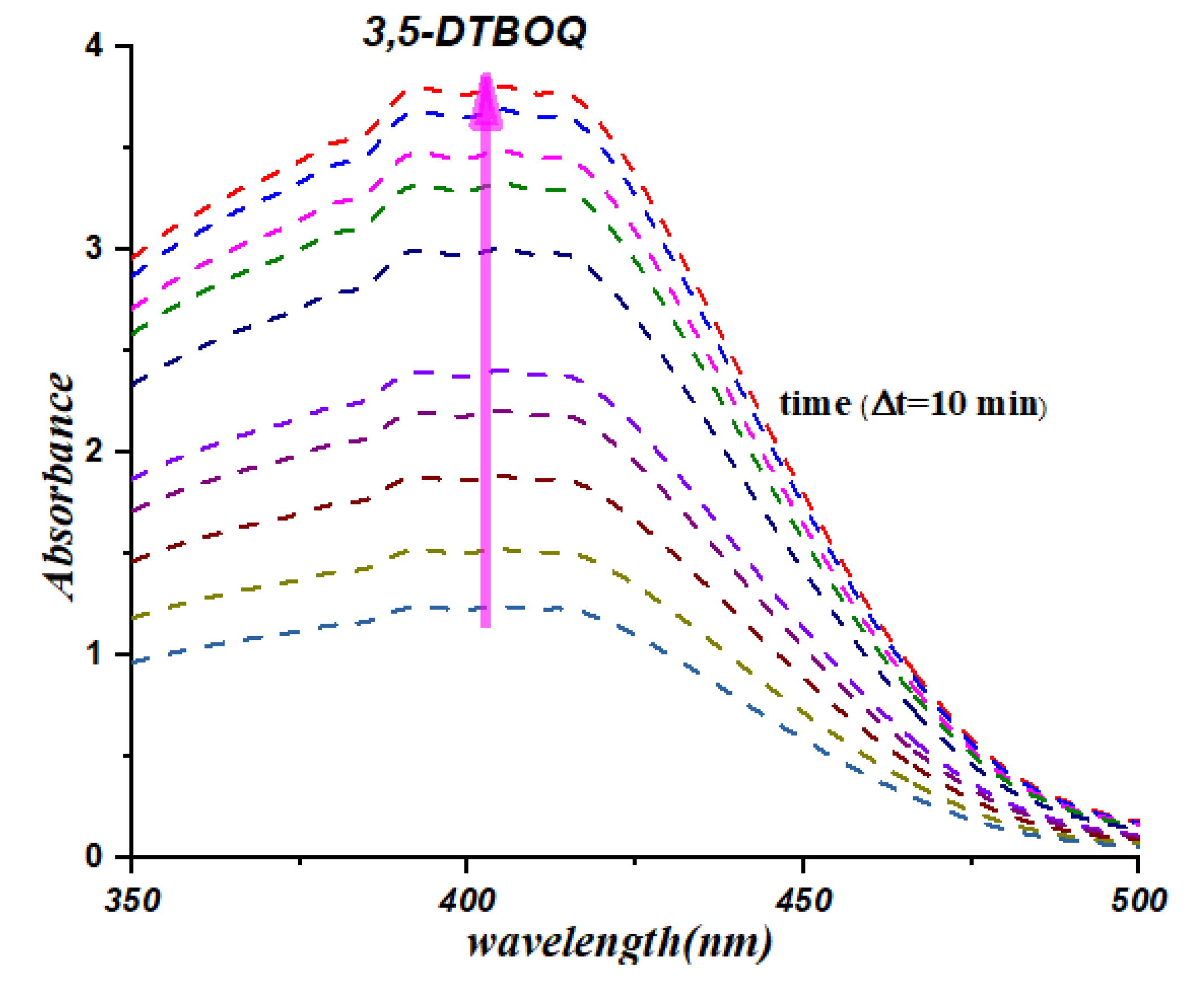
3.2. UV-Vis Spectrophotometric Study
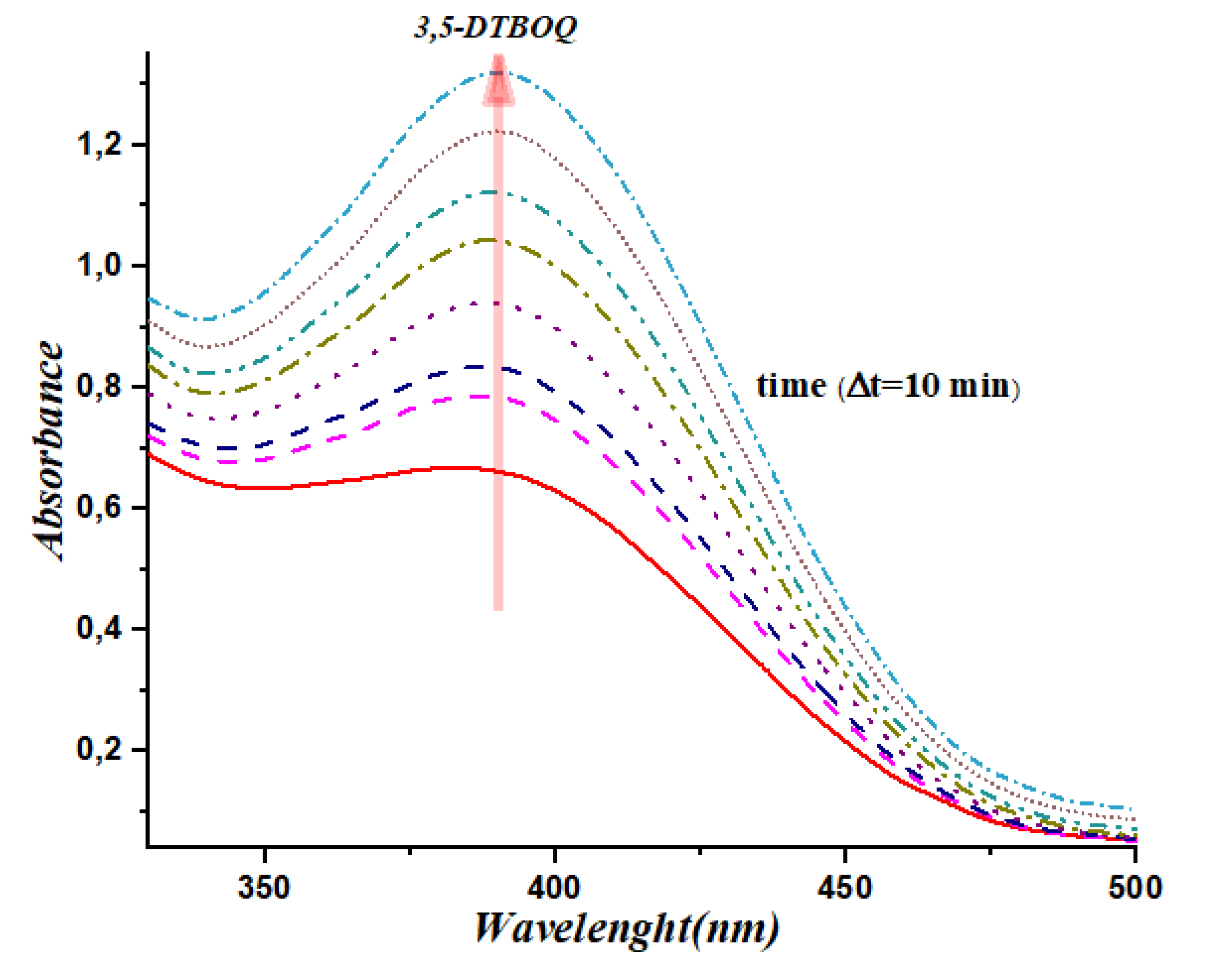
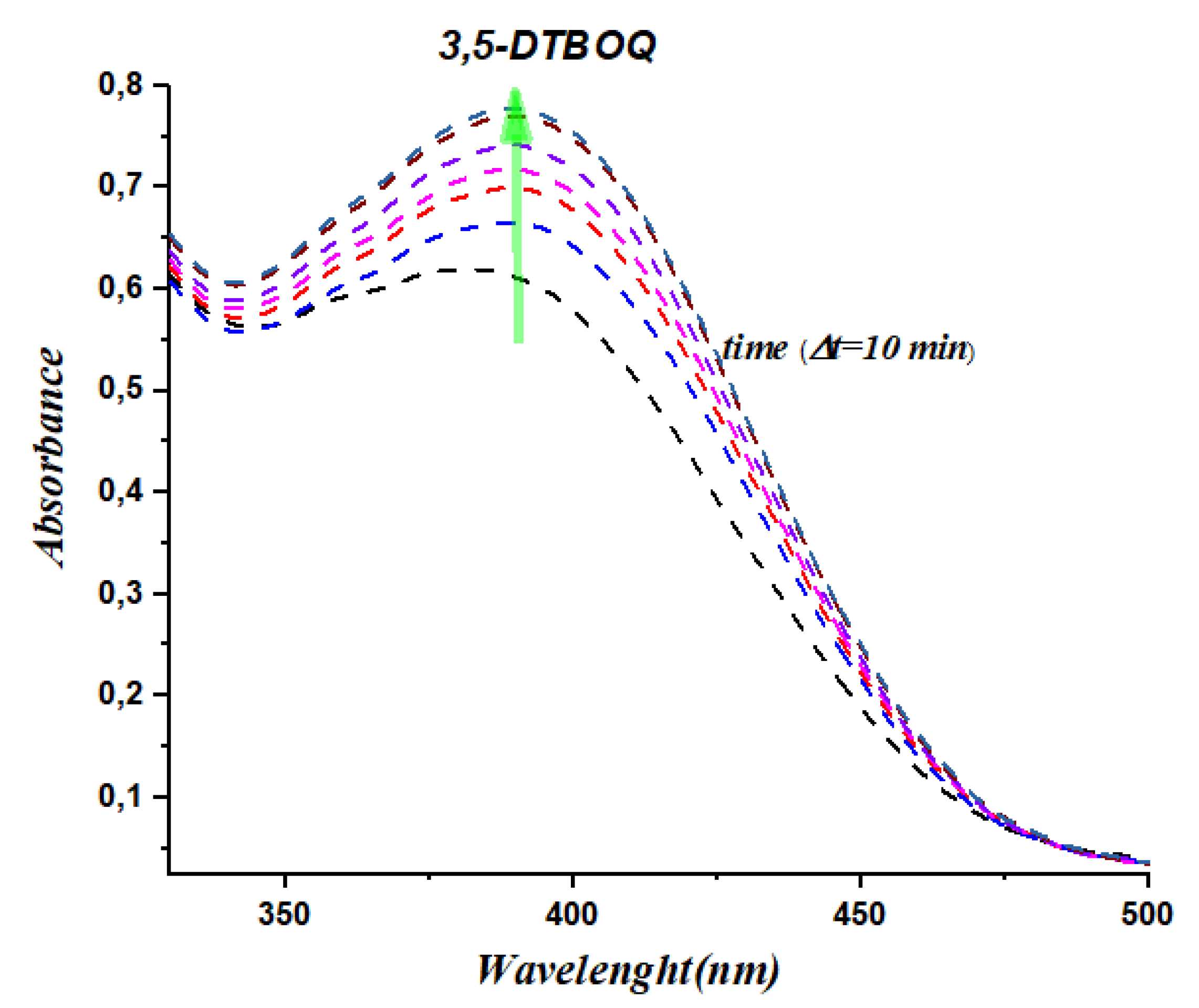
To validate the significant catalytic activity of our synthesized organotin complexes, we conducted kinetic experiments to monitor the formation of 3,5-di-tert-butyl-o-benzoquinone in the presence of the complexes (
C1, C2, C3 and
C5). The evolution of absorbance was recorded at λ= 400nm every 10 minutes at room temperature. The results are presented in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. Our results show that the complexes
C1, C2, C3 and
C5 exhibit significant catalytic activity in the oxidation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-benzoquinone. The appearance of a centered band at 400 nm (
Figure 3 and
Figure 4) clearly indicates the formation of the desired product, which is a testament to the catalytic efficiency of the organotin complexes. The kinetic data obtained provides further evidence of the catalytic activity of our complexes, with the absorbance values increasing steadily over time. Specifically, complexes C8 and C9 exhibited the highest catalytic activity, with the absorbance values increasing more rapidly than the other complexes tested. Certainly, these results demonstrate the significant catalytic activity of our synthesized organotin complexes and provide insights into their potential application in catalysis. Further studies can be conducted to optimize the structure and chemical properties of these complexes for enhanced catalytic activity and selectivity, opening up exciting possibilities for the development of novel catalysts with broad-ranging applications.
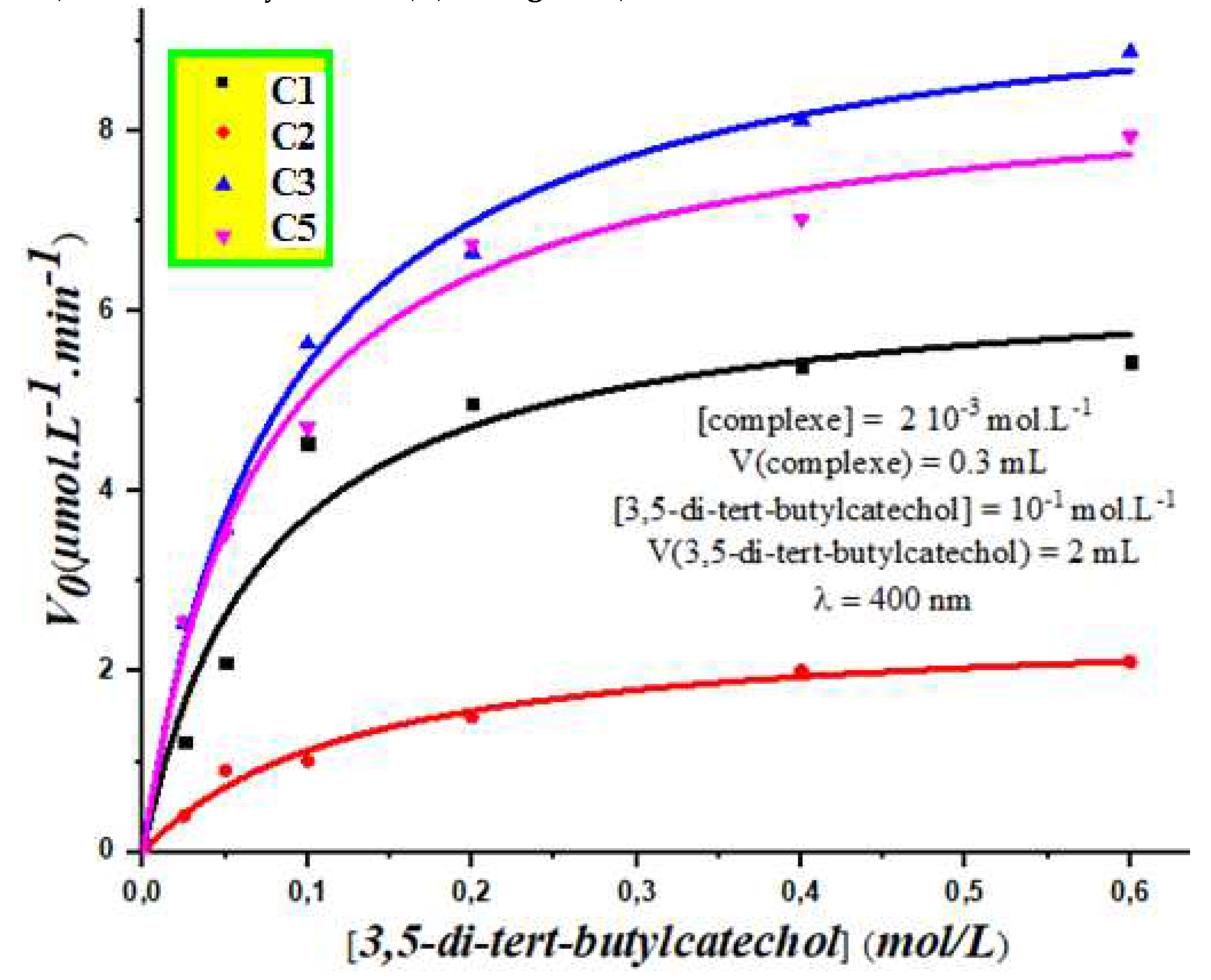
3.3. Vmax and Km Study
A kinetic study is a critical tool for characterizing the behavior of enzymatic reactions, and in this case, the catalytic activity of our synthesized organotin complexes. By measuring the initial rate (V0) of the reaction at different concentrations of the substrate, we were able to construct a graph of Vo (µmol.L-1.min-1) versus the substrate concentration (mol.L-1), which provided us with information on the Km and Vmax constants.
The Km value is a measure of the affinity of the enzyme (or in this case, the complex) for the substrate, whereas the Vmax value represents the maximum velocity of the reaction. These kinetic constants are crucial in understanding the behavior of the reaction and optimizing its conditions. Our study involved mixing a solution of the organotin complexes with the substrate 3,5-di-tert-butylcatechol (3,5-DTBC) at varying concentrations, and monitoring the rate of the reaction over time. The results indicated that the complexes C1, C2, C3 and C5 exhibited significant catalytic activity for the oxidation evalution of 3,5-di-tert-butylcatechol. By determining the Km and Vmax constants for each of the complexes, we were able to gain insights into their catalytic behavior, and evaluate their potential application in various catalytic processes. The obtained results can also help in optimizing reaction conditions and identifying the most effective catalyst for a given substrate. In general, our study demonstrates the power of kinetic studies in characterizing the catalytic,- behavior of enzymes and organometallic complexes, and provides valuable insights for developing more efficient catalytic systems in the future.
In order to further investigate the catalytic activity of our complexes, we conducted a kinetic study to determine the
Km and
Vmax constants of the catechol oxidation reaction. To achieve this, we mixed 0.3 ml of a solution of Sn (IV) complexes C1, C3, C8, and C9 at a concentration of 10
-4 mol/L with a solution of 3,5-di-tert-butylcatechol at a concentration varying from 2.510
-2 mol/L to 0.6 mol/L under ambient conditions. The initial rate (
V0) was then plotted as a function of the substrate concentration (3,5-di-tert-butylcatechol) (see
Figure 7).
From the results obtained, we observed a linear relationship between the initial velocities and the substrate concentration for complexes C8 and C9. This led us to apply the Michaelis-Menten model to determine the kinetic parameters of the best catalyst. Our findings indicate that the rate
Vmax for complexes
C1 and
C3 are 8.80 µmol. L
-1.min
-1 and 7.90 µmol. L
-1.min
-1 respectively (see
Table 2).
Interestingly, we noted a low Km value for complex C1, indicating a strong affinity between the tested catalyst and the substrate (3,5-di-tert-butylcatechol) in DMSO organic solvent. This further confirms that complex C1 demonstrated better performance for the oxidation of the substrate (3,5-di-tert-butylcatechol) in our case. generally, these results validate the significant catalytic activity of our organotin complexes, and highlight their potential as efficient catalysts for various applications in the future.
4. Conclusion
Our study demonstrates the potential of organotin complexes as effective catalysts for the oxidation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-benzoquinone. The kinetic analysis revealed that complexes C1 and C2 are the most efficient catalysts, exhibiting high rates of oxidation with low Km values, indicating strong affinity between the catalysts and the substrate. The results suggest that the catalytic activity of the complexes is influenced by the concentration of the complex and the coordination environment. Furthermore, the mild reaction conditions used in this study, conducted at room temperature and with oxygen as the oxidant, make these complexes attractive candidates for further exploration in catalysis. This study adds to the growing body of knowledge on the use of organotin complexes in catalysis and provides valuable insights into the design of new and effective catalysts for various chemical reactions.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large group Research Project under grant number (RGP2/413/44).
References
- L. Pellerito, L. L. Pellerito, L. Nagy, Organotin (IV) n+ complexes formed with biologically active ligands: equilibrium and structural studies, and some biological aspects. Coordination Chemistry Reviews, 224(1-2), (2002) 111-150. [CrossRef]
- R. C. Poller, The chemistry of organotin compounds, Logos P, (1970).
- V. S. Petrosyan, NMR spectra and structures of organotin compounds. Progress in Nuclear Magnetic Resonance Spectroscopy, 11(2) (1977) 115-148. [CrossRef]
- W. P. Neumann, The organic chemistry of tin, Interscience, London (1970).
- P. G. Harrison, Chemistry of tin, Springer (1989).
- J. Otera, N. J. Otera, N. Dan-oh, H. Nozaki, Unique template effects of distannoxane catalysts in transesterification of diol esters. Tetrahedron, 49(15) (1993) 3065-3074. [CrossRef]
- W. T. Piver, Organotin compounds: industrial applications and biological investigation. Environ Health Perspect., 4 (1973) 61-79. [CrossRef]
- J. J. Zuckerman, R. P. J. J. Zuckerman, R. P. Reisdorf, H. V. Ellis III, R. R. Wilkinson, Organotins in biology and the environment, Organometals and Organometalloids, Chapter 24 (1978) 388-424. [CrossRef]
- S. K. Hadjikakou, N. S. K. Hadjikakou, N. Hadjiliadis, Antiproliferative and anti-tumor activity of organotin compounds. Coordination Chemistry Reviews, 253(1-2) (2009) 235-249. [CrossRef]
- Y. Ellahioui, S. Y. Ellahioui, S. Prashar, S. Gomez-Ruiz, Anticancer applications and recent investigations of metallodrugs based on gallium, tin and titanium. Inorganics, 5(1) (2017) 4. [CrossRef]
- R. A. Alderden, M. D. R. A. Alderden, M. D. Hall, T. W. Hambley, The discovery and development of cisplatin. J. Chem. Educ., 83(5) (2006) 728-734. [CrossRef]
- Y. P. Ho, S. C. Y. P. Ho, S. C. Au-Yeung, K. K. To, Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med Res Rev. 23(5) (2003) 633-655. [CrossRef]
- M. Sirajuddin, S. M. Sirajuddin, S. Ali, Organotin (IV) carboxylates as promising potential drug candidates in the field of cancer chemotherapy. Curr Pharm Des., 22(44) (2016) 6665-6681. [CrossRef]
- C. N. Banti, S. K. C. N. Banti, S. K. Hadjikakou, T. Sismanoglu, N. Hadjiliadis, Anti-proliferative and antitumor activity of organotin (IV) compounds. An overview of the last decade and future perspectives. Journal of inorganic biochemistry, J Inorg Biochem, 194 (2019) 114-152. [CrossRef]
- K. Ovejero-Paredes, D. K. Ovejero-Paredes, D. Díaz-García, V. García-Almodóvar, L. Lozano Chamizo, M. Marciello, M. Díaz-Sánchez, M. Filice, Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers, 12(1) (2020) 187. [CrossRef]
- T. Anasamy, C. F. T. Anasamy, C. F. Chee, L. V. Kiew, L. Y. Chung, In vivo antitumour properties of tribenzyltin carboxylates in a 4T1 murine metastatic mammary tumour model: Enhanced efficacy by PLGA nanoparticles. Eur. J Pharm. Sci., 142 (2020) 105140. [CrossRef]
- R. Barbieri; L. Pellerito; G. Ruisi; M.T. Lo Giudice; F. Huber; G. Atassi, The antitumour activity of diorganotin(IV) complexes with adenine and glycylglycine. Inorganica Chimica Acta 66(3) (1982) L39–L40. [CrossRef]
- M. Nath, R. M. Nath, R. Yadav, G. Eng, P. Musingarimi, Characteristic spectral studies and in vitro Antimicrobial and in vivo multi-infection antifungal activities in mice of new organotin (IV) derivatives of heterocyclic amino acids. Applied organometallic chemistry, 13(1) (1999) 29-37. [CrossRef]
- F. Huber, G. F. Huber, G. Roge, L. Carl, G. Atassi, F. Spreafico, S. Filippeschi, F. Di Bianca, Studies on the anti-tumour activity of di-and tri-organotin (IV) complexes of amino acids and related compounds, of 2-mercaptoethanesulphonate, and of purine-6-thiol. Journal of the Chemical Society, Dalton Transactions, 3 (1985) 523-527. [CrossRef]
- F. Barbieri, M. F. Barbieri, M. Viale, F. Sparatore, G. Schettini, A. Favre, C. Bruzzo, A. Alama, Antitumor activity of a new orally active organotin compound: a preliminary study in murine tumor models. Anti-cancer drugs, 13(6) (2002) 599-604. [CrossRef]
- C. E. Carraher, A. C. E. Carraher, A. Battin, K. R. Shahi, M. R. Roner, Synthesis, structural characterization, and initial evaluation as anticancer drugs of dibutyltin polyamines derived from various 4, 6-diaminopyrimidines. Journal of Inorganic and Organometallic Polymers and Materials, 17(4) (2007) 631-639. [CrossRef]
- M. N. Shuaibu, H. M. N. Shuaibu, H. Kanbara, T. Yanagi, A. Ichinose, D. A. Ameh, J. J. Bonire, A. J. Nok, In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Parasitology research, 91(1) (2003) 5-11. [CrossRef]
- M. Sirajuddin, S. M. Sirajuddin, S. Ali, Synthesis and Characterization of Potential Bioactive Organotins: Azomethine Based Organotin (IV) Complexes, LAP Lambert Academic Publishing, (2013).
- N. Boussalah, R. N. Boussalah, R. Touzani, I. Bouabdallah, S. El Kadiri, S. Ghalem, Synthesis, structure and catalytic properties of tripodal amino-acid derivatized pyrazole-based ligands. Journal of Molecular Catalysis A: Chemical, 306(1-2) (2009) 113-117. [CrossRef]
- Titi, Abderrahim, Saud M. Almutairi, Rachid Touzani, Mouslim Messali, Monique Tillard, Belkheir Hammouti, Mohamed El Kodadi, Driss Eddike, Abdelkader Zarrouk, and Ismail Warad. J. Mol. Struct. 1236 (2021) 130304. [CrossRef]
- 26. Titi A, Shiga T, Oshio H, Touzani R. Synthesis of novel Cl2Co4L6 cluster using 1-hydroxymethyl-3, 5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. Journal of Molecular Structure, 2020.
- Titi A, Warad I, Tillard M, Messali M, Touzani R. Inermolecular interaction in [C6H10N3] 2 [CoCl4] complex: Synthesis, XRD/HSA relation, spectral and catecholase catalytic analysis. Journal of Molecular Structure (2020), 128422. [CrossRef]
- Titi, K. Zaidi, A. Y. A. Alzahrani, M. El Kodadi, E. B. Yousfi, A. Moliterni, B. Hammouti, R. Touzani, M. Abboud, New In Situ Catalysts Based on Nitro Functional Pyrazole Derivatives and Copper (II) Salts for Promoting Oxidation of Catechol to o-Quinone, Catalysts, 13(1) (2023) 162. [CrossRef]
- M. El Boutaybi, A. M. El Boutaybi, A. Titi, A. Y. A. Alzahrani, Z. Bahari, M. Tillard, B. Hammouti, R. Touzani, Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate, Catalysts 12(12) (2022) 1642. [CrossRef]
- Titi A, Oshio, Messali M, Touzani R, Warad I. Synthesis and XRD of Novel Ni 4 (µ 3-O) 4 Twist Cubane Cluster Using Three NNO Mixed Ligands: Hirshfeld, Spectral, Thermal and Oxidation Properties. Journal of Cluster Science (2020), 1-8. [CrossRef]
- Neves, L. M. Rossi, A. J. Bortoluzzi, B. Szpoganicz, C. Wiezbicki, E. Schwingel, S. Ostrovsky, Catecholase activity of a series of dicopper (II) complexes with variable Cu− OH (phenol) moieties. Inorganic chemistry, 41(7) (2002) 1788-1794. [CrossRef]
- Eicken, B. Krebs, J. C. Sacchettini, Catechol oxidase—structure and activity. Current opinion in structural biology, 9(6) (1999) 677-683. [CrossRef]
- Kupán, J. Kaizer, G. Speier, M. Giorgi, M. Réglier, F. Pollreisz, Molecular structure and catechol oxidase activity of a new copper (I) complex with sterically crowded monodentate N-donor ligand. Journal of inorganic biochemistry, 103(3) (2009) 389-395. [CrossRef]
- Guha, T. Chattopadhyay, N. D. Paul, M. Mukherjee, S. Goswami, T. K. Mondal, D. Das, Radical pathway in catecholase activity with zinc-based model complexes of compartmental ligands. Inorganic chemistry, 51(16) (2012) 8750-8759. [CrossRef]
- Mouadili, A. Attayibat, S. Kadiri, S. Radi, R.Touzani, Catecholase activity investigations using in situ copper complexes with pyrazole and pyridine based ligands. Applied Catalysis A: General, 454 (2013) 93-99. [CrossRef]
- M. Dahmani, A. Ettouhami, B. El Bali, A. Yahyi, C. Wilson, K. Ullah, R. Imad, S. Ullah, S. Wajid, F. Arshad, H. Elmsellem. Organotin (IV) derivative of Piperic acid and Phenylthioacetic acid: Synthesis, Crystal structure, Spectroscopic characterizations and Biological activities. Mor. J. Chem. 8(1) (2020) 244-263.
- M. Dahmani, A. M. Dahmani, A. Et-Touhami, A. Yahyi, T. Harit, D. Eddike, M. Tillard, R. Benabbes. Synthesis, characterization, X-ray structure and in vitro antifungal activity of triphenyltin complexes based on pyrazole dicarboxylic acid derivatives. Journal of Molecular Structure 1225 (2020) 129137. [CrossRef]
- M. Dahmani, T. M. Dahmani, T. Harit, A. Et-touhami, A. Yahyi, D. Eddike, M. Tillard, R. Benabbes, Two novel macrocyclic organotin (IV) carboxylates based on bipyrazoledicarboxylic acid derivatives: Syntheses, crystal structures and antifungal activities. Journal of Organometallic Chemistry. 948 (2021) 121913. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).