Submitted:
25 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; Culver, D.A.; Drake, W.; Drent, M.; Gerke, A.K.; Ghobrial, M.; Govender, P.; Hamzeh, N.; James, W.E.; Judson, M.A.; Kellermeyer, L.; Knight, S.; Koth, L.L.; Poletti, V.; Raman, S.V.; Tukey, M.H.; Westney, G.E.; Baughman, R.P. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Franzen, D.P.; Brutsche, M.; Nilsson, J.; Böni, C.; Daccord, C.; Distler, O.; Elsener, D.; Funke-Chambour, M.; Gruner, C.; Hayward-Könnecke, H.; Hostettler, K.E.; Kündig, T.; Ribi, C.; Seebach, J.D.; Seeger, H.; Vrugt, B.; Kolios, A.G.A. Sarcoidosis - a multisystem disease. Swiss Med Wkly. 2022, 152, w30049. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A.; Judson, M.A. New advances in the management of pulmonary sarcoidosis. BMJ. 2019, 367, l5553. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. N Engl J Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Saleem, M.I.; Wall, D.; Gilmartin, J.J.; Rutherford, R.M.; O'Regan, A. Predictive value of C-reactive protein and clinically relevant baseline variables in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016, 33, 331–340. [Google Scholar] [PubMed]
- Zhou, Y.; Zhang, Y.; Zhao, M.; Li, Q.; Li, H. sIL-2R levels predict the spontaneous remission in sarcoidosis. Respir Med. 2020, 171, 106115. [Google Scholar] [CrossRef]
- Belhomme, N.; Jouneau, S.; Bouzillé, G.; Decaux, O.; Lederlin, M.; Guillot, S.; Perlat, A.; Jégo, P. Role of serum immunoglobulins for predicting sarcoidosis outcome: A cohort study. PLoS One. 2018, 13, e0193122. [Google Scholar] [CrossRef]
- Bergantini, L.; Bianchi, F.; Cameli, P.; Mazzei, M.A.; Fui, A.; Sestini, P.; Rottoli, P.; Bargagli, E. Prognostic Biomarkers of Sarcoidosis: A Comparative Study of Serum Chitotriosidase, ACE, Lysozyme, and KL-6. Dis Markers. 2019, 2019, 8565423. [Google Scholar] [CrossRef]
- Ishiyama, M.; Soine, L.A.; Vesselle, H.J. Semi-quantitative metabolic values on FDG PET/CT including extracardiac sites of disease as a predictor of treatment course in patients with cardiac sarcoidosis. EJNMMI Res. 2017, 7, 67. [Google Scholar] [CrossRef]
- Benamore, R.; Kendrick, Y.R.; Repapi, E.; Helm, E.; Cole, S.L.; Taylor, S.; Ho, L.P. CTAS: a CT score to quantify disease activity in pulmonary sarcoidosis. Thorax. 2016, 71, 1161–1163. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Janssen Bonás, M.; Vorselaars, A.D.M.; Veltkamp, M. Biomarkers in the Diagnosis and Prognosis of Sarcoidosis: Current Use and Future Prospects. Front Immunol. 2020, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Publisher Correction: Sarcoidosis. Nat Rev Dis Primers. 2019, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Kobak, S. Catch the rainbow: Prognostic factor of sarcoidosis. Lung India. 2020, 37, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A. The ability to predict the clinical course of pulmonary sarcoidosis from data that is right in front of us. J Bras Pneumol. 2022, 48, e20220012. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zou, L.; Wang, S.; Zeng, T.; Li, P.; Shen, Y.; Chen, L. Performance of Serum Angiotensin-Converting Enzyme in Diagnosing Sarcoidosis and Predicting the Active Status of Sarcoidosis: A Meta-Analysis. Biomolecules. 2022, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Zinchenko, Y.; Starshinova, A.; Kudlay, D.; Kudryavtsev, I.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Sarcoidosis: Progression to the chronic stage and pathogenic based treatment (narrative review). Front Med (Lausanne). 2022, 9, 963435. [Google Scholar] [CrossRef]
- Aleksonienė, R.; Zeleckienė, I.; Matačiūnas, M.; Puronaitė, R.; Jurgauskienė, L.; Malickaitė, R.; Strumilienė, E.; Gruslys, V.; Zablockis, R.; Danila, E. Relationship between radiologic patterns, pulmonary function values and bronchoalveolar lavage fluid cells in newly diagnosed sarcoidosis. J Thorac Dis. 2017, 9, 88–95. [Google Scholar] [CrossRef]
- Aleksonienė, R.; Besusparis, J.; Gruslys, V.; Jurgauskienė, L.; Laurinavičienė, A.; Laurinavičius, A.; Malickaitė, R.; Norkūnienė, J.; Zablockis, R.; Žurauskas, E.; Danila, E. CD31+, CD38+, CD44+, and CD103+ lymphocytes in peripheral blood, bronchoalveolar lavage fluid and lung biopsy tissue in sarcoid patients and controls. J Thorac Dis. 2021, 13, 2300–2318. [Google Scholar] [CrossRef]
- Costabel, U.; Hunninghake, G.W. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999, 14, 735–737. [Google Scholar] [CrossRef]
- Erdal, B.S.; Crouser, E.D.; Yildiz, V.; King, M.A.; Patterson, A.T.; Knopp, M.V.; Clymer, B.D. Quantitative computerized two-point correlation analysis of lung CT scans correlates with pulmonary function in pulmonary sarcoidosis. Chest. 2012, 142, 1589–1597. [Google Scholar] [CrossRef]
- Simmering, J.; Stapleton, E.M.; Polgreen, P.M.; Kuntz, J.; Gerke, A.K. Patterns of medication use and imaging following initial diagnosis of sarcoidosis. Respir Med. 2021, 189, 106622. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.J.; Khalid, U.; Abu Bakar, M.; Butt, F.M.; Ashraf, MB. Sarcoidosis: epidemiology, characteristics, and outcomes over 10 years - a single-center study in Pakistan. Expert Rev Respir Med. 2022, 16, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Casal, A.; Suárez-Antelo, J.; Soto-Feijóo, R.; Ferreiro, L.; Rodríguez-Núñez, N.; Lama, A.; Riveiro, V.; Toubes, M.E.; Lourido, T.; Ricoy, J.; Rábade, C.; Zamarrón, C.; Rodríguez, C.; Abelleira, R.; Álvarez-Dobaño, J.M.; Golpe, A.; de Alegría, A.M.; Antúnez, J.R.; Gude, F.; Valdés, L. Sarcoidosis. Disease progression based on radiological and functional course: Predictive factors. Heart Lung. 2022, 56, 62–69. [Google Scholar] [CrossRef]
- Schimmelpennink, M.C.; Meek, D.B.; Vorselaars, A.D.M.; Langezaal, L.C.M.; van Moorsel, C.H.M.; van der Vis, J.J.; Veltkamp, M.; Grutters, J.C. Characterization of the PF-ILD phenotype in patients with advanced pulmonary sarcoidosis. Respir Res. 2022, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.A.; Strek, M.E.; Patterson, K.C. From granuloma to fibrosis: sarcoidosis associated pulmonary fibrosis. Curr Opin Pulm Med. 2016, 22, 484–91. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, A.R.; Chew, T.W.; Chlipala, E.; Harbord, M.W.N.; Muller, W.A. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol. 2006, 81, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Marelli-Berg, F.M.; Clement, M.; Mauro, C.; Caligiuri, G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci 2013, 126, 2343–2352. [Google Scholar] [CrossRef]
- Lishnevsky, M.; Young, L.C.; Woods, S.J.; Groshong, S.D.; Basaraba, R.J.; Gilchrist, J.M.; Higgins, D.M.; Gonzalez-Juarrero, M.; Bass, T.A.; Muller, W.A.; Schenkel, A.R. Microhemorrhage is an early event in the pulmonary fibrotic disease of PECAM-1 deficient FVB/n mice. Exp Mol Pathol. 2014, 97, 128–136. [Google Scholar] [CrossRef]
- Guedes, A.G.; Jude, J.A.; Paulin, J.; Rivero-Nava, L.; Kita, H.; Lund, F.E.; Kannan, M.S. Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras. Am J Physiol Lung Cell Mol Physiol. 2015, 308, L485–L493. [Google Scholar] [CrossRef]
- Newman, D.K.; Fu, G.; McOlash, L.; Schauder, D.; Newman, P.J.; Cui, W.; Rao, S.; Johnson, B.D.; Gershan, J.A.; Riese, M.J. PECAM-1 (CD31) expression in naïve and memory, but not acutely activated, CD8+ T cells. J Leukoc Biol. 2018, 104, 883–893. [Google Scholar] [CrossRef]
- Ziora, D.; Jastrzębski, D.; Adamek, M.; Czuba, Z.; Kozielski, J.J.; Grzanka, A.; Kasperska-Zajac, A. Circulating concentration of markers of angiogenic activity in patients with sarcoidosis and idiopathic pulmonary fibrosis. BMC Pulm Med. 2015, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Van Seventer, G.A.; Siraganian, R.; Wahl, L.; Shaw, S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989, 143, 2457–63. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol Pathol. 1999, 52, 189–96. [Google Scholar] [CrossRef] [PubMed]
- Rivera, N.V.; Hagemann-Jensen, M.; Ferreira, M.A.R.; Kullberg, S.; Eklund, A.; Martin, N.G.; Padyukov, L.; Grunewald, J. Common variants of T-cells contribute differently to phenotypic variation in sarcoidosis. Sci Rep. 2017, 7, 5623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Long, M.; Yuan, M.; Yin, J.; Luo, W.; Wang, S.; Cai, Y.; Jiang, W.; Chao, J. Macrophage-derived GPNMB trapped by fibrotic extracellular matrix promotes pulmonary fibrosis. Commun Biol. 2023, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Culty, M.; O’Mara, T.E.; Underhill, C.B.; Yeager, H. Jr.; Swartz, R.P. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukoc Biol. 1994, 56, 605–11. [Google Scholar] [CrossRef] [PubMed]
- d'Alessandro, M.; Carleo, A.; Cameli, P.; Bergantini, L.; Perrone, A.; Vietri, L.; Lanzarone, N.; Vagaggini, C.; Sestini, P.; Bargagli, E. BAL biomarkers' panel for differential diagnosis of interstitial lung diseases. Clin Exp Med. 2020, 20, 207–16. [Google Scholar] [CrossRef]
- Sung, S.S.; Fu, S.M.; Rose, C.E. Jr.; Gaskin, F.; Ju, S.T.; Beaty, S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006, 176, 2161–72. [Google Scholar] [CrossRef]
- Beauchamp, N.M.; Yammani, R.D.; Alexander-Miller, M.A. CD8 marks a subpopulation of lung-derived dendritic cells with differential responsiveness to viral infection and toll-like receptor stimulation. J Virol. 2012, 86, 10640–50. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014, 41, 633–45. [Google Scholar] [CrossRef]
- Bernatchez, E.; Gold, M.J.; Langlois, A.; Lemay, A.M.; Brassard, J.; Flamand, N.; Marsolais, D.; McNagny, K.M.; Blanchet, MR. Pulmonary CD103 expression regulates airway inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2015, 308, L816–L826. [Google Scholar] [CrossRef] [PubMed]
- McMaster, S.R.; Wein, A.N.; Dunbar, P.R.; Hayward, S.L.; Cartwright, E.K.; Denning, T.L.; Kohlmeier, J.E. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. 2018, 11, 1071–8. [Google Scholar] [CrossRef]
- Wu, H.; Liao, W.; Li, Q.; Long, H.; Yin, H.; Zhao, M.; Chan, V.; Lau, C.S.; Lu, Q. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun Rev. 2018, 17, 906–911. [Google Scholar] [CrossRef] [PubMed]
- d'Alessandro, M.; Gangi, S.; Cavallaro, D.; Bergantini, L.; Mezzasalma, F.; Cattelan, S.; Baglioni, S.; Abbritti, M.; Cameli, P.; Bargagli, E. CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life (Basel). 2022, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Helmers, R.A.; Dayton, C.S.; Floerchinger, C.; Hunninghake, G.W. Bronchoalveolar lavage in interstitial lung disease: effect of volume of fluid infused. J Appl Physiol (1985). 1989, 67, 1443–6. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Itoh, H.; Kitaichi, M.; Nagai, S.; Izumi, T. Pulmonary sarcoidosis: correlation of CT and histopathologic findings. Radiology. 1993, 189, 105–9. [Google Scholar] [CrossRef] [PubMed]
- Criado, E.; Sánchez, M.; Ramírez, J.; Arguis, P.; de Caralt, T.M.; Perea, R.J.; Xaubet, A. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010, 30, 1567–86. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.G.; van den Heuvel, D.A.; Grutters, J.C. Imaging the inflammatory activity of sarcoidosis. Eur Respir J. 2013, 41, 743–51. [Google Scholar] [CrossRef]
- Distefano, G.; Vancheri, A.; Palermo, M.; Tiralongo, F.; Foti, P.V.; Mauro, L.A.; Vancheri, C.; Basile, A.; Palmucci, S. Morphological Patterns of Sarcoidosis and Clinical Outcome: Retrospective Analysis through a Multidisciplinary Approach. Diagnostics (Basel). 2020, 10, 212. [Google Scholar] [CrossRef]
- Polverosi, R.; Russo, R.; Coran, A.; Battista, A.; Agostini, C.; Pomerri, F.; Giraudo, C. Typical and atypical pattern of pulmonary sarcoidosis at high-resolution CT: relation to clinical evolution and therapeutic procedures. Radiol Med. 2014, 119, 384–92. [Google Scholar] [CrossRef]
- Duan, J.; Xu, Y.; Zhu, H.; Zhang, H.; Sun, S.; Sun, H.; Wang, W.; Xie, S. Relationship between CT activity score with lung function and the serum angiotensin converting enzyme in pulmonary sarcoidosis on chest HRCT. Medicine (Baltimore). 2018, 97, e12205. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Saleem, M.I.; Wall, D.; Gilmartin, J.J.; Rutherford, R.M.; O'Regan, A. Predictive value of C-reactive protein and clinically relevant baseline variables in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016, 33, 331–340. [Google Scholar] [PubMed]
- Silva, A.L.; Melo, N.; Caetano Mota, P.; Lima, B.; Pereira, J.M.; Cunha, R.; Guimarães, S.; Souto-Moura, C.; Morais, A. Pulmonary Sarcoidosis: Prognostic Factors at Diagnosis in Patients from North of Portugal. Reumatol Clin (Engl Ed). 2020, 16, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kirkil, G.; Lower, E.E.; Baughman, R.P. Predictors of Mortality in Pulmonary Sarcoidosis. Chest. 2018, 153, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Danila, E.; Jurgauskiene, L.; Malickaite. R. BAL fluid cells and pulmonary function in different radiographic stages of newly diagnosed sarcoidosis. Adv Med Sci. 2008, 53, 228–33. [Google Scholar] [CrossRef]
- Danila, E.; Zurauskas, E.; Loskutoviene, G.; Zablockis, R.; Nargela, R.; Birzietyte, V.; Valentinaviciene, G. Significance of bronchoscopic lung biopsy in clinical practice. Adv Med Sci. 2008, 53, 11–6. [Google Scholar] [CrossRef]
- Danila, E.; Zurauskas, E. Diagnostic value of epithelioid cell granulomas in bronchoscopic biopsies. Intern Med. 2008, 47, 2121–6. [Google Scholar] [CrossRef]
- Danila, E.; Jurgauskiene, L.; Norkuniene, J.; Malickaite, R. BAL fluid cells in newly diagnosed pulmonary sarcoidosis with different clinical activity. Ups J Med Sci. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Danila, E.; Norkūniene, J.; Jurgauskiene, L.; Malickaite, R. Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clin Respir J. 2009, 3, 214–21. [Google Scholar] [CrossRef]
- Pocienė, I.; Gauronskaitė, R.; Galkauskas, D.; Mainelis, A.; Gruslys, V.; Danila, E. Age as a Risk Factor in the Occurrence of Complications during or after Bronchoscopic Lung Biopsy. Geriatrics (Basel). 2022, 7, 34. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Ozturk, O.G.; Durmaz, A.; Othman Hasan, O.; Guzelbaba, B.; Seydaoglu, G.; Kuleci, S.; Hanta, I.; Erken, E.; Kocabas, A. Early prediction of sarcoidosis prognosis with HLA typing: a 5 year follow-up study. Sarcoidosis Vasc Diffuse Lung Dis. 2018, 35, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Soine, L.A.; Vesselle, H.J. Semi-quantitative metabolic values on FDG PET/CT including extracardiac sites of disease as a predictor of treatment course in patients with cardiac sarcoidosis. EJNMMI Res. 2017, 7, 67. [Google Scholar] [CrossRef]
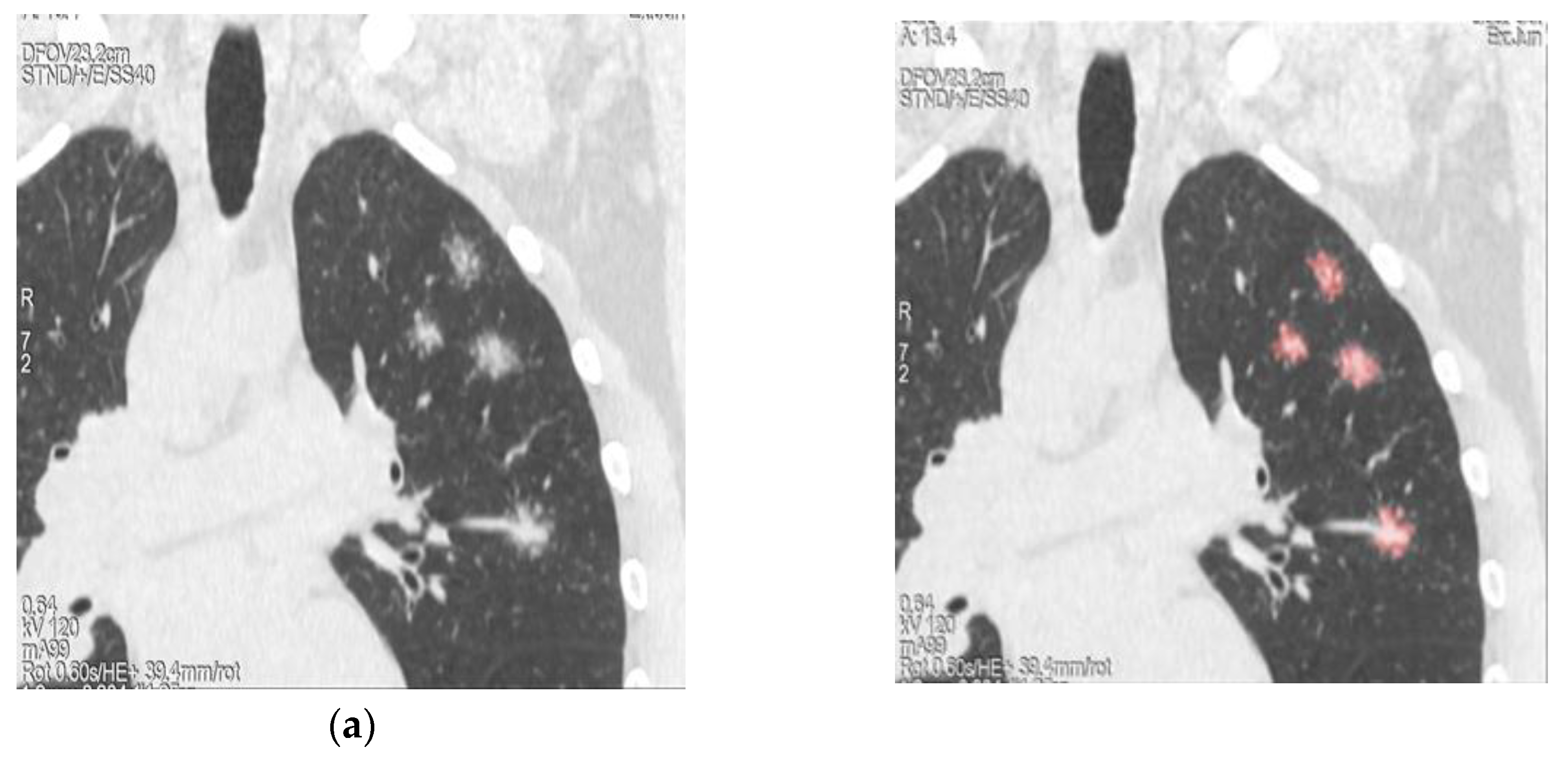
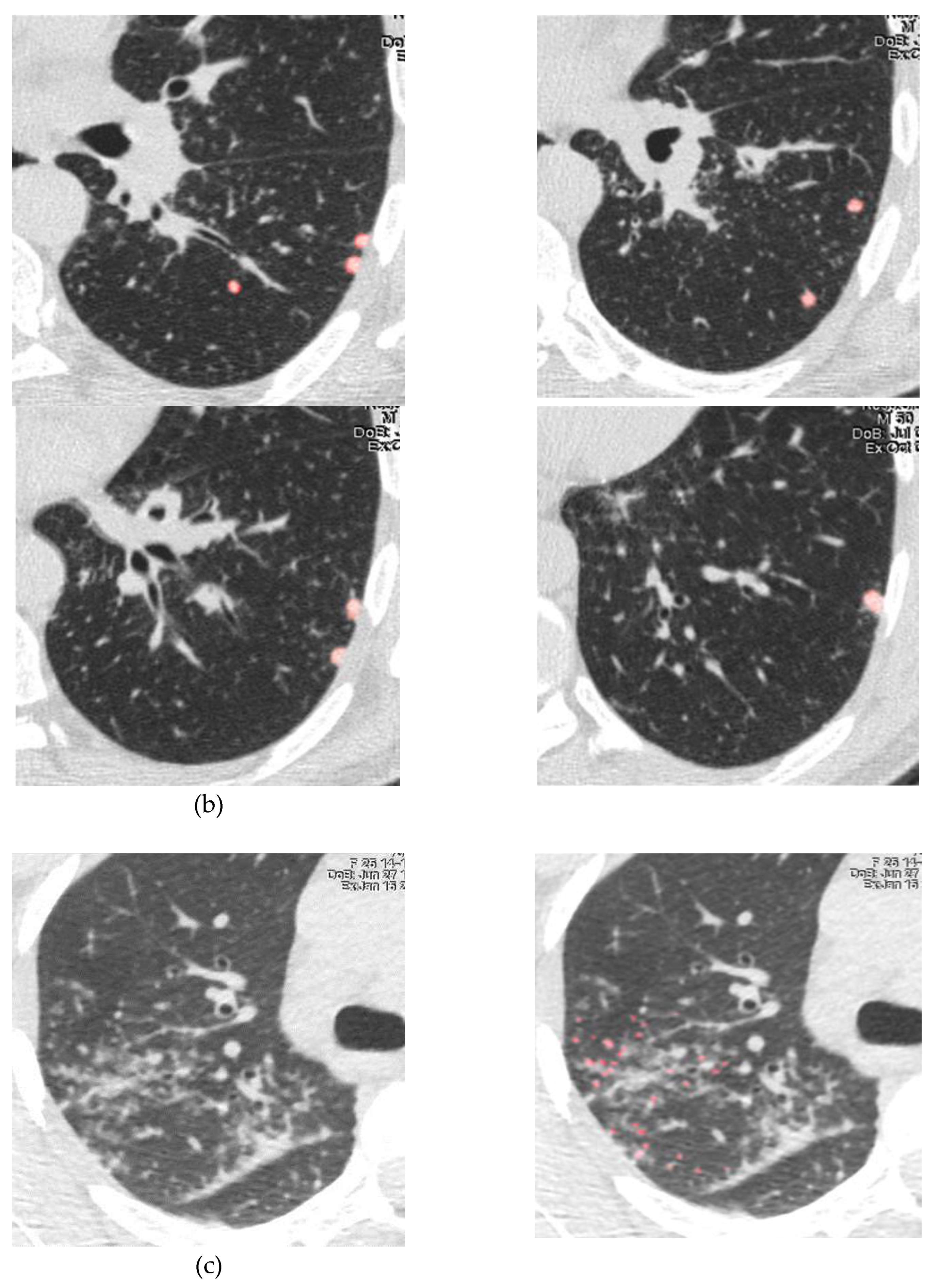
| Demographics | Sarcoidosis patients (n=71) |
|---|---|
| Sex (male/female) | 38/33 |
| Age (years) | 37 (21–68) |
| Löfgren syndrome (yes/no) | 27/44 |
| Smoker (yes/never) | 25/46 |
| FVC, % pred | 104±15 |
| FEV1, % pred | 97±13 |
| FEV1/FVC, % | 79±6 |
| TLC, % pred | 99±12 |
| VC, % pred | 106±14 |
| RV, % pred | 90±21 |
| DLCO, % pred | 76±11 |
| BALF total cells count, x 106/mL | 375±192 |
| BALF macrophages, % | 60.8±19.2 |
| BALF lymphocytes, % | 38.4±19.2 |
| BALF neutrophils, % | 0.5±0.8 |
| BALF eosinophils, % | 0.2±0.3 |
| BALF CD4, % | 69.9±17.7 |
| BALF CD8, % | 18.8±13.3 |
| BALF CD4+/CD8+ | 6.1±4.8 |
| Cells | Blood (n=71) |
BALF (n=71) |
|---|---|---|
| CD4+, % | 41.1±8.5 | 69.9±17.7 |
| CD8+, % | 27.1±9.0 | 18.8±13.3 |
| CD4+/CD8+ | 1.7±0.7 | 6.1±4.8 |
| CD31+CD4+, % | 12.5±6.5 | 5.9±4.5 |
| CD38+CD4+, % | 23.4±9.1 | 24.0±14.1 |
| CD44+CD4+, % | 45.6±9.9 | 75.7±13.4 |
| CD103+CD4+, % | 2.3±6.9 | 8.7±8.2 |
| CD31+CD8+, % | 19.1±7.7 | 10.1±8.5 |
| CD38+CD8+, % | 20.3±7.4 | 5.9±6.5 |
| CD44+CD8+, % | 38.8±11.1 | 20.9±12.5 |
| CD103+CD8+, % | 3.7±4.7 | 13.3±11.3 |
| Cells | Sarcoidosis (n=35) |
|---|---|
| CD4+, total | 7375±8391 |
| CD8+, total | 3873±7067 |
| CD38+, total | 2803.4±5167 |
| CD44+, total | 10322±8094 |
| CD103+, total | 1532±1589 |
| CD4+, % | 19.1±11.7 |
| CD8+, % | 8.1±6.3 |
| CD38+, % | 6.0±6.2 |
| CD44+, % | 27.2±10.3 |
| CD103+, % | 4.3±3.0 |
| CD4+ density, mm2 | 705±519 |
| CD8+ density, mm2 | 315±269 |
| CD38+ density, mm2 | 235±266 |
| CD44+ density, mm2 | 1002±502 |
| CD103+ density, mm2 | 158±118 |
| Collagen, % | 20.2±7.4 |
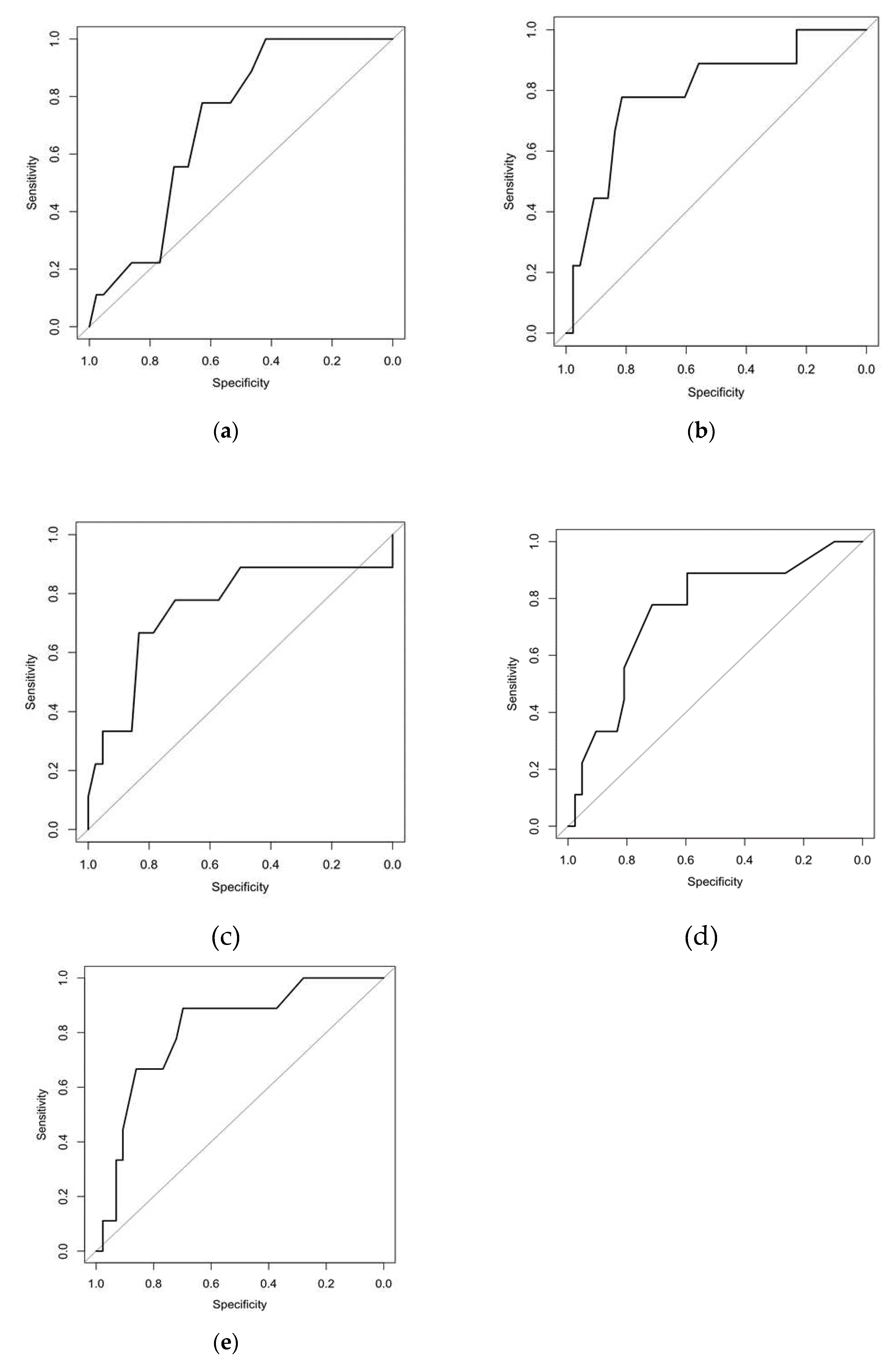
| Criteria | Cut-off | Sp | Sn | AUC | AUC (CI 95%) | OR | CI 95% | P value |
|---|---|---|---|---|---|---|---|---|
| CD4+CD31+ blood, % | ≤14.5 | 0.419 | 1.000 | 0.708 | 0.555; 0.861 | 13.78 | 0.75; 252.06 | 0.020 |
| CD4+CD44+ blood, % | ≤37.5 | 0.814 | 0.778 | 0.795 | 0.622; 0.968 | 15.31 | 2.66; 88.04 | <0.001 |
| CD8+CD31+ BALF, % | ≥13.5 | 0.833 | 0.667 | 0.751 | 0.536; 0.967 | 10.00 | 2.01; 49.83 | 0.010 |
| CD8+CD103+ BALF, % | ≥15.5 | 0.714 | 0.778 | 0.754 | 0.574; 0.933 | 8.75 | 1.59; 48.29 | 0.010 |
| Number of lung nodules | ≥15.0 | 0.698 | 0.889 | 0.810 | 0.658; 0.962 | 18.46 | 2.09; 163.05 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





