Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
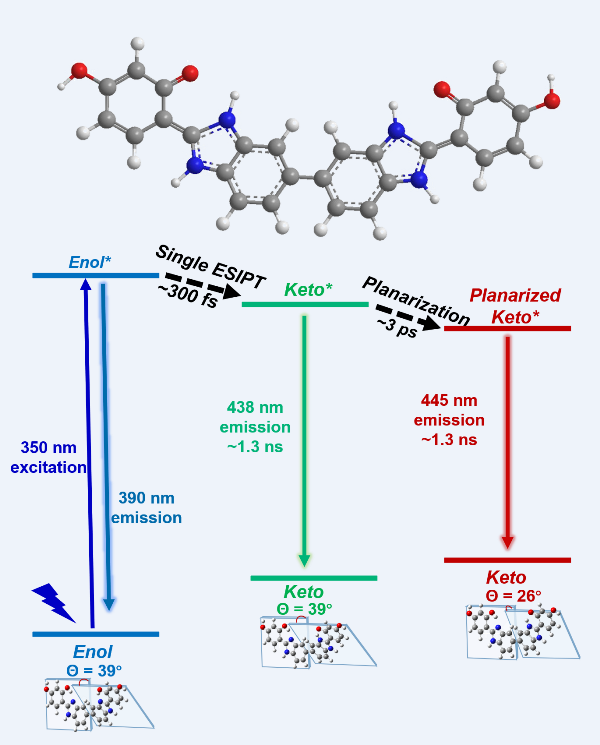
2. Results and Discussion
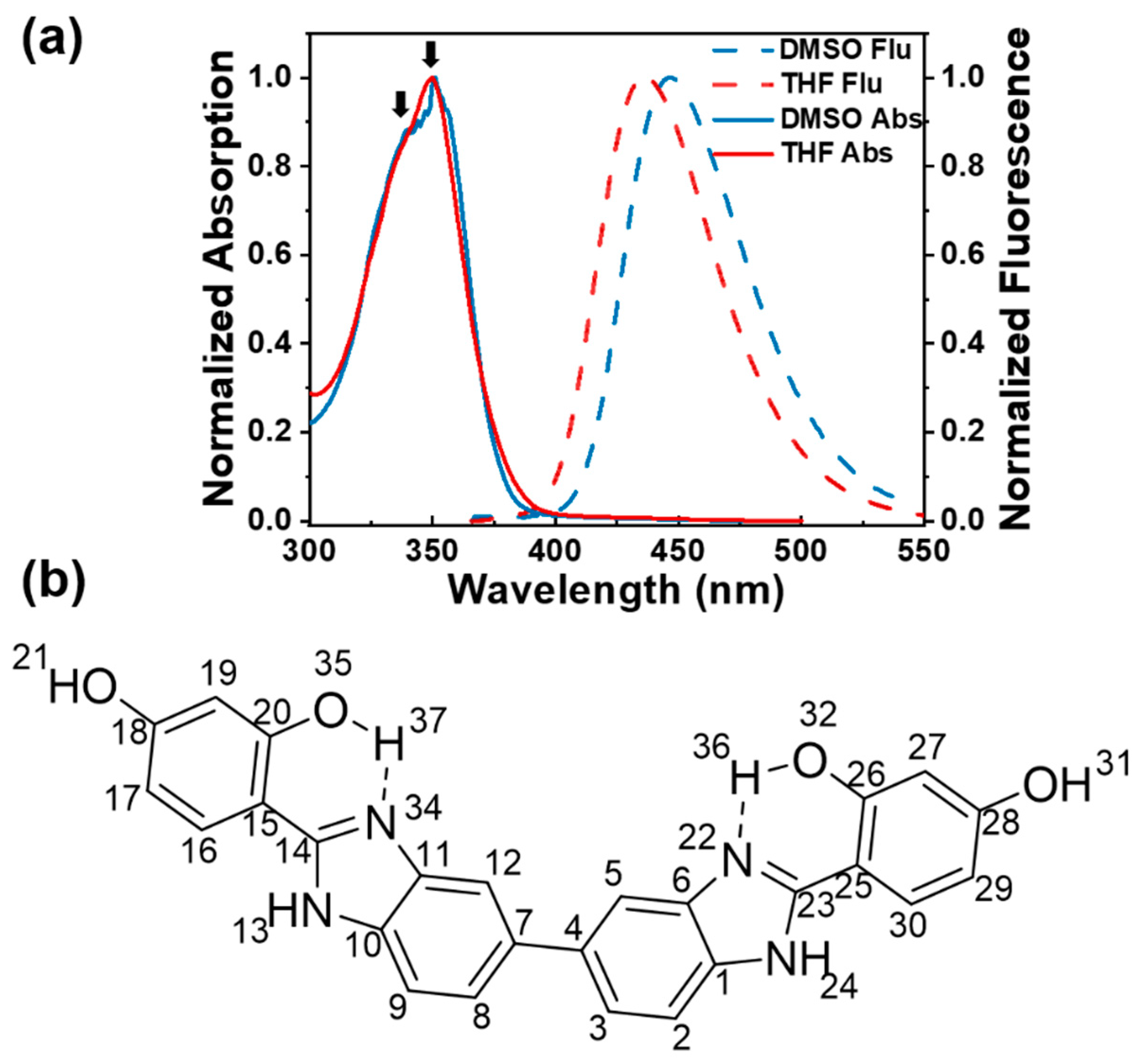
2.1. Steady-State Absorption and Fluorescence Spectroscopy
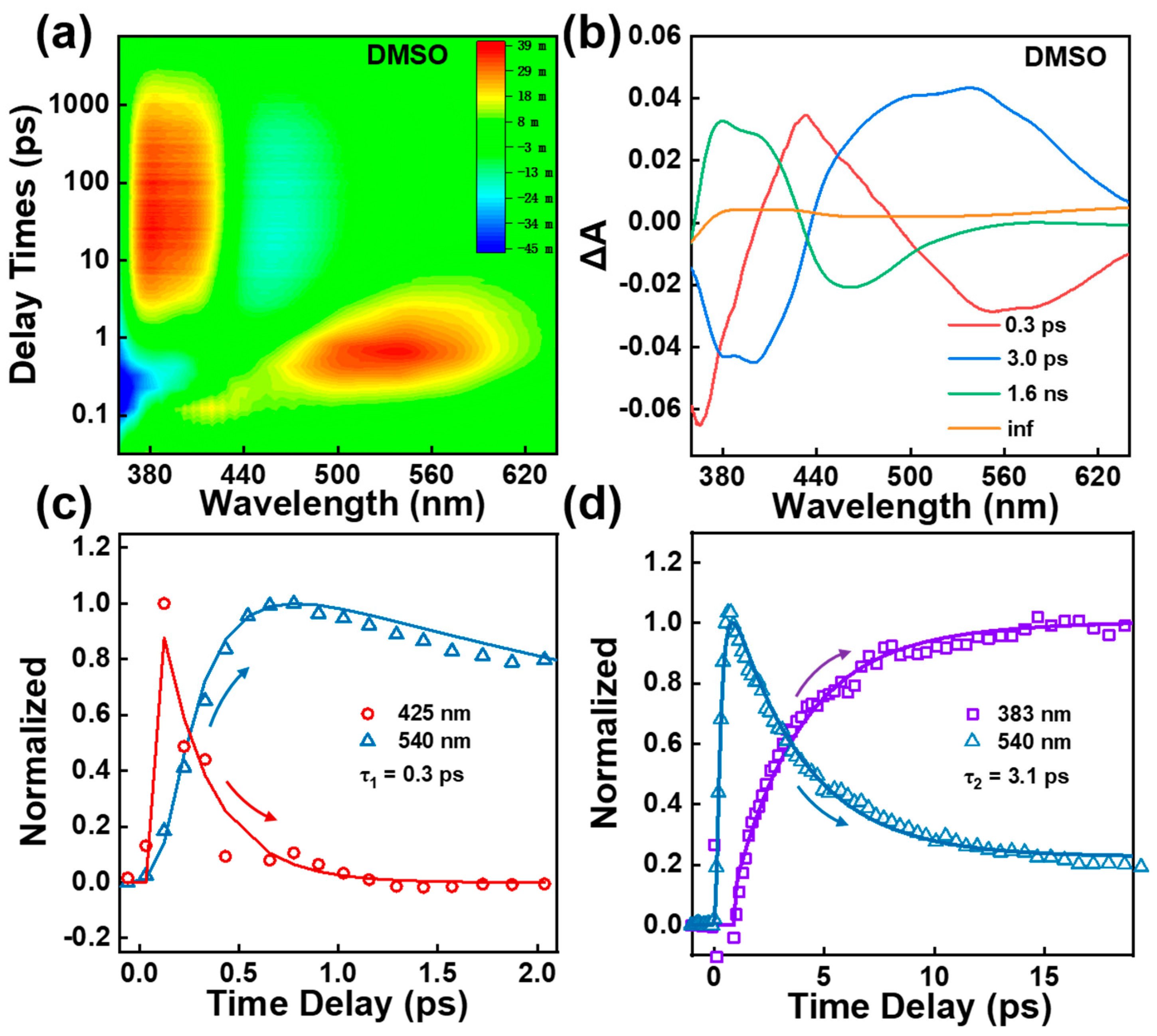
2.2. Femtosecond Transient Absorption Spectroscopy
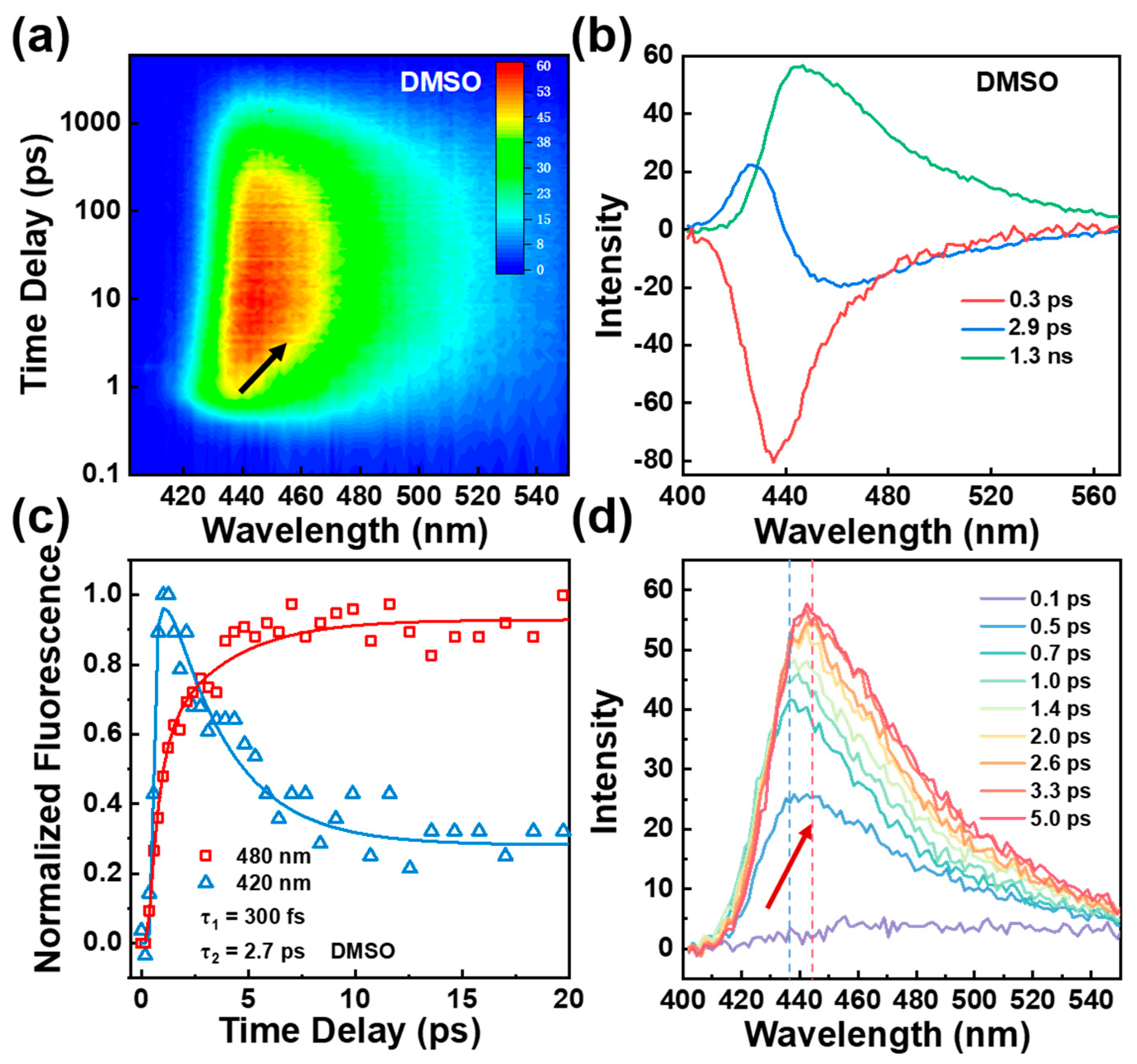
2.3. Femtosecond Fluorescence Up-Conversion Spectroscopy
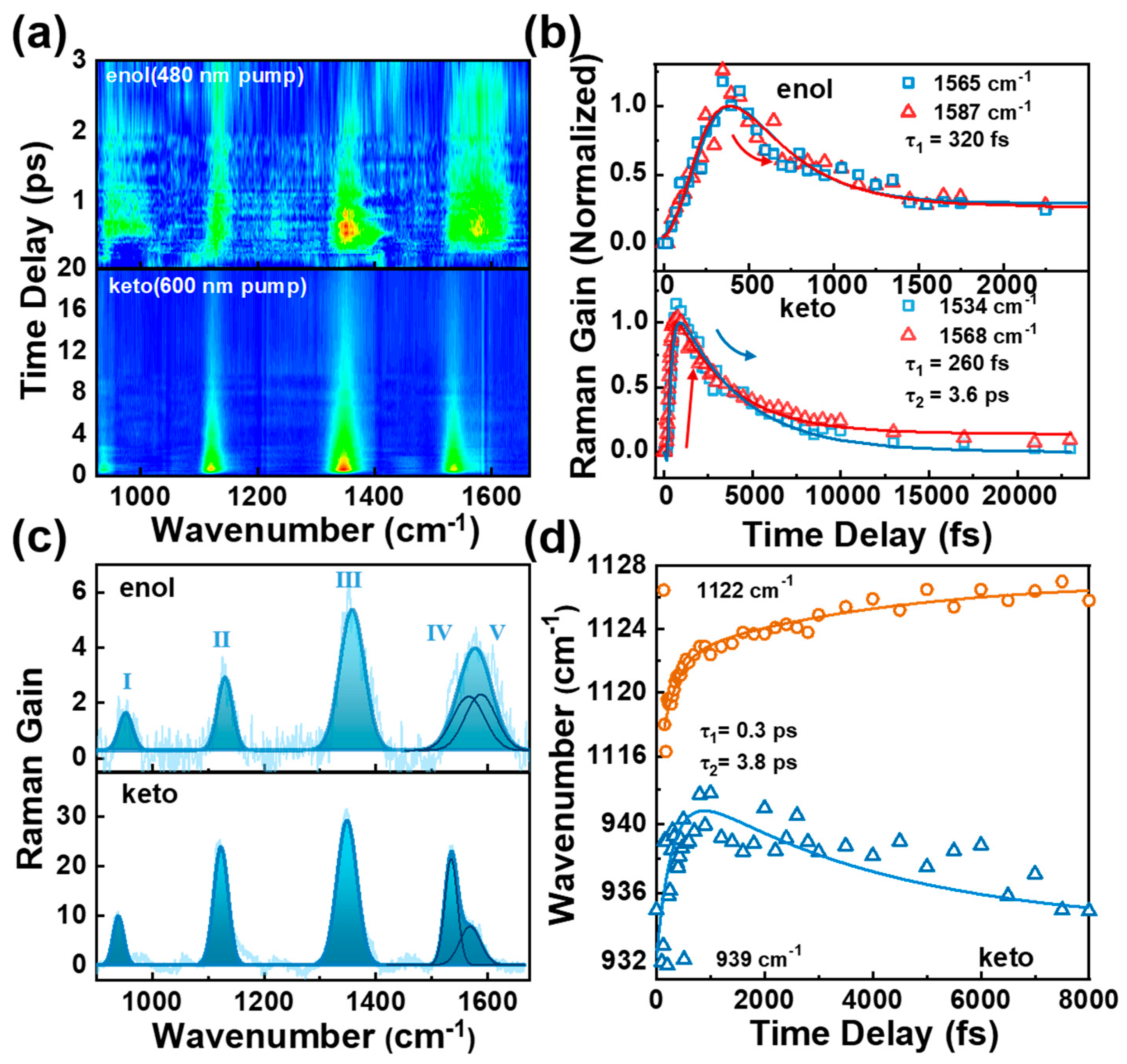
2.4. Femtosecond Raman Stimulated Spectroscopy
3. Materials and Methods
3.1. Synthesis of BBM
3.2. Transient Absorption Spectroscopy
3.3. Time-Resolved Up-Conversion Fluorescence Spectroscopy
3.4. Femtosecond Stimulated Raman Spectroscopy (FSRS)
3.5. Calculations
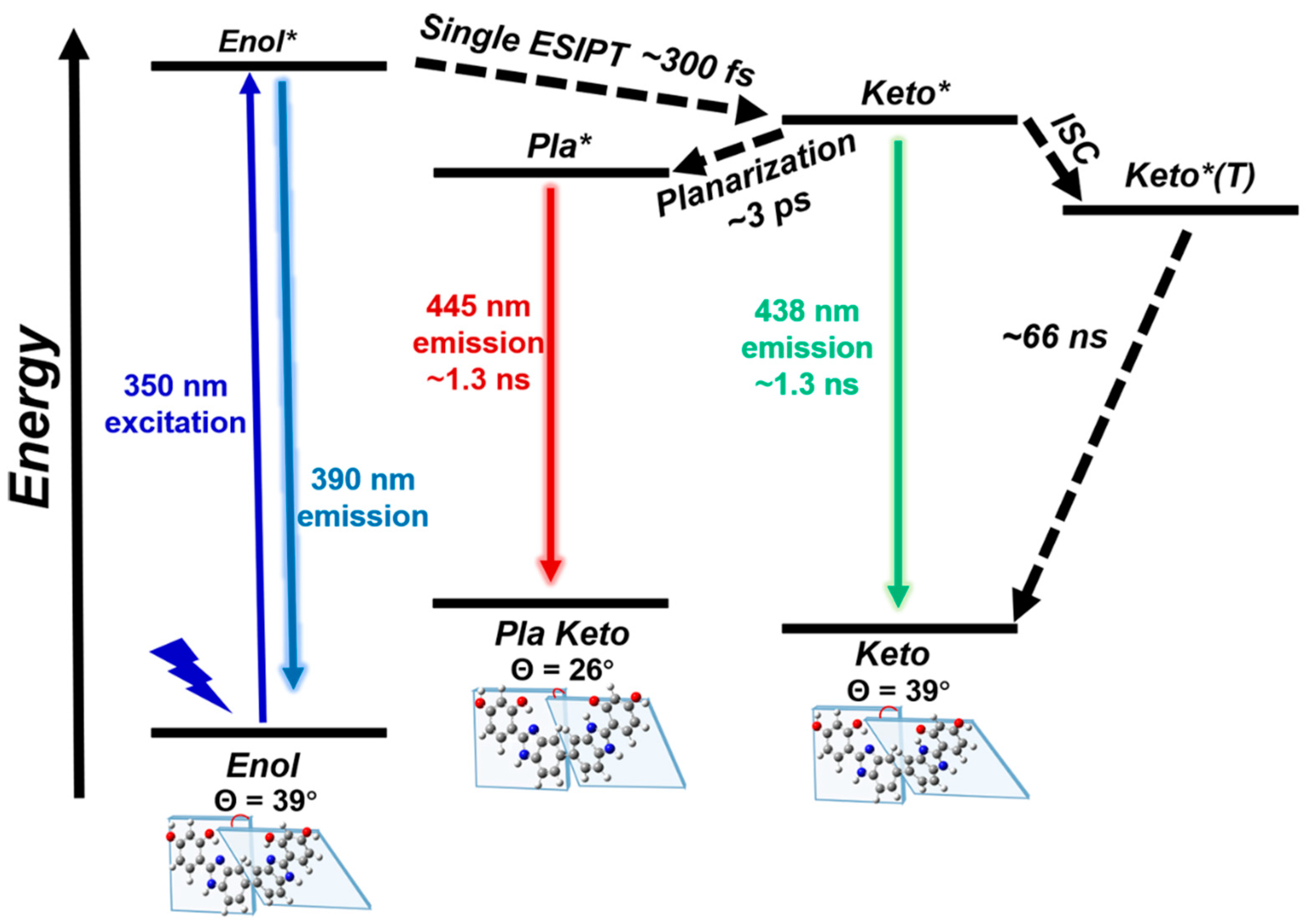
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henary, M.M.; Wu, Y.; Fahrni, C.J. Zinc(II)-selective ratiometric fluorescent sensors based on inhibition of excited-state intramolecular proton transfer. Chem. Eur. J. 2004, 10, 3015–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Dou, B.; Wright, B.; Wang, J.; Pang, Y. Zn2+ binding-enabled excited state intramolecular proton transfer: a step toward new near-infrared fluorescent probes for imaging applications. Adv. Healthcare Mater. 2012, 1, 485–92. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, S.; You, Y.; Baek, K.H.; Ohkubo, K.; Cho, J.; Fukuzumi, S.; Shin, I.; Park, S.Y.; Nam, W. Fluorescent zinc sensor with minimized proton-induced interferences: photophysical mechanism for fluorescence turn-on response and detection of endogenous free zinc ions. Inorg. Chem. 2012, 51, 8760–74. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, K.; Mori, S.; Shinmei, K.; Iwase, H.; Nakano, Y.; Fujii, Y. Investigation of substitution effect on fluorescence properties of Zn2+ elective ratiometric fluorescent compounds: 2-(2’-Hydroxyphenyl)benzimidazole derivatives. Talanta 2016, 146, 575–84. [Google Scholar] [CrossRef] [PubMed]
- Bag, R.; Sikdar, Y.; Sahu, S.; Das Mukhopadhyay, C.; Drew, M.G. B.; Goswami, S. Benzimidazole based ESIPT active chemosensors enable nano–molar detection of Cu2+ in 90% aqueous solution, MCF–7 cells, and plants. J. Photochem. Photobiol. A 2022, 431. [Google Scholar] [CrossRef]
- Barbatti, M.; Aquino, A.J.; Lischka, H.; Schriever, C.; Lochbrunner, S.; Riedle, E. Ultrafast internal conversion pathway and mechanism in 2-(2’-hydroxyphenyl)benzothiazole: a case study for excited-state intramolecular proton transfer systems. PCCP 2009, 11, 1406–15. [Google Scholar] [CrossRef] [PubMed]
- de Vivie-Riedle, R.; De Waele, V.; Kurtz, L.; Riedle, E. Ultrafast excited-state proton transfer of 2-(2’-hydroxyphenyl)benzothiazole: Theoretical analysis of the skeletal deformations and the active vibrational modes. J. Phys. Chem. A 2003, 107, 10591–10599. [Google Scholar] [CrossRef]
- Lochbrunner, S.; Wurzer, A.J.; Riedle, E. Ultrafast excited-state proton transfer and subsequent coherent skeletal motion of 2-(2’-hydroxyphenyl)benzothiazole. J. Chem. Phys. 2000, 112, 10699–10702. [Google Scholar] [CrossRef]
- Aly, S.M.; Usman, A.; AlZayer, M.; Hamdi, G.A.; Alarousu, E.; Mohammed, O.F. Solvent-dependent excited-state hydrogen transfer and intersystem crossing in 2-(2’-hydroxyphenyl)-benzothiazole. J. Phys. Chem. B 2015, 119, 2596–603. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Abou-Zied, O.K.; Yu, C.; Romesberg, F.E.; Glasbeek, M. Femtosecond fluorescence upconversion studies of excited-state proton-transfer dynamics in 2-(2 ‘-hydroxyphenyl)benzoxazole (HBO) in liquid solution and DNA. Chem. Phys. Lett. 2003, 367, 599–608. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Seo, J.; Park, S.Y.; Joo, T. Excited State Intramolecular Proton Transfer and Charge Transfer Dynamics of a 2-(2 ‘-Hydroxyphenyl)benzoxazole Derivative in Solution. J. Phys. Chem. A 2010, 114, 5618–5629. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.R.; Rodriguez, M.C. R.; Mosquera, M.; Rodriguez-Prieto, F. Excited-state intramolecular proton transfer in 2-(3’-hydroxy-2’-pyridyl)benzoxazole. Evidence of coupled proton and charge transfer in the excited state of some o-hydroxyarylbenzazoles. J. Phys. Chem. A 2007, 111, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.R.; Rodriguez, M.C. R.; Mosquera, M.; Rodriguez-Prieto, F. Rotamerism, tautomerism, and excited-state intramolecular proton transfer in 2-(4’-N,N-diethylamino-2’-hydroxyphenyl)benzimidazoles: Novel benzimidazoles undergoing excited-state intramolecular coupled proton and charge transfer. J. Phys. Chem. A 2008, 112, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.; Laermer, F.; Elsaesser, T. Femtosecond Studies of Excited-State Proton and Deuterium Transfer in Benzothiazole Compounds. J. Phys. Chem. 1991, 95, 10391–10395. [Google Scholar] [CrossRef]
- Iravani, M.; Omidyan, R. Photochromism of 2-(2-Hydroxyphenyl) Benzothiazole (HBT) and Its Derivatives: A Theoretical Study. J. Phys. Chem. A 2018, 122, 3182–3189. [Google Scholar] [CrossRef]
- Pijeau, S.; Foster, D.; Hohenstein, E.G. Excited-State Dynamics of 2-(2’-Hydroxyphenyl)benzothiazole: Ultrafast Proton Transfer and Internal Conversion. J. Phys. Chem. A 2017, 121, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, O.F.; Luber, S.; Batista, V.S.; Nibbering, E.T. Ultrafast branching of reaction pathways in 2-(2’-hydroxyphenyl)benzothiazole in polar acetonitrile solution. J. Phys. Chem. A 2011, 115, 7550–8. [Google Scholar] [CrossRef]
- Alarcos, N.; Gutierrez, M.; Liras, M.; Sanchez, F.; Douhal, A. An abnormally slow proton transfer reaction in a simple HBO derivative due to ultrafast intramolecular-charge transfer events. PCCP 2015, 17, 16257–69. [Google Scholar] [CrossRef]
- Kayal, S.; Roy, K.; Lakshmanna, Y.A.; Umapathy, S. Probing the effect of solvation on photoexcited 2-(2’-hydroxyphenyl)benzothiazole via ultrafast Raman loss spectroscopic studies. J. Chem. Phys. 2018, 149, 044310. [Google Scholar] [CrossRef]
- Wei, J.; Wu, Y.; Pu, R.; Shi, L.; Jiang, J.; Du, J.; Guo, Z.; Huang, Y.; Liu, W. Tracking Ultrafast Structural Dynamics in a Dual-Emission Anti-Kasha-Active Fluorophore Using Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. Lett. 2021, 12, 4466–4473. [Google Scholar] [CrossRef]
- Xu, W.; Wei, L.; Wang, Z.; Zhu, R.; Jiang, J.; Liu, H.; Du, J.; Weng, T.-C.; Zhang, Y.-B.; Huang, Y.; Liu, W. Tracking Ultrafast Fluorescence Switch-On and Color-Tuned Dynamics in Acceptor–Donor–Acceptor Chromophore. J. Phys. Chem. B 2021, 125, 10796–10804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Zhang, G.; Kong, J.; Niu, X.; Chan, J.M. W.; Liu, W.; Xia, A. Direct Tracking Excited-State Intramolecular Charge Redistribution of Acceptor-Donor-Acceptor Molecule by Means of Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. B 2021, 125, 4456–4464. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Han, F.; Smith, C.; Fang, C. Ultrafast conformational dynamics of pyranine during excited state proton transfer in aqueous solution revealed by femtosecond stimulated Raman spectroscopy. J. Phys. Chem. B 2012, 116, 10535–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Tang, L.; Oscar, B.G.; Chen, C. Capturing Structural Snapshots during Photochemical Reactions with Ultrafast Raman Spectroscopy: From Materials Transformation to Biosensor Responses. J. Phys. Chem. Lett. 2018, 9, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, L.; Oscar, B.G.; Wang, Y.; Chen, C.; Fang, C. Tracking Ultrafast Vibrational Cooling during Excited-State Proton Transfer Reaction with Anti-Stokes and Stokes Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. Lett. 2017, 8, 997–1003. [Google Scholar] [CrossRef]
- Hao, X.; Han, S.; Zhu, J.; Hu, Y.; Chang, L.Y.; Pao, C.W.; Chen, J.L.; Chen, J.M.; Haw, S.C. A bis-benzimidazole PMO ratiometric fluorescence sensor exhibiting AIEE and ESIPT for sensitive detection of Cu2+. RSC Adv. 2019, 9, 13567–13575. [Google Scholar] [CrossRef] [PubMed]
- G. J.Woolfe; M.Melzig; S.Schneider; F.Dörr, The role of tautomeric and rotameric species in the photophysics of 2-(2′-hydroxyphenyl)benzoxazole. Chemical Physics 1983, 77, 213–221. [Google Scholar] [CrossRef]
- Abou-Zied, O.K.; Jimenez, R.; Thompson, E.H. Z.; Millar, D.P.; Romesberg, F.E. Solvent-Dependent Photoinduced Tautomerization of 2-(2’-Hydroxyphenyl)benzoxazole. J. Phys. Chem. A 2002, 106, 3665–3672. [Google Scholar] [CrossRef]
- Chou, P.T.; Studer, S.L.; Martinez, M.L. Studies of the Triplet-State of 2-(2’-Hydroxyphenyl)Benzothiazole. Chem. Phys. Lett. 1991, 178, 393–398. [Google Scholar] [CrossRef]
- Ohshima, A.; Momotake, A.; Nagahata, R.; Arai, T. Enhancement of the large Stokes-shifted fluorescence emission from the 2-(2’-hydroxyphenyl)benzoxazole core in a dendrimer. J. Phys. Chem. A 2005, 109, 9731–9736. [Google Scholar] [CrossRef]
- Horng, M.L.; Gardecki, J.A.; Maroncelli, M. Rotational dynamics of coumarin 153: Time-dependent friction, dielectric friction, and other nonhydrodynamic effects. J. Phys. Chem. A 1997, 101, 1030–1047. [Google Scholar] [CrossRef]
- Horng, M.L.; Gardecki, J.A.; Papazyan, A.; Maroncelli, M. Subpicosecond Measurements of Polar Solvation Dynamics - Coumarin-153 Revisited. J. Phys. Chem. 1995, 99, 17311–17337. [Google Scholar] [CrossRef]
- Maus, M.; Rettig, W.; Bonafoux, D.; Lapouyade, R. Photoinduced intramolecular charge transfer in a series of differently twisted donor - Acceptor biphenyls as revealed by fluorescence. J. Phys. Chem. A 1999, 103, 3388–3401. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.L.; Zhang, Z.; Chen, Y.A.; Chao, W.C.; Su, J.; Tian, H.; Chou, P.T. Snapshotting the Excited-State Planarization of Chemically Locked N,N’-Disubstituted Dihydrodibenzo[a,c]phenazines. J. Am. Chem. Soc. 2017, 139, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.G.; Chen, Y.; Wu, C.H.; Chen, Y.A.; Chen, M.C.; Lin, J.A.; Huang, C.Y.; Su, J.; Tian, H.; Chou, P.T. Phenothiazine Scope: Steric Strain Induced Planarization and Excimer Formation. Angew. Chem. Int. Ed. 2019, 58, 13297–13301. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Abeywickrama, C.S.; Huo, D.; Kong, J.; Tao, M.; Xia, A.; Pang, Y.; Wan, Y. Solvation Controlled Excited-State Planarization in a Push–Pull Pyrene Dye. J. Phys. Chem. C 2020, 124, 8550–8560. [Google Scholar] [CrossRef]
- Fang, C.; Frontiera, R.R.; Tran, R.; Mathies, R.A. Mapping GFP structure evolution during proton transfer with femtosecond Raman spectroscopy. Nature 2009, 462, 200–U74. [Google Scholar] [CrossRef]
- Liu, W.M.; Wang, Y.L.; Tang, L.T.; Oscar, B.G.; Zhu, L.D.; Fang, C. Panoramic portrait of primary molecular events preceding excited state proton transfer in water. Chem. Sci. 2016, 7, 5484–5494. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; J. Tomasi, C. <Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar]
- Miertuš, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Pascual-ahuir, J.L.; Silla, E.; Tuñon, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J. Comput. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. gaussian 09, Revision d. 01, Gaussian. Inc. Wallingford CT 2009, 201. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



