Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
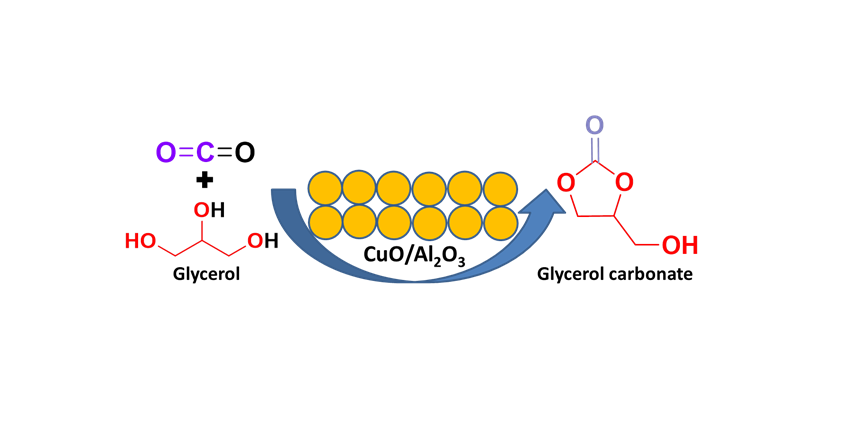
Keywords:
1. Introduction
2. Result and discussion
2.1. Effect of supports
2.2. Effect of CuO loading amount and calcination temperature
2.3. Characterization
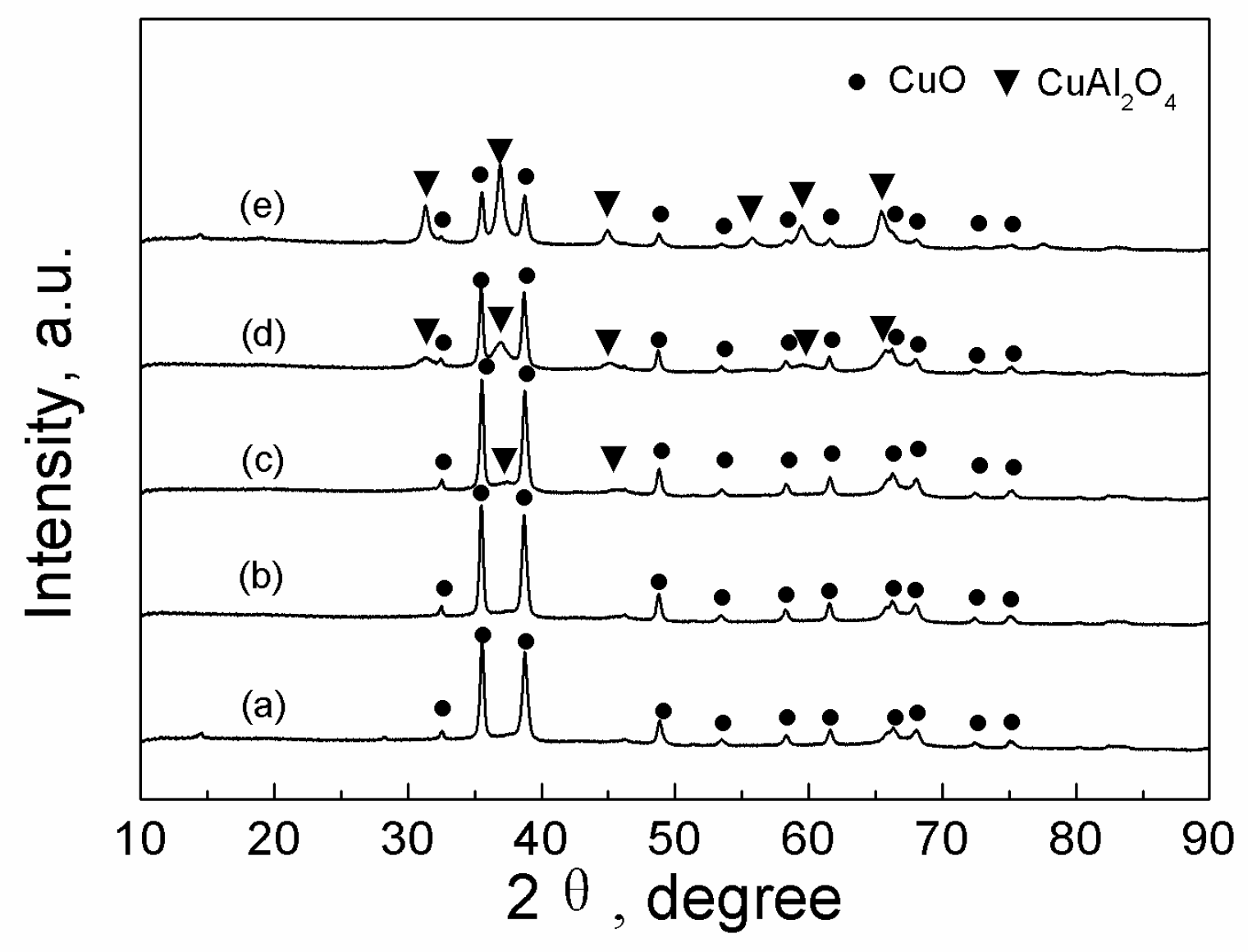
2.3.1. XRD
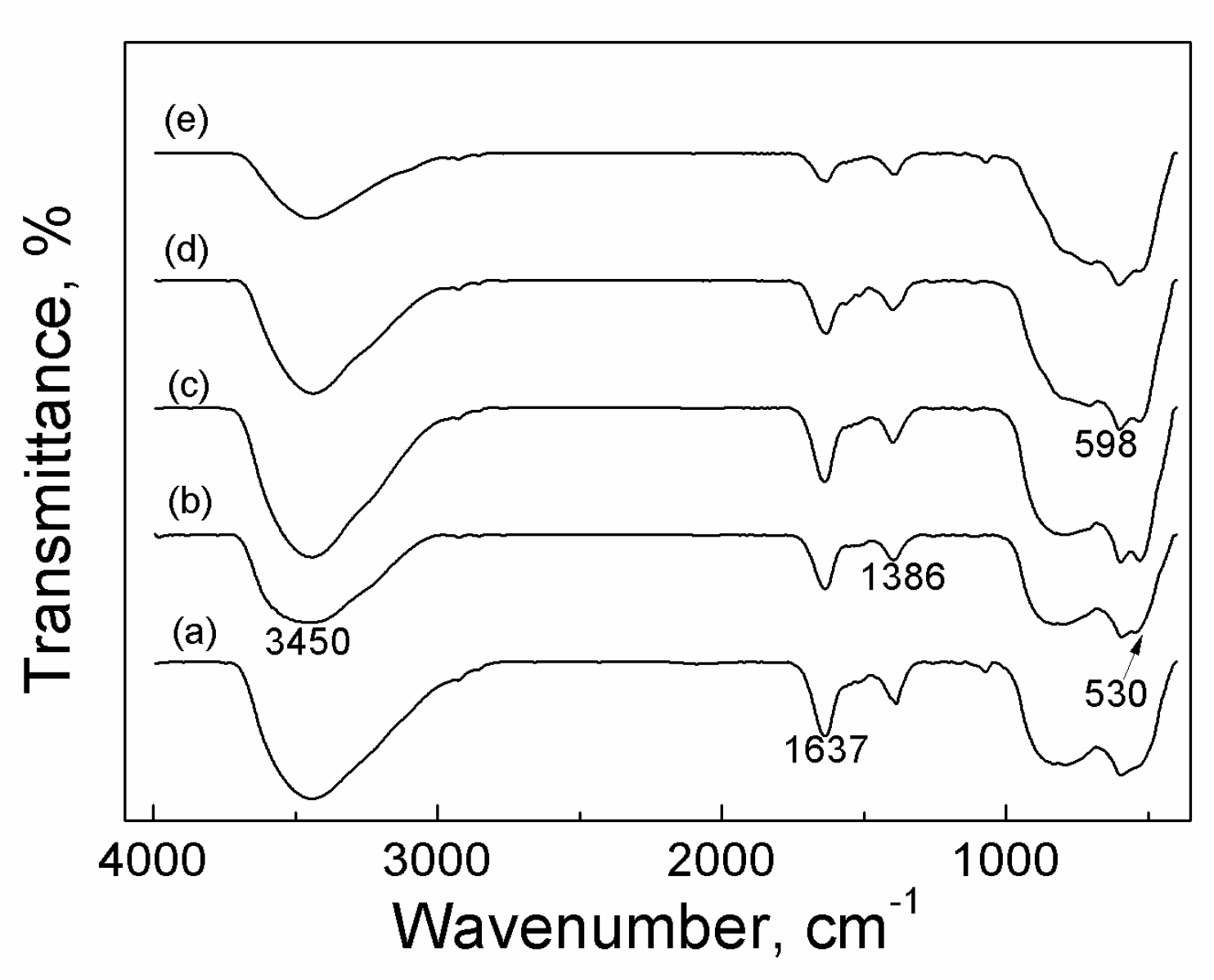
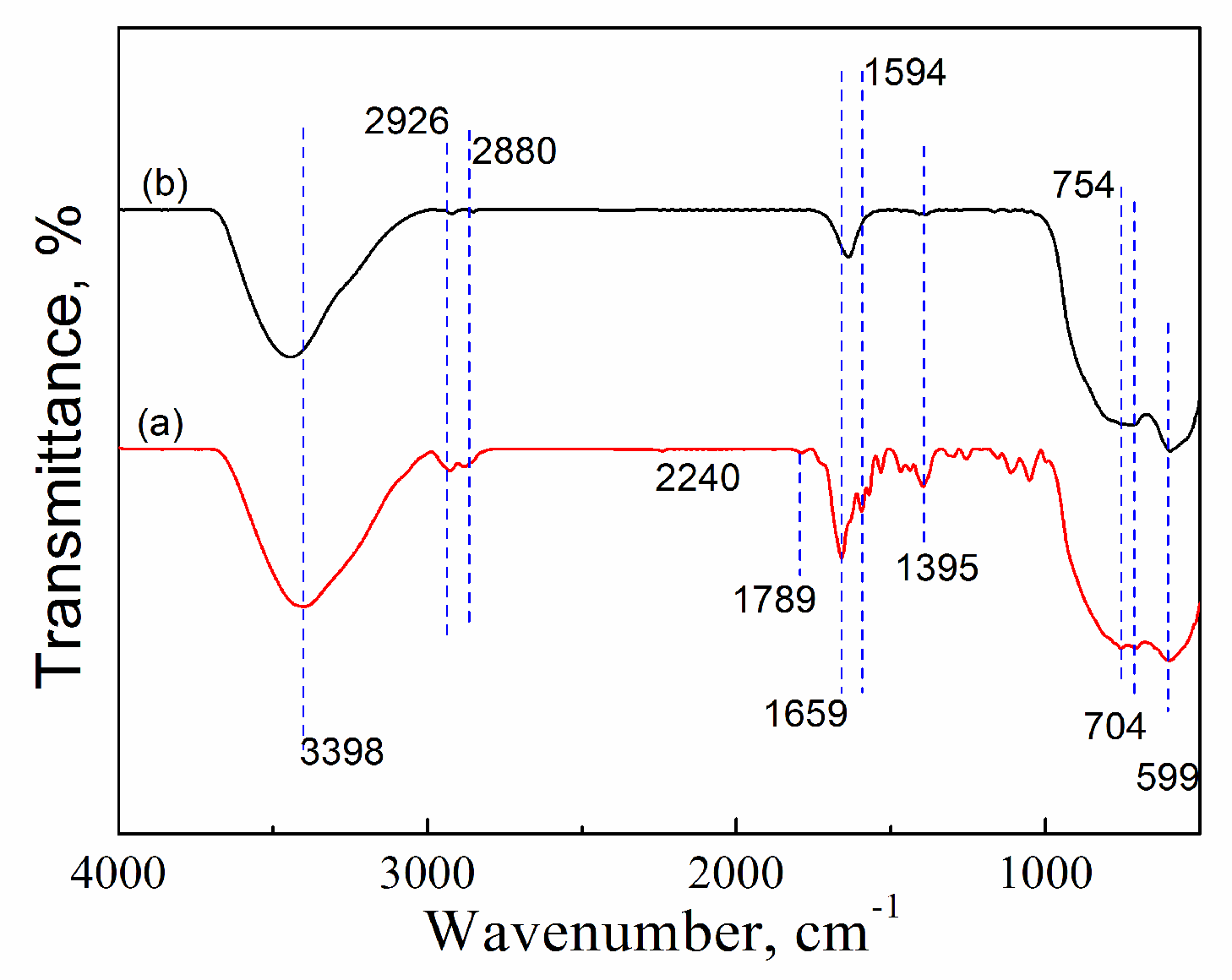
2.3.2. FTIR
2.3.3. SEM
2.3.4. BET
2.3.5. CO2-TPD
2.4. Effect of reaction condition
2.4.1. Effect of reaction temperature
2.4.2. Effect of CO2 initial pressure
2.4.3. Effect of reaction time
2.4.4. Effect of weight of catalyst
2.5. Stability of CuO/Al2O3(30%, 700) catalyst
2.6. Proposed reaction mechanism
2.7. Comparison of the catalytic activity between different catalysts
3. Materials and Methods
3.1. Chemicals
3.2. Supported CuO-based nanoparticle catalyst preparation
3.3. Catalyst characterization
3.4. Reaction procedure
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Guedes, P.H.P.S.; Luz, R.F.; Cavalcante, R.M.; Young, A.F. Process simulation for technical and economic evaluation of acrolein and glycerol carbonate production from glycerol. Biomass Bioenerg. 2023, 168, 106659. [Google Scholar] [CrossRef]
- Wang, H.; Lu, P. Liquid−liquid equilibria for the system dimethyl carbonate + methanol + glycerol in the temperature range of (303.15 to 333.15) K. J. Chem. Eng. Data 2012, 57, 582–589. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Liu, Y.; Yang, C. KNO3/CaO as cost-effective heterogeneous catalyst for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. J. Ind. Eng. Chem. 2015, 28, 334–343. [Google Scholar] [CrossRef]
- Lu, P.; Wang, H.; Hu, K. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over the extruded CaO-based catalyst. Chem. Eng. J. 2013, 228, 147–154. [Google Scholar] [CrossRef]
- Ye, X.; Wang, W.; Zhao, X.; Wen, T.; Li, Y.; Ma, Z.; Wen, L.; Ye, J.; Wang, Y. The role of the KCaF3 crystalline phase on the activity of KF/CaO biodiesel synthesis catalyst. Catal. Commun. 2018, 116, 72–75. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. A green and facile production of catalysts from waste red mud for the one-pot synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2019, 7, 102888. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpä, M. Recent advancement in biodiesel production methodologies using various feedstock: a review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Li, W.; Sreerangappa, R.; Estager, J.; Monbaliu, J.C.M.; Debecker, D.P.; Luis, P. Application of pervaporation in the bio-production of glycerol carbonate. Chemical Engineering & Processing: Process Intensification 2018, 132, 127–136. [Google Scholar]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Shao, S.; Li, W.; Du, S.; Chen, X.; Chao, C.; Fan., X. Effect of ionic radius and valence state of alkali and alkaline earth metals on promoting the catalytic performance of La2O3 catalysts for glycerol carbonate production. Chem. Eng. J. 2023, 458, 141486. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J. Obtaining glycerol carbonate and glycols using thermomorphic systems based on glycerol and cyclic organic carbonates: Kinetic studies. J. Ind. Eng. Chem. 2018, 63, 124–132. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; He, D. Catalytic synthesis of glycerol carbonate from biomass-based glycerol and dimethyl carbonate over Li-La2O3 catalysts. Appl. Catal. A: Gen. 2018, 564, 234–242. [Google Scholar] [CrossRef]
- Wan, Y.; Lei, Y.; Lan, G.; Liu, D.; Li, G.; Bai, R. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over DABCO embedded porous organic polymer as a bifunctional and robust catalyst. Appl. Catal. A: Gen. 2018, 562, 267–275. [Google Scholar] [CrossRef]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Cleaner Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Fernandes, G.P.; Yadav, G.D. Selective glycerolysis of urea to glycerol carbonate using combustion synthesized magnesium oxide as catalyst. Catal. Today 2018, 309, 153–160. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Jiang, C.; Wang, Y.; Ma, J. Synthesis of glycidol and glycerol carbonate from glycerol and dimethyl carbonate using deep-eutectic solvent as a catalyst. Chem. Eng. J. 2022, 442, 136196. [Google Scholar] [CrossRef]
- Das, A.; Shi, D.; Halder, G.; Rokhum, S.L. Microwave-assisted synthesis of glycerol carbonate by transesterification of glycerol using Mangifera indica peel calcined ash as catalyst. Fuel 2022, 330, 125511. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A: Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Zhang, J.; He, D. Surface properties of Cu/La2O3 and its catalytic performance in the synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide. J. Colloid Interface Sci. 2014, 419, 31–38. [Google Scholar] [CrossRef]
- Elhaj, E.; Wang, H.; Gu, Y. Functionalized quaternary ammonium salt ionic liquids (FQAILs) as an economic and efficient catalyst for synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Molecular Catalysis 2019, 468, 19–28. [Google Scholar] [CrossRef]
- George, J.; Patel, Y.; Pillai, S.M.; Munshi, P. Methanol assisted selective formation of 1,2-glycerol carbonate from glycerol and carbon dioxide using nBu2SnO as a catalyst. J. Mol. Catal. A: Chem. 2009, 304, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, H.; He, D. Transformation of CO2 and glycerol to glycerol carbonate over CeO2-ZrO2 solid solution—effect of Zr doping. Biomass and Bioenergy 2018, 118, 74–83. [Google Scholar] [CrossRef]
- Liu, J.; He, D. Transformation of CO2 with glycerol to glycerol carbonate by a novel ZnWO4-ZnO catalyst. Journal of CO2 Utilization 2018, 26, 370–379. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Pianzolli, R.; Machado, L.C.; Miranda, J.L.; Turci, C.C.; Guerra, A.C.O.; Souza-Aguiar, E.F.; Mota, C.J.A. Metal-impregnated zeolite Y as efficient catalyst for the direct carbonation of glycerol with CO2. Appl. Catal. A: Gen. 2015, 504, 187–191. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Mota, C.J.A. Direct carbonation of glycerol with CO2 catalyzed by metal oxides. ChemPhysChem 2017, 18, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodynamics 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Vieville, C.; Yoo, J.W.; Pelet, S.; Mouloungui, Z. Synthesis of glycerol carbonate by direct carbonatation of glycerol in supercritical CO2 in the presence of zeolites and ion exchange resins. Catalysis Letters 1998, 56, 245–247. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Aresta, M.; Ethiraj, J.; Fragale, C.; Nocito, F. Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes. Tetrahedron 2011, 67, 1308–1313. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Pastore, C. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: the role of the catalyst, solvent and reaction conditions. J. Mol. Catal. A: Chem. 2006, 257, 149–153. [Google Scholar] [CrossRef]
- Zhang, J.; He, D. Synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide over Cu catalysts: the role of supports. J. Chem. Technol. Biotechnol. 2015, 90, 1077–1085. [Google Scholar] [CrossRef]
- Li, H.; Xin, C.; Jiao, X.; Zhao, N.; Xiao, F.; Li, L.; Wei, W.; Sun, Y. Direct carbonylation of glycerol with CO2 to glycerol carbonate over Zn/Al/La/X (X=F, Cl, Br) catalysts: the influence of the interlayer anion. J. Mol. Catal. A: Chem. 2015, 402, 71–78. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Gao, P.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal. Sci. Technol. 2013, 3, 2801–2809. [Google Scholar] [CrossRef]
- Park, C.; Nguyen-Phu, H.; Shin, E.W. Glycerol carbonate with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: preferred reaction route depending on crystalline structure. Molecular Catalysis 2017, 435, 99–109. [Google Scholar] [CrossRef]
- Ma, J.; Song, J.; Liu, H.; Liu, J.; Zhang, Z.; Jiang, T.; Fan, H.; Han, B. One-pot conversion of CO2 and glycerol to value-added products using propylene oxide as the coupling agent. Green Chem. 2012, 14, 1743–1748. [Google Scholar] [CrossRef]
- Su, X.; Lin, W.; Cheng, H.; Zhang, C.; Wang, Y.; Yu, X.; Wu, Z.; Zhao, F. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate. Green Chem. 2017, 19, 1775–1781. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, H.; Yao, X.; Dong, L.; Chen, Y. Investigation of the physicochemical properties of CuO/Sm2O3/γ-Al2O3 catalysts and their activity for NO removal by CO. J. Mol. Catal. A: Chem. 2016, 420, 34–44. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Shokrani, R.; Abdollahifar, M. On the solution combustion synthesis of copper based nanocatalysts for steam methanol reforming: Effect of precursor, ultrasound irradiation and urea/nitrate ratio. J. Mol. Catal. A: Chem. 2016, 421, 222–234. [Google Scholar] [CrossRef]
- Sodeifian, G.; Behnood, R. Application of microwave irradiation in preparation and characterization of CuO/Al2O3 nanocomposite for removing MB dye from aqueous solution. Journal of Photochemistry and Photobiology A: Chemistry 2017, 342, 25–34. [Google Scholar] [CrossRef]
- Ha, J.; Ryu, H.; Lee, W.; Bae, J. Efficient photoelectrochemical water splitting using CuO nanorod/Al2O3 heterostructure photoelectrodes with different Al layer thicknesses. Physica B 2017, 519, 95–101. [Google Scholar] [CrossRef]
- Ajamein, H.; Haghighi, M.; Alaei, S. Influence of propylene glycol/nitrates ratio on microwave-assisted combustion synthesis of CuO-ZnO-Al2O3 nanocatalyst: structural and catalytic properties toward hydrogen production from methanol. Materials Research Bulletin 2018, 102, 142–152. [Google Scholar] [CrossRef]
- Luo, M.; Fang, P.; He, M.; Xie, Y. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A: Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Shaheen, W.M. Thermal solid-solid interaction and catalytic properties of CuO/Al2O3 system treated with ZnO and MoO3. Thermochimica Acta 2002, 385, 105–116. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Farhadi, M. Synthesis and characterization of spinel-type CuAl2O4 nanocrystalline by modified sol–gel method. J. Sol-Gel Sci. Technol. 2009, 51, 48–52. [Google Scholar] [CrossRef]
- Lv, W.; Liu, B.; Qiu, Q.; Wang, F.; Luo, Z.; Zhang, P.; Wei, S. Synthesis, characterization and photocatalytic properties of spinel CuAl2O4 nanoparticles by a sonochemical method. Journal of Alloys and Compounds 2009, 479, 480–483. [Google Scholar] [CrossRef]
- Tomishige, K.; Ikeda, Y.; Sakaihori, T.; Fujimoto, K. Catalytic properties and structure of zirconia catalysts for direct synthesis of dimethyl carbonate from methanol and carbon dioxide. J. Catal. 2000, 192, 355–362. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A: Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. Production of glycerol carbonate from glycerol with aid of ionic liquid as catalyst, Chem. Eng. J. 2016, 297, 128–138. [Google Scholar]
- Liu, Y. Synthesis of glycerol carbonate from glycerol and carbon dioxide over cobalt acetate catalyst. Speciality Petrochemicals 2014, 31, 25–27. (in chinese). [Google Scholar]
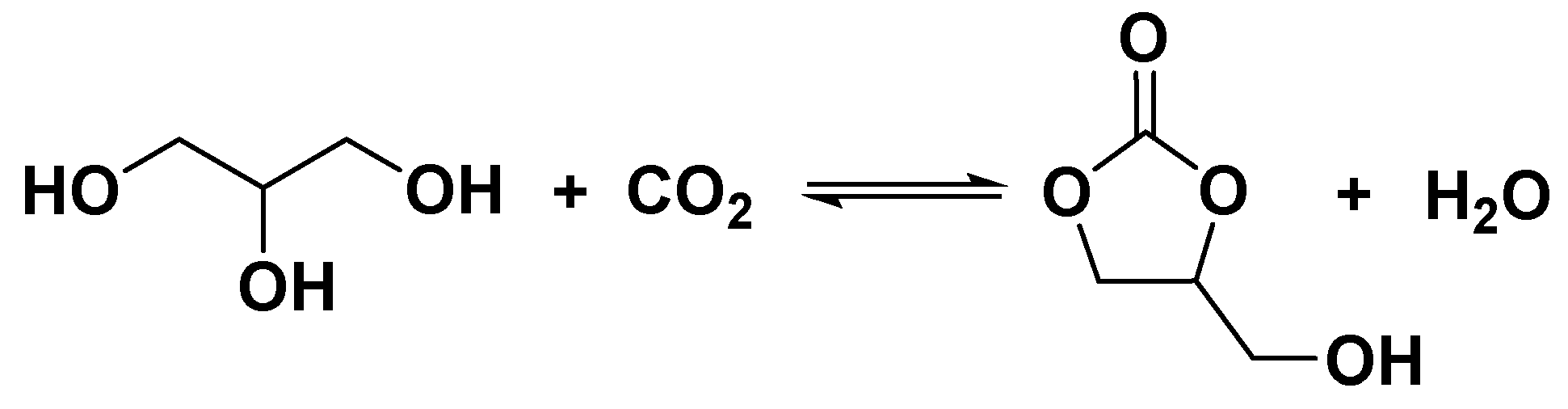

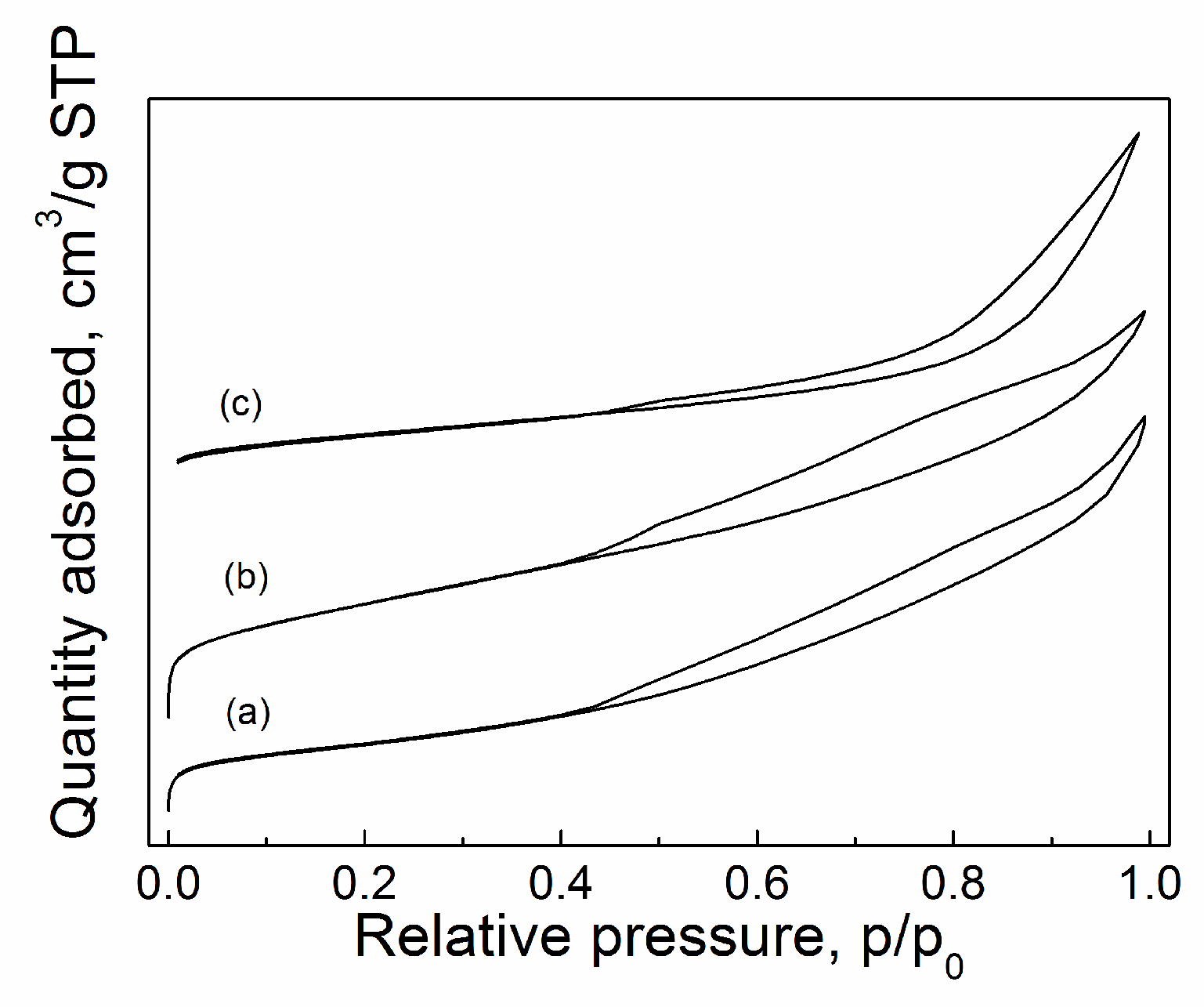
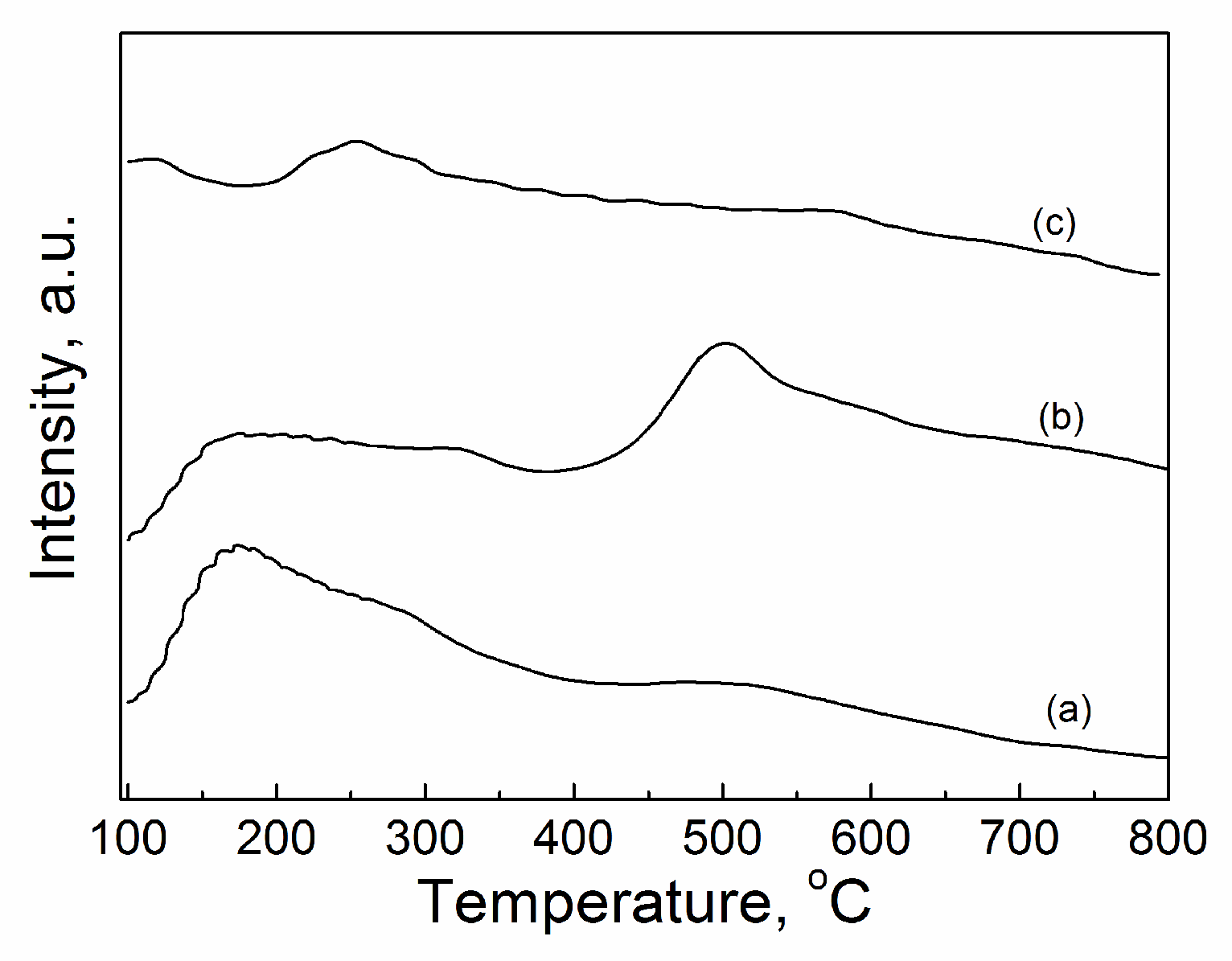
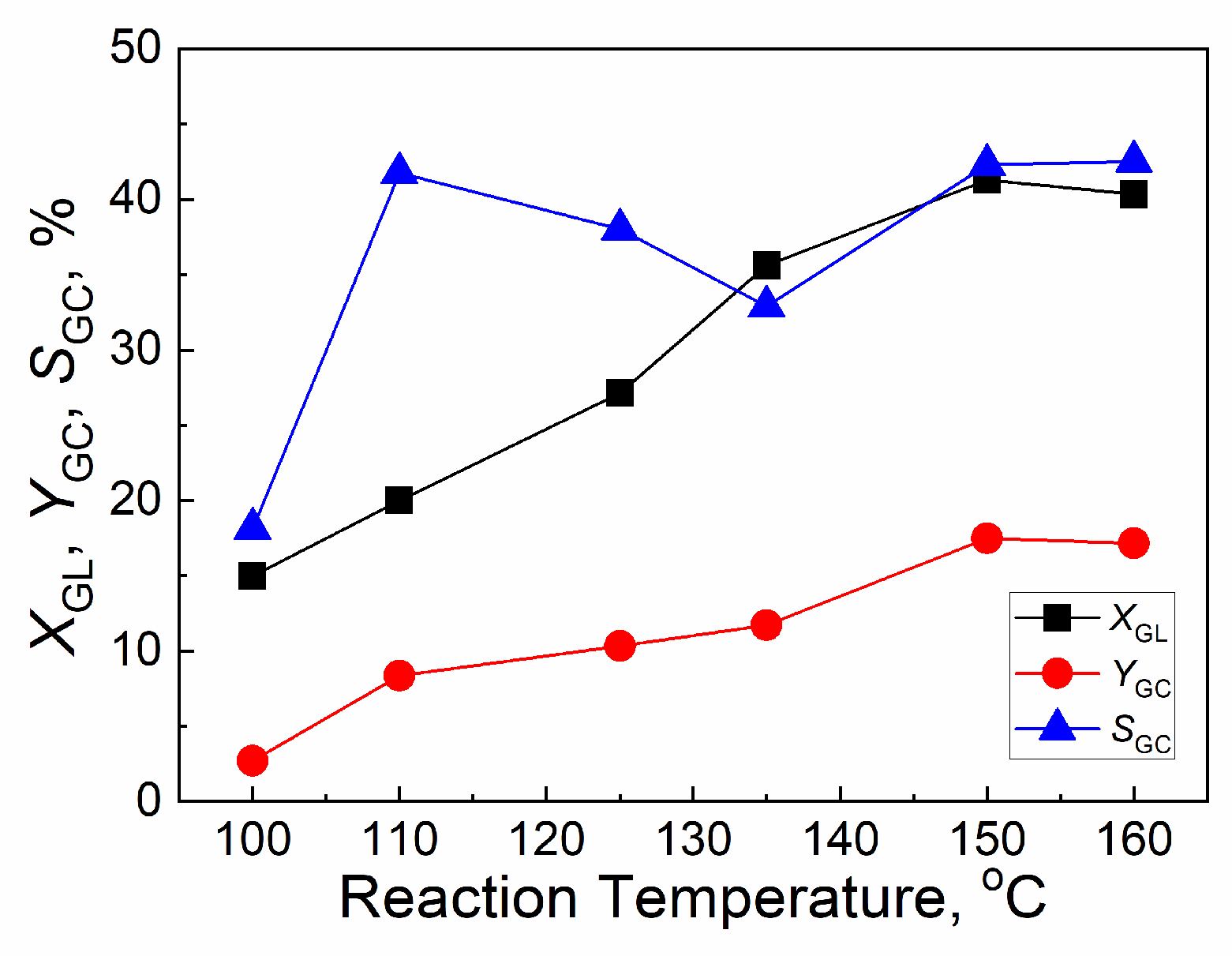
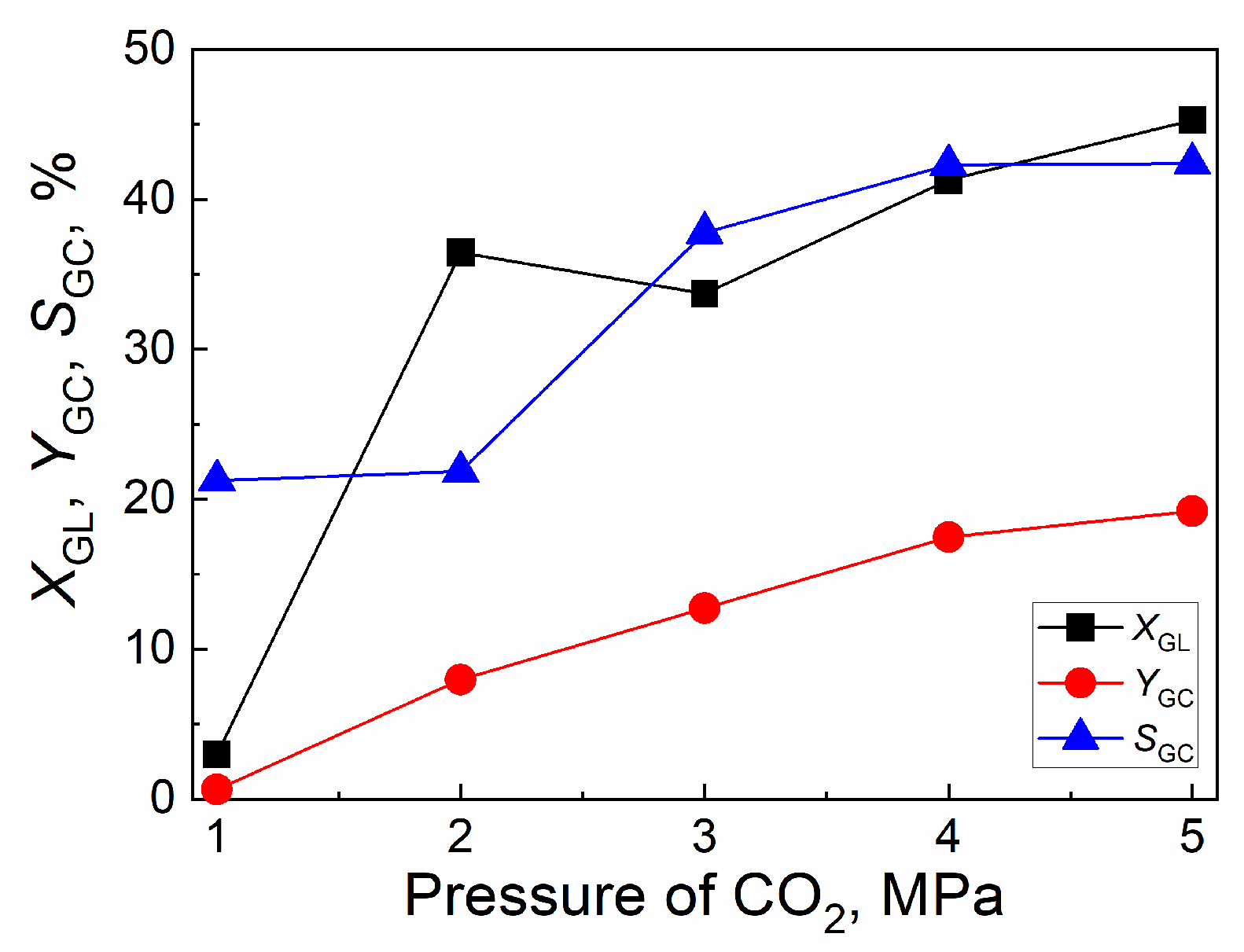
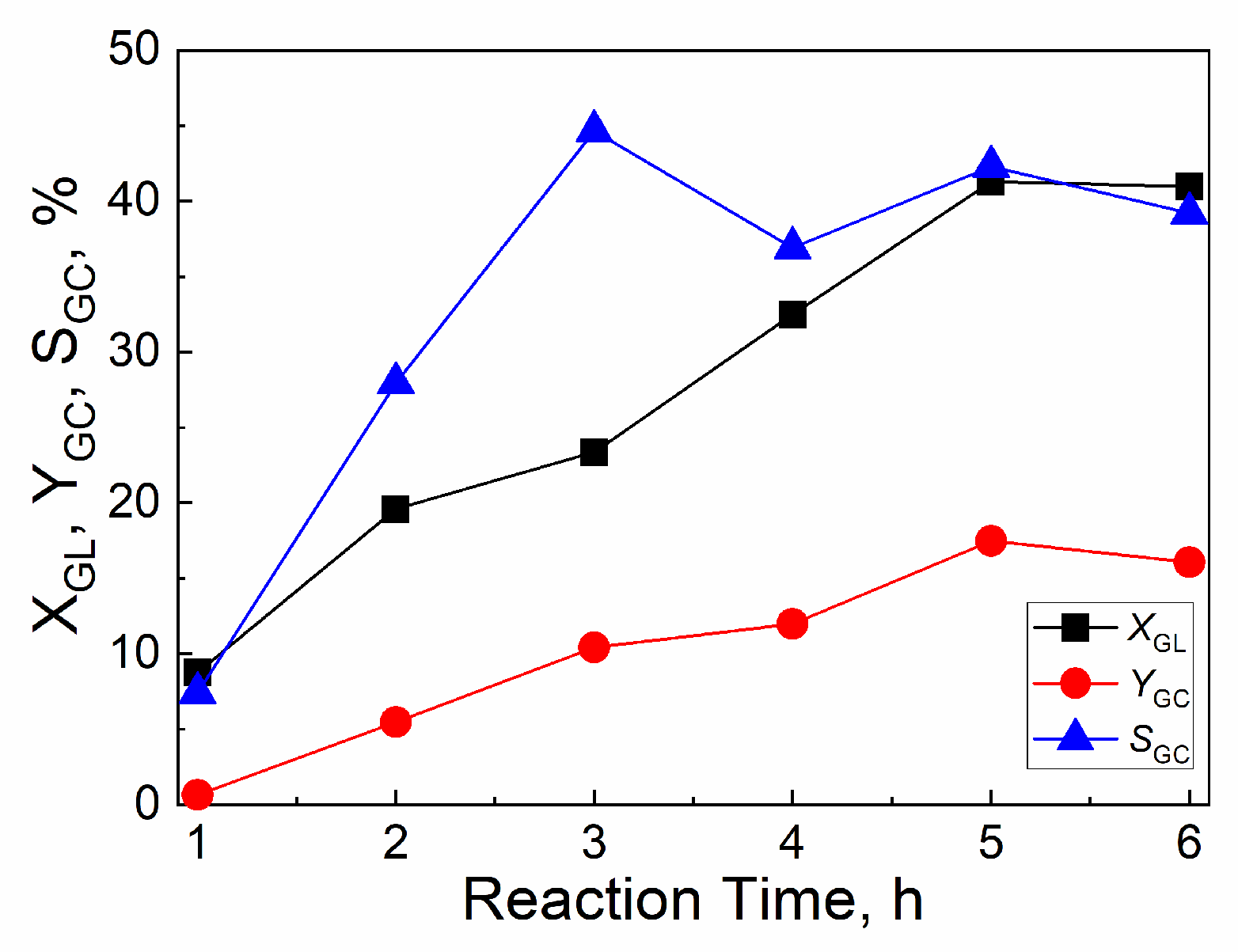
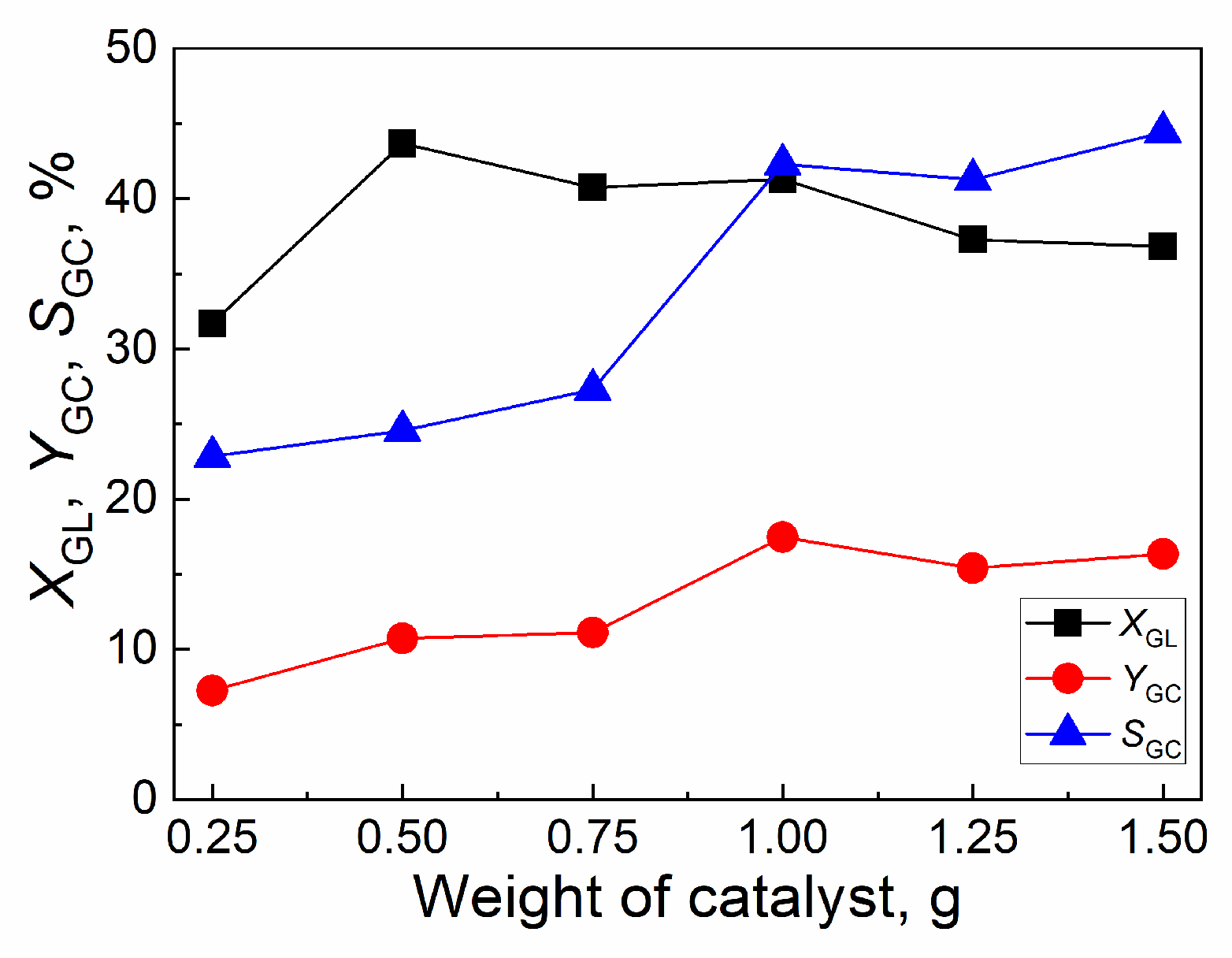
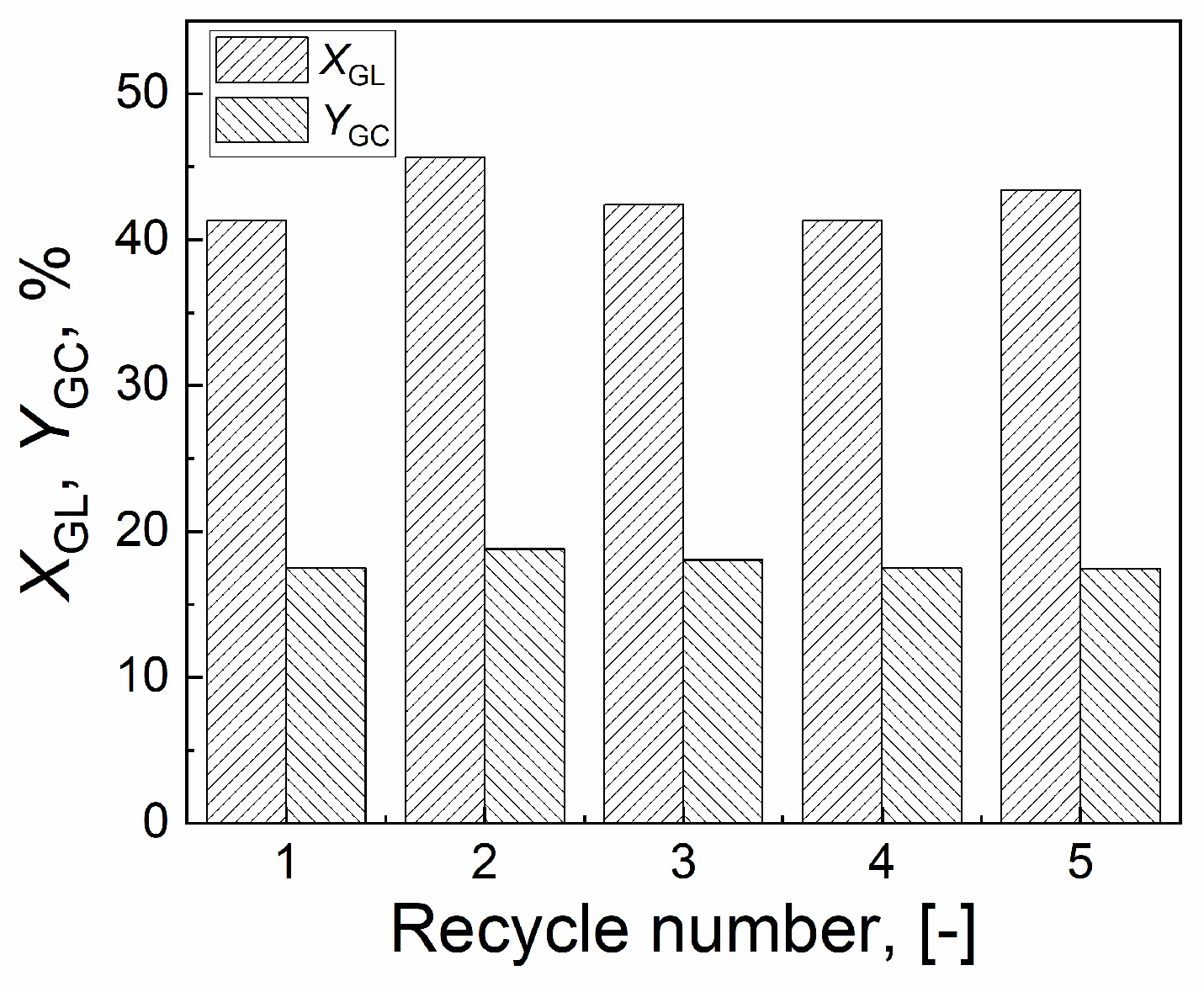
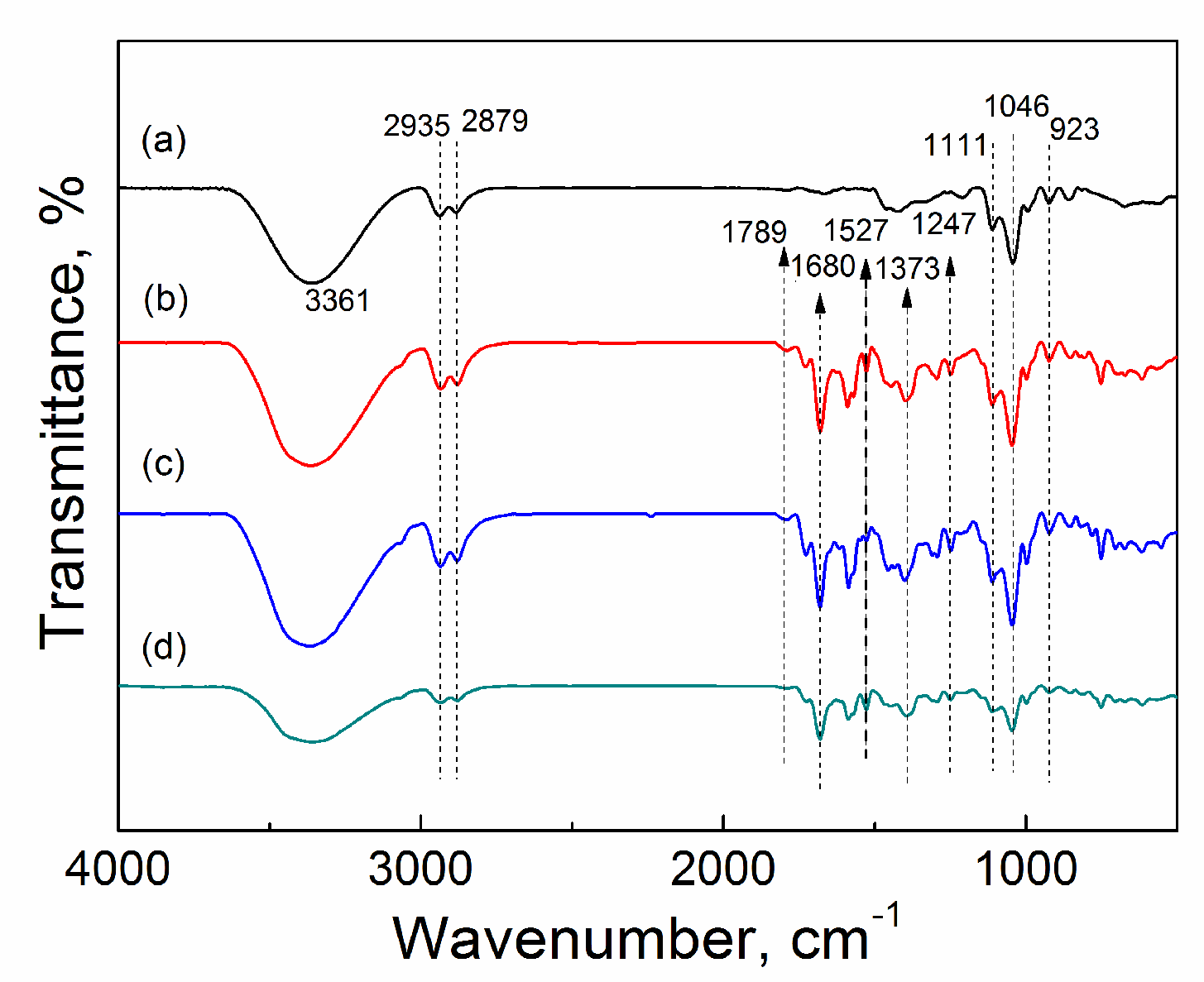
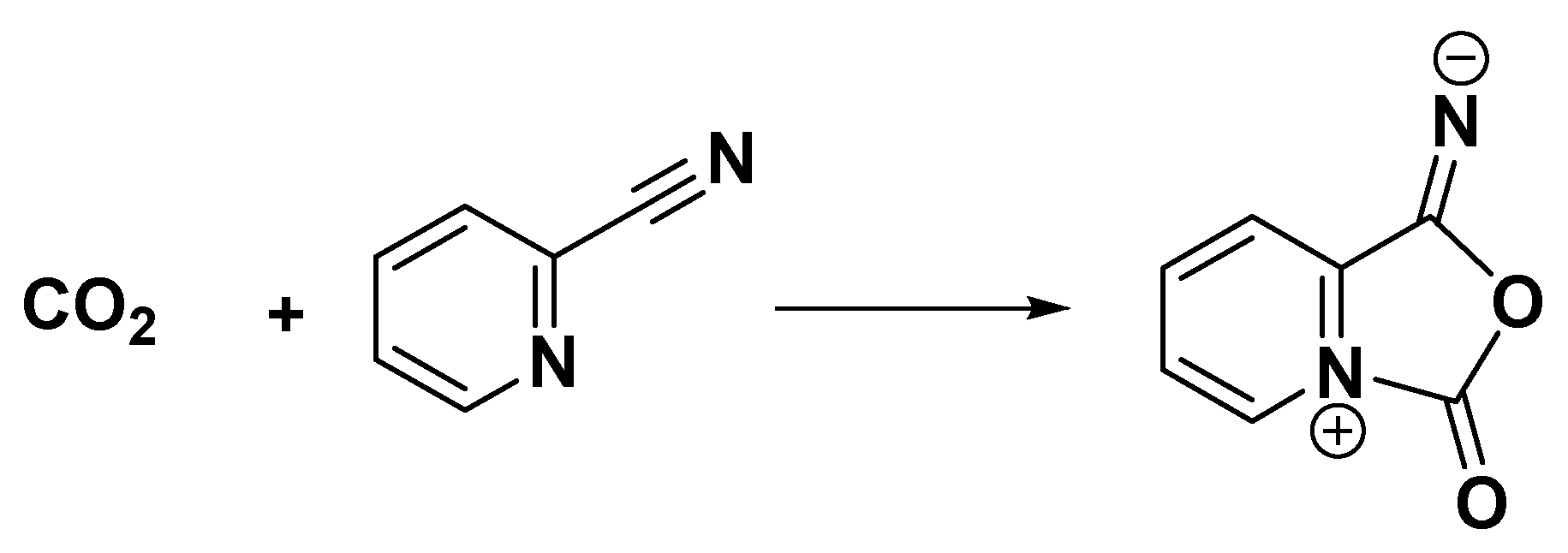

| Catalyst | Tcal(oC) | WCuO(wt%) | XGL(%) | YGC(%) | SGC(%) |
|---|---|---|---|---|---|
| CuO/GO(30%, 500) | 500 | 30 | 49.0 | 14.6 | 29.7 |
| CuO/AC(30%, 500) | 500 | 30 | 21.4 | 8.9 | 41.4 |
| CuO/SiO2(30%, 500) | 500 | 30 | 38.2 | 10.1 | 26.5 |
| CuO/GE(30%, 500) | 500 | 30 | 49.0 | 13.4 | 27.3 |
| CuO/Al2O3(30%, 500) | 500 | 30 | 37.8 | 15.0 | 39.8 |
| Catalyst | Tcal(oC) | WCuO(wt%) | XGL(%) | YGC(%) | SGC(%) |
|---|---|---|---|---|---|
| CuO/Al2O3(5%, 500) | 500 | 5 | 14.6 | 2.8 | 19.2 |
| CuO/Al2O3(10%, 500) | 500 | 10 | 17.9 | 6.0 | 33.5 |
| CuO/Al2O3(20%, 500) | 500 | 20 | 29.4 | 10.0 | 34.1 |
| CuO/Al2O3(30%, 500) | 500 | 30 | 37.8 | 15.0 | 39.8 |
| CuO/Al2O3(40%, 500) | 500 | 40 | 51.6 | 15.3 | 29.7 |
| CuO/Al2O3(30%, 400) | 400 | 30 | 58.0 | 15.1 | 26.0 |
| CuO/Al2O3(30%, 600) | 600 | 30 | 44.0 | 13.6 | 30.9 |
| CuO/Al2O3(30%, 700) | 700 | 30 | 41.3 | 17.5 | 42.4 |
| CuO/Al2O3(30%, 800) | 800 | 30 | 33.5 | 14.2 | 42.4 |
| Catlysts | SBET (m2/g) | Total pore volume (cm3/g) | Average pore diameter (nm) | Basic site amount (umol/g) | |||
|---|---|---|---|---|---|---|---|
| < 200 oC | 200~400 oC | >400 oC | total | ||||
| CuO/Al2O3(30%,600) | 111.29 | 0.269 | 7.98 | 171.18(0.19) a | 367.13(0.42) | 337.15(0.39) | 875.45 |
| CuO/Al2O3(30%,700) | 170.52 | 0.277 | 6.65 | 193.86(0.18) | 184.90(0.17) | 703.66(0.65) | 1082.42 |
| CuO/Al2O3(30%,800) | 92.70 | 0.240 | 11.42 | 4.91(0.02) | 90.16(0.27) | 233.86(0.71) | 328.93 |
| Catalysts | T(°C) | Time(h) | P(MPa) | Cat.(wt%) | Dehydrant | XGL(%) | YGC(%) | TOFb | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CeO2 | 150 | 5 | 4 | 186.8 | 2-cyanopyride | - | 78.9 | 0.92 | [18] |
| La2O2CO3–ZnO | 170 | 12 | 4 | 5 | acetonitrile | 30.3 | 14.3 | 2.59 | [31] |
| CHT-Cl | 170 | 12 | 4 | 3.0 | acetonitrile | 35.5 | 16.7 | 4.97 | [30] |
| ZnY | 180 | 3 | 10 | 2.0 | no | - | 5.8 | 10.58 | [24] |
| Cu/MCM-41 | 150 | 3 | 7 | 1.7 | acetonitrile | 18.7 | 1.8 | 3.81 | [29] |
| CeO2-nanopolyhedra | 170 | 12 | 10 | 7.4 | 2-cyanopyride | 35.5 | 14.2 | 1.74 | [35] |
| ZnO | 180 | 12 | 15 | 0.6 | no | - | 8.1 | 11.35 | [25] |
| Co(OAc)2 | 180 | 6 | 2 | 2.5 | acetonitrile | 36.7 | 4.6 | 3.33 | [48] |
| ZnWO4-ZnO | 150 | 6 | 5 | 54.4 | no | - | 6.5 | 0.22 | [23] |
| CuO/Al2O3(30%, 700) | 150 | 5 | 4 | 43.6 | 2-cyanopyride | 41.3 | 17.5 | 0.87 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




