1. Introduction
The ability to predict the likelihood of a live birth after single fresh embryo transfer is important for treatment planning and managing patient expectation, particularly in the first in vitro fertilisation (IVF) cycle. Cryopreservation of supernumerary embryos is often regarded as an important prognostic variable and a surrogate marker of IVF success. It increases the cumulative pregnancy rate from a single IVF stimulation (Veeck et al., 1993), permits the transfer of fewer embryos per attempt (Tiitinen et al., 2001), and decreases the costs associated with multiple IVF stimulation cycles.
The overall reduction in the mean number of embryos transferred over the last decade, in conjunction with improvement in extended embryo culture and cryopreservation techniques, have led to an increase in the availability of surplus embryos for cryopreservation (Marsh et al., 2012).
Previous large studies have examined the association between the number of oocytes retrieved and cleavage-stage embryos available, and the odds of a live birth following a fresh embryo transfer (Sjogren et al. 1992; Sunkara et al., 2011; Steward et al., 2014; Baker et al., 2015). However, the number of oocytes retrieved does not always predict the number of blastocysts eventually available for transfer and freezing, and therefore could be a less reliable marker of embryonic implantation potential and IVF outcome. The number of supernumerary blastocysts suitable for freezing is therefore perceived as a stronger predictor of cycle outcome. Whilst previous studies have described the association between the occurrence of a live birth and embryo availability (Hill et al., 2013; Papanikolou et al.,2019), the effect of patient’s age, which is often the biggest confounder of success within an IVF cycle, on this association has not been rigorously studied.
The aim of this study was to examine the relationship between the number of supernumerary blastocysts cryopreserved and the likelihood of achieving a live birth following elective fresh autologous single blastocyst transfer in patients having their first IVF cycle, stratified according to patient age.
2. Materials and Methods
2.1. Study Design and Participants
Anonymised data on IVF cycles performed at the Assisted Conception Unit, at Guy’s and St. Thomas’ Hospital in London, United Kingdom, were collected between July 2006 and June 2018 and retrospectively analysed. This cohort study included records of all patients who had their first IVF and intra-cytoplasmic sperm injection (ICSI) cycle culminating in a fresh autologous single blastocyst transfer on day 5 after oocyte fertilisation. All patients included within this study who had at least one supernumerary blastocyst cryopreserved underwent elective single blastocyst transfer.
To minimise potential confounders, strict inclusion and exclusion criteria were utilised. Only couples undergoing their first fresh stimulation cycle of IVF or ICSI were included in the study. Cycles involving pre-implantation genetic testing (PGT), oocyte donation, transfer of cleavage-stage or more than one fresh embryo or total embryo freezing due to increased risk of ovarian hyperstimulation syndrome were excluded.
Patient demographics and cycle characteristics, including female age, controlled ovarian stimulation protocol, type and total dose of gonadotrophins administered, method of oocyte fertilisation (IVF vs ICSI), number of oocytes retrieved and normally fertilised, and number of supernumerary blastocysts cryopreserved in the fresh cycle were analysed.
Since age is known to influence the likelihood of achieving a live birth after fresh embryo transfer (Scheffer et al., 2017), we stratified patients based on their age into three groups (younger than 35 years, 35-39 years and 40 years and older). The time period of July 2006 till June 2018 was selected as no major changes in clinical protocols, culture conditions or laboratory equipment were made during this time period.
2.2. Ovarian Stimulation and Oocyte Retrieval
Follicle stimulating hormone (FSH) injections were started at a dose of 150-450 IU daily for multi-follicular ovarian stimulation in either the mid-luteal long down regulation or the short gonadotropin releasing hormone (GnRH) antagonist protocol as described previously (Sunkara et al., 2007; Sunkara et al., 2014). The choice of the controlled ovarian stimulation protocol and daily dose of the follicle stimulating hormone (FSH) injections was based on female age, basal follicle stimulating hormone (FSH) level, AFC and anti-mullerian hormone (AMH) level as described elsewhere (Sunkara et al., 2014; El-Toukhy et al., 2016).
Ovarian response was monitored by transvaginal ultrasound scanning and, where required, serum estradiol level measurements and oocyte maturation was induced using either 10,000IU of urinary-derived HCG (Pregnyl, Merck-Serono, Halle, Germany) or 250µg of recombinant HCG (Ovitrelle, Merck-Serono, Darmstadt, Germany) when at least three 18mm follicles were seen on ultrasound scan.
Transvaginal ultrasound-guided retrieval of cumulus-oocyte complexes was performed using GE Logic V1 portable scanning system (GE Healthcare Ultrasound, Milwaukee, WI, USA) 36 hours after the HCG injection.
2.3. Embryo Assessment
Standard IVF or ICSI was used for oocyte fertilisation as clinically indicated and embryos were cultured till day 5 after fertilisation if at least 2 embryos were deemed to be of good quality on day 3 after fertilisation as previously described (Naji et al., 2018). On day 5 after fertilisation, fresh blastocyst stage embryos were assigned grades according to strict morphological criteria (Gardner and Schoolcraft, 1999; Gardner and Balaban, 2006), which were not changed during the study period. According to our laboratory practice and unpublished audit data, an embryo was considered suitable for transfer or freezing if it had reached the expanded blastocyst stage with prominent and compact inner cell mass and many identical trophectoderm cells forming a continuous layer [blastocyst grade 3CC or higher] and showed no signs of degeneration (Gardner and Schoolcraft, 1999; Gardner and Balaban, 2006).
All patients included in the study had single embryo transfer in accordance to national regulations (Harbotle et al. 2015). All embryo transfers were performed on day 5 after fertilisation under transabdominal ultrasound guidance using GE Voluson 730 system (GE Healthcare Ultrasound) on day 5 after fertilisation. Supernumerary blastocysts of grade 3CC or higher that showed no signs of degeneration were cryopreserved on day 5 or 6 after fertilisation. A standard slow freezing protocol, employing 1,2-propanediol (PROH) and sucrose as cryoprotectants, (Lassalle et al., 1985; Edgar et al., 2000) or vitrification using Cryolock device (Biotech Inc., GA, USA) and Vitrolife vitrification medium (FUJIFILM Irvine Scientific, Co. Wicklow, Ireland) was used for blastocyst cryopreservation. The luteal phase was supported with 400 mg progesterone pessaries (Cyclogest, Actavis UK Ltd., Barnstaple, UK) twice daily commencing on the day of oocyte retrieval until 8 weeks’ gestation.
2.4. Outcome Measure
The outcomes of each treatment cycle included pregnancy, clinical pregnancy and live birth. Pregnancy was confirmed by a positive urine hCG test 11 days after blastocyst transfer. A clinical pregnancy was defined as the observation of a gestational sac on ultrasound scanning 5 weeks after embryo transfer. Live birth was defined as the delivery of a live born baby after 23 completed weeks of gestation. For this study, live birth was the primary outcome of interest.
2.5. Statistical Analysis
The study data were presented as mean ± interquartile range (IQR) or percentage. Statistical analysis was performed using STATA version 16 (STATA Corp., Texas, USA). The probability of live birth between patients with supernumerary blastocysts cryopreserved and those without was performed through a chi-square analysis. Continuous parameters were compared using Wilcoxon rank-sum tests. A multivariate logistic regression analysis was used to examine the association between livebirth and whether or not supernumerary blastocysts were available for cryopreservation after controlling for important confounders, including female age, protocol and type of gonadotrophins used for ovarian stimulation, total dose of gonadotrophins administered, and number of oocytes retrieved.
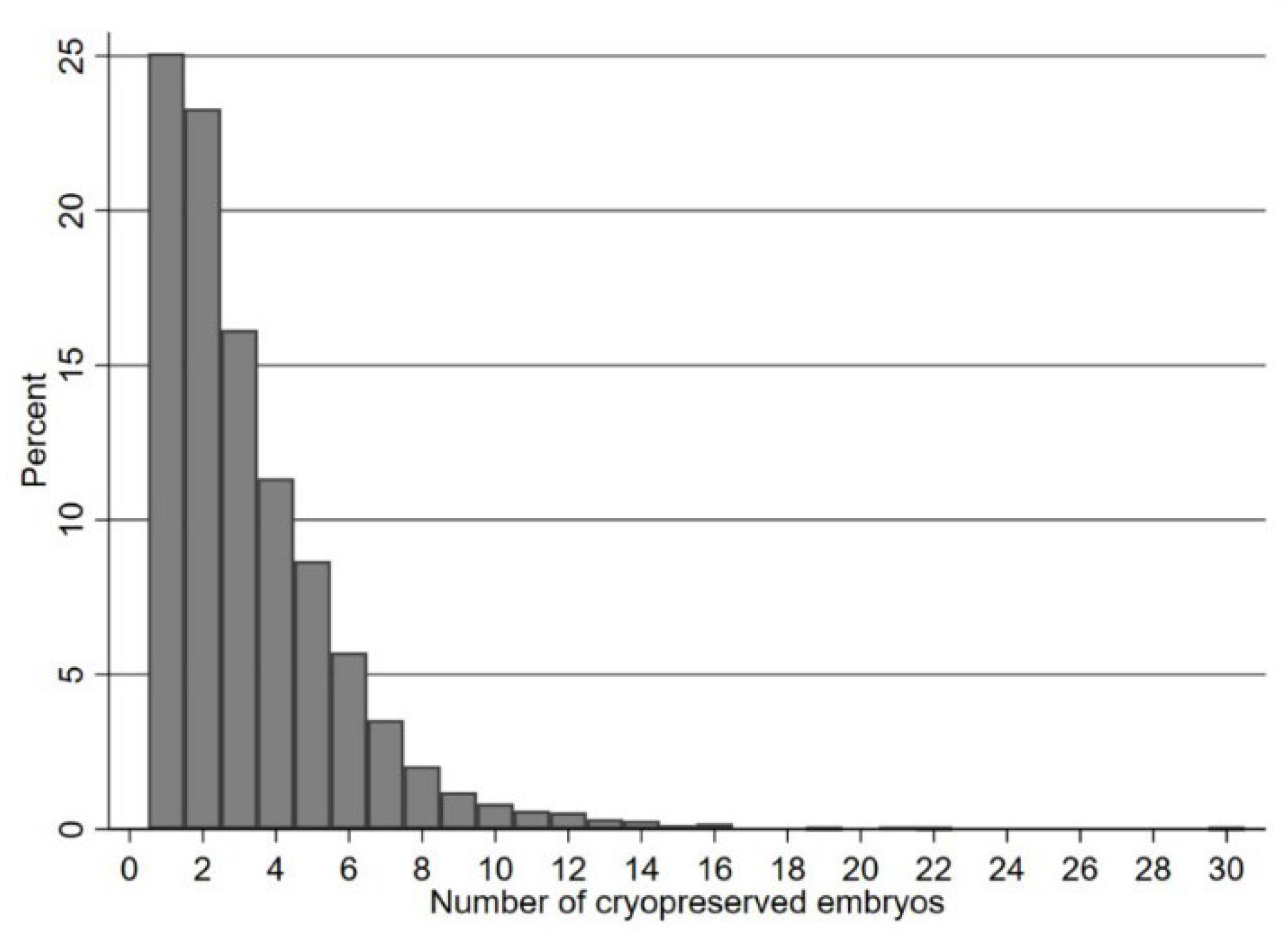
To assess the association between the number of supernumerary blastocysts cryopreserved and probability of a live birth outcome, fractional logistic regressions (Royston and Altman, 1994) were modelled using a cubic polynomial transformation of the number of supernumerary blastocysts cryopreserved; stratified by female age group. With the exception of the histogram of number of blastocysts, the x-axes and row numbers in figures and tables have been truncated at 15 for legibility, since a small number of cycles (n=12) had over 15 (16-30) supernumerary blastocysts cryopreserved. Shapiro-Wilk test was performed to assess and confirm the normality of continuous variables.
2.6. Ethics
Ethical approval was received for this study from the local research ethics committee (ref: 01121432/2019). Our study involved neither therapeutic intervention nor change of our routine IVF protocols or data collection. Each couple gave written informed consent for the use of their data anonymously for audit and research purposes upon enrolment into our IVF programme and before starting an IVF cycle in accordance with the UK Human Fertilisation and Embryology Authority (HFEA) regulations.
3. Results
A total of 10,015 fresh autologous non-PGT IVF/ICSI cycles performed in our centre between July 2006 and June 2018 were eligible for inclusion in the study. The mean female age of couples included in the study cohort was 35.1 ± 4.4 years (range 18-45) and was normally distributed throughout the data set.
The mean daily dose of FSH used for ovarian stimulation was 283.8± 54.5iu (range 112.5-450), and the mean duration of ovarian stimulation was 11.2± 2.1 days. In 64% of cycles, ICSI was the method used for oocyte fertilization. The mean number of normally fertilized oocytes was 12.1 ± 7.0. Of the 10,015 first fresh IVF/ICSI cycles included in the study, 5,992 (60%) had a single blastocyst transfer with no supernumerary blastocysts available for cryopreservation, whilst 4023 cycles (40%) had between 1 and 30 (mean 3.3 ± 2.5) supernumerary blastocysts cryopreserved.
Figure 1 depicts the frequency distribution of the number of cryopreserved blastocysts in those 4023 cycles.
The overall clinical pregnancy and live birth rates in the study were 36% and 30%, respectively. Cycles in which one or more supernumerary blastocysts were cryopreserved had a significantly higher live birth rate compared to those without blastocyst cryopreservation (38% vs. 24%, P<0.0001, Table 1). After adjusting for important confounders, the likelihood of achieving a live birth following a single blastocyst transfer in cycles where one or more supernumerary blastocysts were cryopreserved was significantly higher compared to those without blastocyst cryopreservation (adjusted odds ratio (aOR) 1.76, 95% CI: 1.61-1.92, P<0.0001).
Table 1.
Baseline characteristics and IVF cycle outcome in those having supernumerary cryopreserved embryos vs those who did not. Values are provided as median (IQR) or as percentage.
Table 1.
Baseline characteristics and IVF cycle outcome in those having supernumerary cryopreserved embryos vs those who did not. Values are provided as median (IQR) or as percentage.
| Factor |
No Blastocysts Cryopreserved |
Supernumerary Blastocysts Cryopreserved |
p-Value |
| Number |
5992 |
4023 |
|
| Age, median (IQR) |
36.0 (33.0, 39.0) |
34.0 (31.5, 37.0) |
<0.001 |
| Age Group |
|
|
|
| <35 |
2256 (38%) |
2119 (53%) |
<0.001 |
| 35-39 |
2970 (49%) |
1728 (43%) |
|
| ≥40 |
766 (13%) |
176 (4%) |
|
| Baseline FSH (IU) Level, median (IQR) |
6.3 (5.3, 7.7) |
6.3 (5.3, 7.6) |
0.15 |
| Type of Gonadotrophins used |
|
|
|
| Recombinant |
5347 (89.2%) |
3688 (91.7%) |
<0.001 |
| Urinary-derived |
645 (10.8%) |
335 (8.3%) |
|
| Daily Dose of FSH, median (IQR) |
277 (150.0, 300.0) |
225.0 (150.0, 300.0) |
<0.001 |
| Duration of Ovarian Stimulation, median (IQR) |
11.0 (10.0, 12.0) |
11.0 (10.0, 12.0) |
0.33 |
| Total Dose of FSH, median (IQR) |
2700 (1800, 3600) |
2250 (1650, 3150) |
<0.001 |
| Number of Oocyctes Retrieved, median (IQR) |
9.0 (6.0, 14.0) |
14.0 (10.0, 18.0) |
<0.001 |
| Method of oocyte fertilisation |
|
|
|
| IVF |
2105 (35%) |
1461 (36%) |
|
| ICSI |
3887 (65%) |
2562 (64%) |
|
| No. of normally-fertilised oocytes, median (IQR) |
5.0 (3.0, 8.0) |
9.0 (6.0, 12.0) |
<0.001 |
| Clinical Pregnancy |
1568(28%) |
1612 (44%) |
<0.001 |
| Live Birth |
1436 (24%) |
1518 (38%) |
<0.001 |
3.1. Association of Live Birth Rate with Number of Supernumerary Blastocysts Cryopreserved
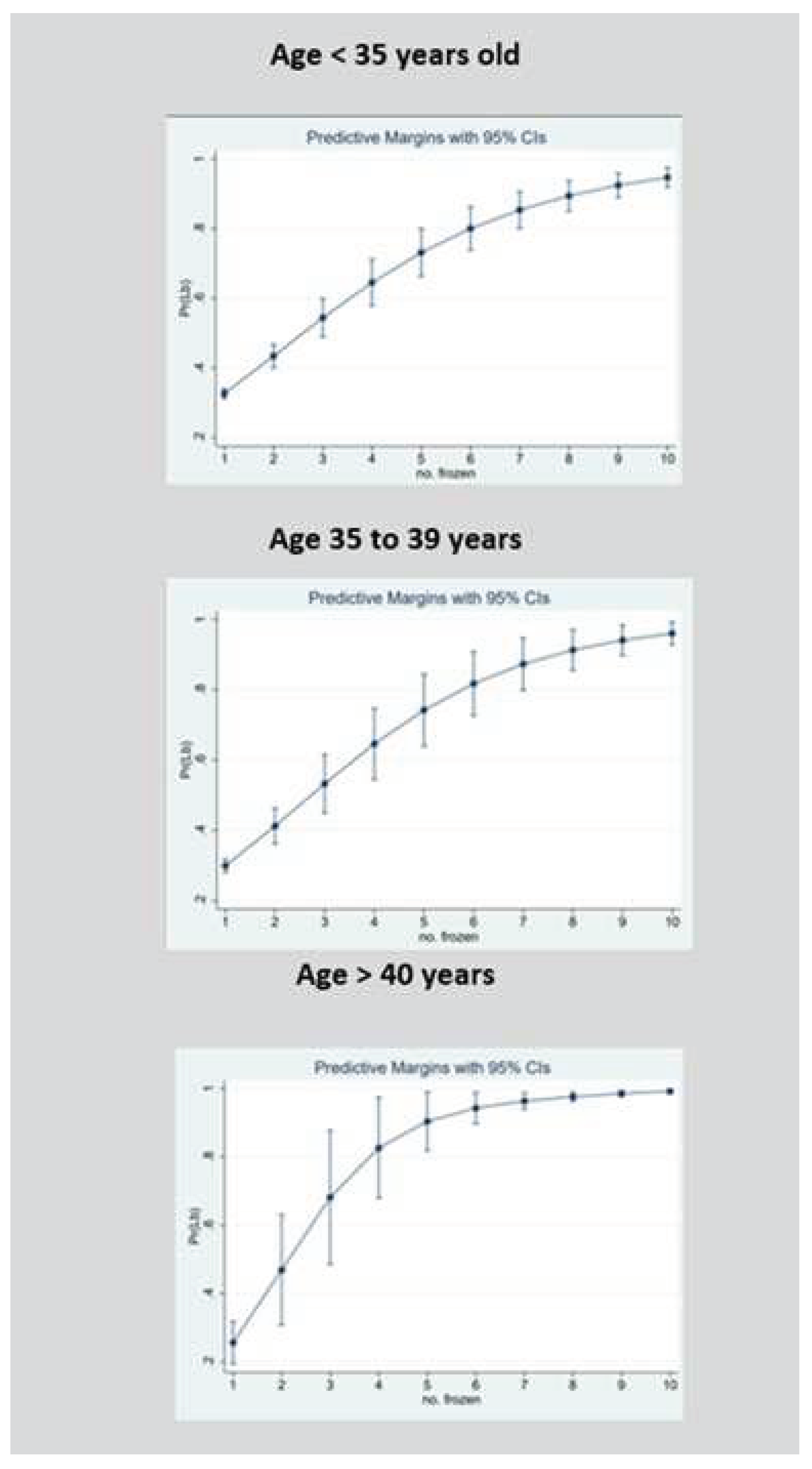
We further analyzed the relationship between the number of supernumerary blastocysts cryopreserved and the odds of a live birth following fresh single blastocyst transfer using a cubed polynomial regression model. To aid clinical relevance, we stratified the regression model according to three female age groups (<35, 35-39 and ≥40 years).
Figure 2 shows the probability of a live birth in relation to the number of blastocysts cryopreserved within each age group. The confidence intervals of the predicted live birth rates began to converge at 10 or more cryopreserved blastocysts. Given the statistical convergence and the relatively small sample size above 10 cryopreserved embryos, these intervals are not displayed. All three age groups had statistically significant cubed number of blastocysts terms, indicating departure from a strictly linear relationship, and favoring a curvi-linear association instead (
Figure 2).
3.2. Probability of Live Birth when Female Age is below 35 Years
For those aged below 35 years, the likelihood of having a live birth increased for each additional blastocyst cryopreserved in a linear progression from 0.33 (95% CI 0.31–0.34) if one supernumerary blastocyst was cryopreserved to 0.80 (95% CI 0.74–0.86; P<.0001) if 6 blastocysts were cryopreserved, with an average increase in the live birth rate of 7.8% with each additional blastocyst cryopreserved up to 6. The likelihood of having a live birth then increased non-linearly, to 0.95 (95% CI 0.92–0.97; P<.0001) if 10 or more blastocyst were cryopreserved, with an average increase in the live birth rate of 3.8% with each additional blastocyst cryopreserved after 6 up to 10 or more.
3.3. Probability of Live Birth when Female Age is between 35 and 39 Years
For those aged between 35-39 years. the likelihood of having a live birth increased for each additional blastocyst cryopreserved in a linear progression from 0.30 (95% CI 0.28–0.32) if one supernumerary blastocyst was cryopreserved to 0.82 (95% CI 0.73–0.91; P<.0001) if 6 blastocysts were cryopreserved, with an average increase in the live birth rate of 8.6% with each additional blastocyst cryopreserved up to 6. The likelihood of having a live birth then increased non-linearly to 0.96 (95% CI 0.93–0.99; P<.0001) if 10 or more blastocyst were cryopreserved, with an average increase in the live birth rate of 3.5% with each additional blastocyst cryopreserved after 6 up to 10 or more.
3.4. Probability of Live Birth when Female Age is Aged 40 Years and Above
The likelihood of having a live birth increased for each additional blastocyst cryopreserved in a linear progression from 0.26 (95% CI 0.19–0.32) if one supernumerary blastocyst was cryopreserved to 0.83 (95% CI 0.68–0.97; P<.0001) if 4 blastocysts were cryopreserved, with an average increase in the live birth rate of 14.3% with each additional blastocyst cryopreserved up to 4. The likelihood of having a live birth then increased non-linearly to 0.99 (95% CI 0.98–0.99; P<.0001) if 10 or more blastocyst were cryopreserved, with an average increase in the live birth rate of 2.7% with each additional blastocyst cryopreserved after 4 up to 10 or more.
4. Discussion
To date, this is the largest study evaluating the relationship between the number of supernumerary blastocysts cryopreserved and the probability of a live birth after day 5 autologous non-PGT single fresh blastocyst transfer in women having their first IVF/ICSI cycle, and the impact of age on this relationship, using data from one IVF centre. Our results indicate that achieving blastocyst cryopreservation is associated with a significantly higher live birth rate and that there is a curvi-linear positive correlation between the number of supernumerary blastocysts cryopreserved and the probability of a live birth until 10 or more blastocysts are cryopreserved. This correlation remained valid at different age groups, suggesting that a cohort effect of human embryos exists in IVF/ICSI cycles at various age groups, including those aged 40 years and older (Romanski et al., 2018). It also indicates that ovarian stimulation may not have a detrimental impact on either oocyte/embryo quality or endometrial receptivity, lending support to similar observations in recent studies (Drakopoulos et al., 2016; Hariton et al., 2017; Polyzos et al., 2018)
The results of the present study are consistent with previous studies reporting a positive correlation between achieving embryo cryopreservation and clinical IVF outcomes, after both cleavage-stage embryo (Stern et al., 2009) and blastocyst transfer (Mullin et al., 2012). Our results also corroborate those of a much smaller study (Hill et al., 2013), which included 655 fresh autologous single blastocyst transfers in good prognosis patients and reported a positive correlation between the number of supernumerary blastocysts cryopreserved and the odds of a live birth after the fresh blastocyst transfer. However, this study did not limit the analysis to patients having their first IVF/ICSI cycle, included patients who had day 6 blastocyst transfer and did not evaluate the results according to specific age groups (Hill et al., 2013).
In addition, our results are consistent with the study of Papanikolou et al. (2019), which included 244 women who had blastocyst-stage embryo transfer and reported that the highest live birth rate after the first transfer was achieved in women who had the most number of blastocysts cryopreserved (7 or more blastocysts) compared to those who had fewer blastocysts cryopreserved.
The results of the present study contrast with those of a recent report (Smeltzer et al., 2019) which suggested that the likelihood of a live birth after single blastocyst transfer increased with the increase in the total number of blastocysts available up to five blastocysts, then decreased for every additional blastocyst available thereafter in patients up to age 35 years, but not in those aged 36-40 years. Unlike our study, the study of Smeltzer and colleagues analysed the number of all, rather than supernumerary, blastocysts generated in an IVF/ICSI cycle and excluded patients above the age of 40 years. Furthermore, the drop in live birth rate after five supernumerary embryos generated is not consistent with recent reports demonstrating an increase in the cumulative live birth rate with the increased availability of oocytes and embryos in the fresh cycle (Sunkara et al., 2011; McLernon et al., 2016; Drakopoulos et al., 2016; Vaughan et al., 2017; Ben-Nagi et al., 2019; Zhang et al., 2019). In addition, the dataset analysed in the study of Smeltzer and colleagues (2019) was based on a national database registry, reflecting different clinical and laboratory IVF practices, thus potentially introducing a degree of performance and reporting bias. Furthermore, the study of Smeltzer and colleagues suggested that patients aged 35 years or younger who have a total of more than 5 blastocysts available in a given IVF/ICSI should undergo PGT for aneuploidy (PGT-A) to better select the blastocyst suitable for transfer, whereas a recent randomised trial (Munne et al., 2019) demonstrated that PGT-A did not improve the overall pregnancy outcomes in women who have 2 or more blastocysts suitable for biopsy, particularly in those aged 35 years or younger.
Our study results are clinically relevant as they can be used to improve counselling of couples at the time of their first fresh embryo transfer by providing a realistic perspective of the probability of delivering a baby after single blastocyst transfer based on female age and the number of supernumerary blastocysts cryopreserved. Understanding the association between the probability of a live birth and the number of supernumerary blastocysts cryopreserved, could support a practice of single fresh blastocyst transfer when multiple supernumerary blastocysts are cryopreserved irrespective of patient age, and thus help to reduce the temptation to transfer more than one blastocyst, thereby reducing the overall risk of multiple births.
The strengths of the present study include the analysis of a large number of IVF/ICSI cycles performed without significant changes in clinical or laboratory protocols. In addition, by only including patients undergoing single blastocyst transfer in their first IVF/ICSI cycle and excluding patients undergoing cleavage-stage embryo transfer, PGT, or receiving donated oocytes, we analysed a relatively homogenous cohort of patients, thereby accounting for potential treatment modality confounders, as well as removing the influence of previous IVF cycles on the utilisation of embryos available in a second or subsequent cycle. Furthermore, we stratified our results by age group to guide clinical practice more precisely and enable clinicians to provide better counselling to patients embarking on their first fresh embryo transfer.
In conclusion, the present study results demonstrate that the number of supernumerary blastocysts cryopreserved in the first autologous fresh IVF/ICSI cycle can be a key factor in determining the probability of achieving a live birth after single blastocyst transfer across different age groups. This information can be helpful in patient counselling and promoting single embryo transfer to reduce the risk of multiple births.
Author Contributions
T El-Toukhy conceived the idea and contributed to the writing of the manuscript. M.Y. Beebeejaun analysed the data and contributed to the writing of the manuscript. T. Copeland provided statistical support and contributed to the writing of the manuscript. L. Polanski contributed to the writing of the manuscript. All authors approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial: or not-for-profit sectors.
Acknowledgments
We thank all the clinical and laboratory staff at our centre who provided patient care during the study period, and above all, the patients whom their treatment cycles were analysed.
Declarations of interest
None.
References
- Ben-Nagi, J.; Jones, B.; Naja, R.; Amer, A.; Sunkara, S.; SenGupta, S.; Serhal, P. Live birth rate is associated with oocyte yield and number of biopsied and suitable blastocysts to transfer in preimplantation genetic testing (PGT) cycles for monogenic disorders and chromosomal structural rearrangements. Eur J Obstet Gynecol Reprod Biol. 2019, 10, 100055. [Google Scholar] [CrossRef]
- Edgar, D.; Bourne, H.; Speirs, A.; McBain, J. A quantitative analysis of the impact of cryopreservation on the implantation potential of human early cleavage stage embryos. Hum. Reprod. 2000, 15, 175–179. [Google Scholar] [CrossRef]
- El-Toukhy, T.; Campo, R.; Khalaf, Y.; Tabanelli, C.; Gianaroli, L.; Gordts, S.S.; et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): A multicentre, randomised controlled trial. Lancet 2016, 387, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Schoolcraft, W.B. In vitro culture of human blastocyst. (1999) In: Jansen R, Mortimer D (eds). Towards Reproductive Certainty: Fertility and Genetics Beyond 1999. UK: Parthenon Publishing Carnforth, 378–388.
- Gardner, D.K.; Balaban, B. Choosing Between Day 3 and Day 5 Embryo Transfers. Clin. Obstet. Gynecol. 2006, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Harbottle, S.; Hughes, C.; Cutting, R.; Roberts, S.; Brison, D.; On Behalf Of The Association Of Clinical Embryologists; The British Fertility Society. Elective Single Embryo Transfer: An update to UK Best Practice Guidelines. Human Fertility 2015, 18, 165–183. [CrossRef] [PubMed]
- Hariton, E.; Kim, K.; Mumford, S.L.; Palmor, M.; Bortoletto, P.; Cardozo, E.R.; Karmon, A.E.; Sabatini, M.E.; Styer, A.K. Total number of oocytes and zygotes are predictive of live birth pregnancy in fresh donor oocyte in vitro fertilization cycles. Fertil. Steril. 2017, 108, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Richter, K.S.; Heitmann, R.J.; Lewis, T.D.; DeCherney, A.H.; Graham, J.R.; Widra, E.; Levy, M.J. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil. Steril. 2013, 99, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Lasalle, B.; Testart, J.; Renard, J. Human embryo features that influence the success of cryopreservation with the use of 1,2-propanediol. Fertil. Steril. 1985, 44, 645–651. [Google Scholar] [CrossRef]
- Marsh, C.A.; Farr, S.L.; Chang, J.; Kissin, D.M.; Grainger, D.A.; Posner, S.F.; Macaluso, M.; Jamieson, D.J. Trends and factors associated with the Day 5 embryo transfer, assisted reproductive technology surveillance, USA, 2001–2009. Hum. Reprod. 2012, 27, 2325–2331. [Google Scholar] [CrossRef]
- McLernon, D.J.; Steyerberg, E.W.; Velde, E.R.T.; Lee, A.J.; Bhattacharya, S. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ 2016, 355, i5735. [Google Scholar] [CrossRef]
- Mullin, C.; Berkeley, A.S.; Grifo, J.A. Supernumerary Blastocyst Cryopreservation: A key Prognostic Indicator for Patients Opting for an Elective Single Blastocyst Transfer (eSBT). J. Assist. Reprod. Genet. 2012, 29, 783–788. [Google Scholar] [CrossRef]
- Munné, S.; Kaplan, B.; Frattarelli, J.L.; Child, T.; Nakhuda, G.; Shamma, F.N.; Silverberg, K.; Kalista, T.; Handyside, A.H.; Katz-Jaffe, M.; et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil. Steril. 2019, 112, 1071–1079. [Google Scholar] [CrossRef]
- Naji, O.; Moska, N.; Dajani, Y.; El-Shirif, A.; El-Ashkar, H.; Hosni, M.M.; Khalil, M.; Khalaf, Y.; Bolton, V.; El-Toukhy, T. Early oocyte denudation does not compromise ICSI cycle outcome: a large retrospective cohort study. Reprod. Biomed. Online 2018, 37, 18–24. [Google Scholar] [CrossRef]
- Papanikolaou, E.; Chartomatsidou, T.; Timotheou, E.; Tatsi, P.; Katsoula, E.; Vlachou, C.; Asouchidou, I.; Zafeiratis, O.; Najdecki, R. In Freeze-All Strategy, Cumulative Live Birth Rate (CLBR) Is Increasing According to the Number of Blastocysts Formed in Women <40 Undergoing Intracytoplasmic Sperm Injection (ICSI). Front. Endocrinol. 2019, 10, 427. [Google Scholar] [CrossRef]
- Drakopoulos, P.; Blockeel, C.; Stoop, D.; Camus, M.; de Vos, M.; Tournaye, H.; Polyzos, N. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum. Reprod. 2016, 31, 370–376. [Google Scholar] [CrossRef]
- Polyzos, N.P.; Drakopoulos, P.; Parra, J.; Pellicer, A.; Santos-Ribeiro, S.; Tournaye, H.; Bosch, E.; Garcia-Velasco, J. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil. Steril. 2018, 110, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Romanski, P.A.; Goldman, R.H.; Farland, L.V.; Srouji, S.S.; Racowsky, C. The association between quality of supernumerary embryos in a cohort and implantation potential of the transferred blastocyst. J. Assist. Reprod. Genet. 2018, 35, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G. Regression Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. J. R. Stat. Soc. Ser. C (Applied Stat. 1994, 43, 429–453. [Google Scholar] [CrossRef]
- Scheffer, J.B.; Scheffer, B.B.; de Carvalho, R.F.; Rodrigues, J.; Grynberg, M.; Lozano, D.H.M. Age as A Predictor of Embryo Quality Regardless of The Quantitative Ovarian Response. Int J Fertil Steril. 2017, 11, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, A.; Sjöblom, P.; Hamberger, L. Culture of human spare pre-embryos: Association between blastocyst formation and pregnancy. J Assist Reprod Genet. 1992, 9, 41–44. [Google Scholar] [CrossRef]
- Smeltzer, S.; Acharya, K.; Truong, T.; Pieper, C.; Muasher, S. Clinical pregnancy (CP) and live birth (LB) increase significantly with each additional fertilized oocyte up to nine, and decline after that: An analysis of 15,803 first fresh in vitro fertilization cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2019, 112, 520–526. [Google Scholar] [PubMed]
- StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.
- Stern, J.E.; Goldman, M.B.; Hatasaka, H.; MacKenzie, T.A.; Surrey, E.S.; Racowsky, C. Optimizing the number of cleavage stage embryos to transfer on day 3 in women 38 years of age and older: a Society for Assisted Reproductive Technology database study. Fertil. Steril. 2009, 91, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.G.; Lan, L.; Shah, A.A.; Yeh, J.S.; Price, T.M.; Goldfarb, J.M.; Muasher, S.J. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: An analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014, 101, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.K.; Coomarasamy, A.; Khalaf, Y.; Braude, P. A three-arm randomised controlled trial comparing Gonadotrophin Releasing Hormone (GnRH) agonist long regimen versus GnRH agonist short regimen versus GnRH antagonist regimen in women with a history of poor ovarian response undergoing in vitro fertilisation (IVF) treatment: Poor responders intervention trial (PRINT). Reprod. Health 2007, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Javier Zamora, J.; Coomarasamy, A. Association between the number of eggs and live birth in IVF treatment: An analysis of 400,135 treatment cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Coomarasamy, A.; Faris, R.; Braude, P.; Khalaf, Y. Long gonadotropin-releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: a randomized controlled trial. Fertil. Steril. 2014, 101, 147–153. [Google Scholar] [CrossRef]
- Tiitinen, A.; Halttunen, M.; Härkki, P.; Vuoristo, P.; Hyden-Granskog, C. Elective single embryo transfer: the value of cryopreservation. Hum. Reprod. 2001, 16, 1140–1144. [Google Scholar] [CrossRef]
- Baker, V.L.; Brown, M.B.; Luke, B.; Smith, G.W.; Ireland, J.J. Gonadotropin dose is negatively correlated with live birth rate: Analysis of more than 650,000 assisted reproductive technology cycles. Fertil. Steril. 2015, 104, 1145–1152. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Leung, A.; Resetkova, N.; Ruthazer, R.; Penzias, A.S.; Sakkas, D.; Alper, M.M. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil. Steril. 2016, 107, 397–404. [Google Scholar] [CrossRef]
- Veeck, L.L.; Amundson, C.H.; Brothman, L.J.; DeScisciolo, C.; Maloney, M.K.; Muasher, S.J.; Jones, H.W. Significantly enhanced pregnancy rates per cycle through cryopreservation and thaw of pronuclear stage oocytes. Fertil. Steril. 1993, 59, 1202–1207. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, T.; Tian, H.; Li, X.; Wang, D.; Wan, X.; Wang, Q.; Mao, X.; La, X. Use of Cumulative Live Birth Rate per Total Number of Embryos to Calculate the Success of IVF in Consecutive IVF Cycles in Women Aged ≥35 Years. BioMed Res. Int. 2019, 2019, 6159793. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).