Submitted:
21 April 2023
Posted:
23 April 2023
You are already at the latest version
Abstract
Keywords:
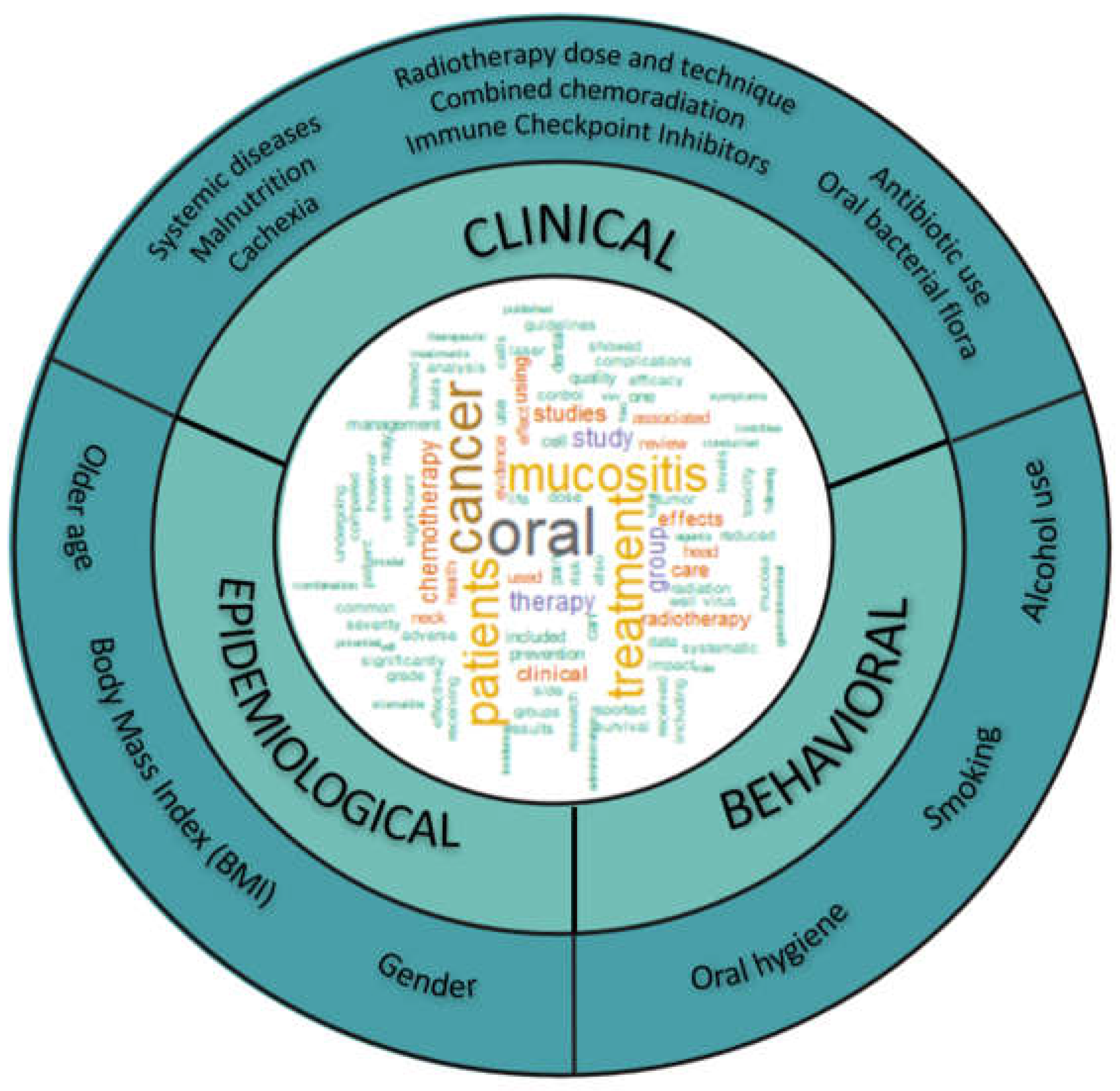
1. Introduction
2. "Omics" approaches may help in risk assessment and developing personalized and more effective therapies OM
2.1. Genomics
2.1.1. Hypothesis-driven Approach
2.1.2. Candidate-Gene Studies
2.1.3. Pathway-Based
| Year | First Author | Sample Size | Sample | Phenotype | Genes | SNPs |
|---|---|---|---|---|---|---|
| 2022 | Schack [46] | Discovery=1183 Danish Cohort Replication=597 Danish Cohort Validation=235 Asian Cohort |
Buffy coats | Mucositis 0: no 1: erythema 2: patchy 3: confluent 4:ulceration |
STING1 | rs1131769 |
| 2020 | Mlak [42] | 60 | Peripheral blood | Mucositis RTOG/EORTC |
TNFRS1 A | rs767455 |
| 2020 | Mlak [43] | 62 | Peripheral blood | Mucositis RTOG/EORTC |
TNF alpha | rs1799964 |
| 2020 | Yang [47] | 960 560 |
Blood | RTOG/EORTC | TNKS | rs117157809 |
| 2018 | Brzozowska [44] | 62 | Peripheral blood |
Mucositis RTOG/EORTC |
APEH | rs4855883 |
| 2018 | Brzozowska [41] | 58 | Peripheral blood |
Mucositis RTOG/EORTC |
TNFRS1 A | rs4149570 |
| 2018 | Brzozowska [40] | 65 | Peripheral blood | Mucositis RTOG/EORTC |
GHRL | Rs1629816 |
| 2017 | Chen [45] | 114 | Peripheral blood | Mucositis RTOG/VRS |
XRCC1 | rs25487 |
| 2017 | Reyes-Gibby [39] | 885 | Peripheral blood | Oral Mucositis (ICD) | RB1 | rs2227311 |
2.1.4. Genome-wide Approach
2.2. Microbiomics of OM
2.3. Metabolomics
2.4. Proteomics and transcriptomics
| Year | First Author | Phenotype | Samples | Sample size | Methods | Targets | Results |
|---|---|---|---|---|---|---|---|
| Metabolomics. | |||||||
| Animal Models | |||||||
| 2021 | Geng [59] | Mucositis (0-5) | Serum from OM rat model, induced with 5-FU and 10% acetic acid |
30 rats | UHPLC | Cholic acid, linoleic acid, 4-pyridoxic acid, LysoPC | Shuanghua Baihe tablets improve inflammatory symptoms of oral mucositis. |
| 2020 | Chen | Induced oral ulcers and degree of healing | Serum from OM rat model, induced with 15% chloral hydrate |
42 rats |
LC-QTOF/MS | 5-HT, GABA | Kouyanqing granules attenuate the symptoms of oral ulcers worsened by sleep deprivation through regulation of the neuroimmunoendocrine system, oxidative stress levels, and tryptophan metabolism. |
| Clinical Samples | |||||||
| 2021 | Yatsuoka [56] | NCI CTCAE | Saliva | 9 HNC |
CE-TOF-MS | tryptophan, D-glucose, D-glutamate, GABA, 2-AB | Pre-treatment concentrations of gamma-aminobutyric acid and 2-aminobutyric acids were higher in the high-grade OM group. |
| 2021 | Yang [57] | NRS 0-10 | Peripheral blood | 10 NPC |
UHPLC-MS/MS | 9-HEPE, 15-HETE | MOM promotes the release of anti-inflammatory lipids to reduce tissue damage; enhancement of 9S-HEPE and 15-HETE in all radiation doses. |
| Microbiomics | |||||||
| 2020 | Reyes-Gibby [7] | NCI CTCAE | buccal mucosal | 66 Locoregional HNSCC |
16S rRNA |
Cardiobacterium, Granulicatella, Prevotella, Fusobacterium, Streptococcus , Megasphaera, Cardiobacterium |
Genera abundance was associated with the hazard for the onset of severe OM. |
| 2020 | Vesty [61] | WHO | saliva and oral swabs | 19 HNC |
NGS | Fusobacterium, Haemophilus, Tannerella, Porphyromonas and Eikenella, Candida | Gram-negative bacteria on the buccal mucosa may influence susceptibility to developing OM. |
| 2019 | Subramaniam and Muthukrishnan [54] | WHO | unstimulated whole saliva | 24 HNSCC |
16S rRNA | Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae | The bacterial isolates obtained during and at the end of therapy appeared to express a higher level of antibiotic-resistance genes (VIM2, MCR-1, TET[K], blaKPC) than those isolated at the onset of therapy. |
| 2018 | Hou [53] | RTOG | oral swabs | 19 NPC |
16S rRNA | Prevotella, Fusobacterium, Treponema, Porphyromonas | Prevotella, Fusobacterium, Treponema and Porphyromonas showed dynamic synchronous variations in abundance throughout the course of radiation therapy, frequently coinciding with the onset of severe mucositis. |
| 2017 | Zhu [62] | RTOG | oral or retropharyngeal mucosa swabs | 41 NPC |
16S rRNA | Firmicutes, Proteobacteria, Bacteroidetes, Fusobacteria, Actinobacteria, Spirochaetes, Cyanobacteria, Verrucomicrobia, Acidobacteria, TM7, Deinococcus-Thermus and SR1 | Oral microbiota changes correlate with the progression and aggravation of radiotherapy-induced mucositis in patients with nasopharyngeal carcinoma. |
| Microbiota | |||||||
| 2018 | Almstahl [63] | WHO | Swab culture | 33 HNC |
Culture | Neisseria, Fusobacterium, Prevotella, Candida | Levels of Neisseria decreased and mucosal pathogens increased during RT; 2 years post-treatment, Fusobacterium and Prevotella decreased; growth of Candida increased |
| 2018 | Gaetti-Jardim [64] | NCI CTCAE | Supra and subgingival biofilms | 28 HNC |
Culture | Candida, Enterobacteriaceae | Candida and family Enterobacteriaceae showed increased prevalence with RT, and were associated with the occurrence of mucositis and xerostomia |
| Transcriptomics | |||||||
| Animal Models | |||||||
| 2021 | Geng [59] | Mucositis (0-5) | Serum from OM rat model, induced with 5-FU and 10% acetic acid |
30 rats |
Whole genome sequencing | ALOX15, CYP2J2, CYP1A1, ALOX15, GATM, ALAS2, PLA2G5 | Shuanghua Baihe tablets improve inflammatory symptoms of oral mucositis. |
| 2021 | Saul-McBeth [65] | Induced oral ulcers, % damage | OM mice model; Induced with head and neck irradiation |
3 mice |
RNA Seq | IL-17RA | IL-17RA provides protection during HNI-induced OM by preventing excess inflammation during ulceration phase of OM. |
| Clinical Samples | |||||||
| 2018 | Mlak [66] | RTOG/EORTC | Plasma | 60 HNC |
Microarray | RRM1 | RRM1 gene expression in cfRNA allows for estimating risk of severe OM. |
| Proteomics | |||||||
| 2015 | Jehmlich [67] | NCI CTC v3 | Unstimulated whole saliva | 50 HNC |
MS | RPL18A, C6orf115, PRTN3, RPS20, FGB, ARPC1B, PLBD1, GGH, ANXA6, FGG, ANP32E, CTSG, PTGR1, SERPINA1, MDH2, CORO1A, HSPE1, BAHCC1 CP, MMP9, GCA, PLYRP1, SCGB2A1, GPI, PPIC, QRDL, HIST1H4A, HNRNPA2B1, ATP5B, LTA4H, TIMP1, TKT, RPL10A, AZU1, MMP8, RPLP2, ARPC4, CAT, S100A8, B2M, SERPING1, CYBB, ELANE, C3, CALML5, ITIHRPS15A, ACTR2 | 48 proteins differed significantly between OM group and non-OM group. 17 proteins displayed increased levels and 31 proteins decreased in level in OM. |
3. Conclusions
4. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J Clin 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin 2016, 66, 203–239. [Google Scholar] [CrossRef] [PubMed]
- Devices@Fda, A.U. Available online: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm (accessed on 1 April 2023).
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front Oncol 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gibby, C.C.; Wang, J.; Zhang, L.; Peterson, C.B.; Do, K.A.; Jenq, R.R.; Shelburne, S.; Shah, D.P.; Chambers, M.S.; Hanna, E.Y.; et al. Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer 2020, 126, 5124–5136. [Google Scholar] [CrossRef]
- Sunaga, T.; Nagatani, A.; Fujii, N.; Hashimoto, T.; Watanabe, T.; Sasaki, T. The association between cumulative radiation dose and the incidence of severe oral mucositis in head and neck cancers during radiotherapy. Cancer Rep (Hoboken) 2021, 4, e1317. [Google Scholar] [CrossRef] [PubMed]
- de Pauli Paglioni, M.; Faria, K.M.; Palmier, N.R.; Prado-Ribeiro, A.C.; RB, E.D.; da Graça Pinto, H.; Treister, N.S.; Epstein, J.B.; Migliorati, C.A.; Santos-Silva, A.R.; et al. Patterns of oral mucositis in advanced oral squamous cell carcinoma patients managed with prophylactic photobiomodulation therapy-insights for future protocol development. Lasers Med Sci 2021, 36, 429–436. [Google Scholar] [CrossRef]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur J Oncol Nurs 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Elad, S.; Zadik, Y. Chronic oral mucositis after radiotherapy to the head and neck: a new insight. Support Care Cancer 2016, 24, 4825–4830. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Melkonian, S.C.; Hanna, E.Y.; Yeung, S.J.; Lu, C.; Chambers, M.S.; Banala, S.R.; Gunn, G.B.; Shete, S.S. Cohort study of oncologic emergencies in patients with head and neck cancer. Head Neck 2017, 39, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.S.; Dutra, B.E.; Garcia-Rodriguez, V.; Panneerselvam, K.; Abraham, F.O.; Zou, F.; Ma, W.; Grivas, P.; Thompson, J.A.; Altan, M.; et al. Clinical Characteristics and Outcomes of Oral Mucositis Associated With Immune Checkpoint Inhibitors in Patients With Cancer. J Natl Compr Canc Netw 2021, 19, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Epstein, J.B.; Raber-Durlacher, J.; Donnelly, P.; Strahilevitz, J. The antimicrobial effect of Iseganan HCl oral solution in patients receiving stomatotoxic chemotherapy: analysis from a multicenter, double-blind, placebo-controlled, randomized, phase III clinical trial. J Oral Pathol Med 2012, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, N.; Sonis, S.T.; Villa, A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women's Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer 2021, 127, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Davy, C.; Heathcote, S. A systematic review of interventions to mitigate radiotherapy-induced oral mucositis in head and neck cancer patients. Support Care Cancer 2021, 29, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, H.M.; Alsini, A.Y.; Alsubaie, K.M.; Abu-Zaid, A.; Alzahrani, F.R.; Sayed, S.; Pathak, A.K.; Alqahtani, K.H. Glutamine for prevention and alleviation of radiation-induced oral mucositis in patients with head and neck squamous cell cancer: Systematic review and meta-analysis of controlled trials. Head Neck 2021, 43, 3199–3213. [Google Scholar] [CrossRef]
- Al-Rudayni, A.H.M.; Gopinath, D.; Maharajan, M.K.; Veettil, S.K.; Menon, R.K. Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Curr Oncol 2021, 28, 2852–2867. [Google Scholar] [CrossRef]
- Di Fede, O.; Canepa, F.; Maniscalco, L.; Tozzo, P.; Matranga, D.; Giuliana, G. Prevention and the treatment of oral mucositis: the efficacy of sodium bicarbonate vs other agents: a systematic review. BMC Oral Health 2023, 23, 4. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent J (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Zadik, Y.; Arany, P.R.; Fregnani, E.R.; Bossi, P.; Antunes, H.S.; Bensadoun, R.J.; Gueiros, L.A.; Majorana, A.; Nair, R.G.; Ranna, V.; et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3969–3983. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- World Health, O. WHO handbook for reporting results of cancer treatment. 1979.
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis, N.C.I.D.D.o.C.T. CTEP Cancer Therapy Evaluation Program. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 1 April 2023).
- Villa, A.; Vollemans, M.; De Moraes, A.; Sonis, S. Concordance of the WHO, RTOG, and CTCAE v4.0 grading scales for the evaluation of oral mucositis associated with chemoradiation therapy for the treatment of oral and oropharyngeal cancers. Support Care Cancer 2021, 29, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Byhardt, R.; Stetz, J.; Gwede, C.; Corn, B.; Fu, K.; Gunderson, L.; McCormick, B.; Morrisintegral, M.; Rich, T.; et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000, 47, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Development of a staging system for chemotherapy-induced stomatitis. Western Consortium for Cancer Nursing Research. Cancer Nurs 1991, 14, 6–12.
- Eilers, J.; Berger, A.M.; Petersen, M.C. Development, testing, and application of the oral assessment guide. Oncol Nurs Forum 1988, 15, 325–330. [Google Scholar] [PubMed]
- Sonis, S.T.; Eilers, J.P.; Epstein, J.B.; LeVeque, F.G.; Liggett, W.H., Jr.; Mulagha, M.T.; Peterson, D.E.; Rose, A.H.; Schubert, M.M.; Spijkervet, F.K.; et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 1999, 85, 2103–2113. [Google Scholar] [CrossRef]
- Stiff, P.J.; Emmanouilides, C.; Bensinger, W.I.; Gentile, T.; Blazar, B.; Shea, T.C.; Lu, J.; Isitt, J.; Cesano, A.; Spielberger, R. Palifermin reduces patient-reported mouth and throat soreness and improves patient functioning in the hematopoietic stem-cell transplantation setting. J Clin Oncol 2006, 24, 5186–5193. [Google Scholar] [CrossRef]
- Stiff, P.J.; Erder, H.; Bensinger, W.I.; Emmanouilides, C.; Gentile, T.; Isitt, J.; Lu, Z.J.; Spielberger, R. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant 2006, 37, 393–401. [Google Scholar] [CrossRef]
- Slade, G.D. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol 1997, 25, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Mendoza, T.R.; Chambers, M.S.; Burkett, V.S.; Garden, A.S.; Hessell, A.C.; Lewin, J.S.; Ang, K.K.; Kies, M.S.; Gning, I.; et al. The M. D. Anderson symptom inventory-head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis. Int J Radiat Oncol Biol Phys 2008, 72, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Kushner, J.A.; Lawrence, H.P.; Shoval, I.; Kiss, T.L.; Devins, G.M.; Lee, L.; Tenenbaum, H.C. Development and validation of a Patient-Reported Oral Mucositis Symptom (PROMS) scale. J Can Dent Assoc 2008, 74, 59. [Google Scholar] [PubMed]
- Institute, N.H.G.R. The Human Genome Project. Available online: https://www.genome.gov/human-genome-project (accessed on 1 April 2023).
- Bates, S.A. Telomere-to-Telomere (T2T) consortium reasearchers to discuss completion of a human genome sequence. Available online: https://www.genome.gov/news/media-adivsory/telomere-to-telomere-t2t-consortium-researchers-to-discuss-completion-of-human-genome-sequence (accessed on 1 April 2023).
- Reyes-Gibby, C.C.; Melkonian, S.C.; Wang, J.; Yu, R.K.; Shelburne, S.A.; Lu, C.; Gunn, G.B.; Chambers, M.S.; Hanna, E.Y.; Yeung, S.J.; et al. Identifying novel genes and biological processes relevant to the development of cancer therapy-induced mucositis: An informative gene network analysis. PLoS ONE 2017, 12, e0180396. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Homa-Mlak, I.; Mlak, R.; Golebiowski, P.; Mazurek, M.; Ciesielka, M.; Malecka-Massalska, T. Polymorphism of regulatory region of GHRL gene (-2531C>T) as a promising predictive factor for radiotherapy-induced oral mucositis in patients with head neck cancer. Head Neck 2018, 40, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Powrozek, T.; Homa-Mlak, I.; Mlak, R.; Ciesielka, M.; Golebiowski, P.; Malecka-Massalska, T. Polymorphism of Promoter Region of TNFRSF1A Gene (-610 T > G) as a Novel Predictive Factor for Radiotherapy Induced Oral Mucositis in HNC Patients. Pathol Oncol Res 2018, 24, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Golebiowski, P.; Korzeb, D.; Rahnama-Hezavah, M.; Malecka-Massalska, T. Polymorphism of TNFRSF1 A may act as a predictor of severe radiation-induced oral mucositis and a prognosis factor in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol 2020, 130, 283–291.e282. [Google Scholar] [CrossRef] [PubMed]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Golebiowski, P.; Sobieszek, G.; Malecka-Massalska, T. The relationship between TNF-alpha gene promoter polymorphism (- 1211 T > C), the plasma concentration of TNF-alpha, and risk of oral mucositis and shortening of overall survival in patients subjected to intensity-modulated radiation therapy due to head and neck cancer. Support Care Cancer 2020, 28, 531–540. [Google Scholar] [CrossRef]
- Brzozowska, A.; Mlak, R.; Homa-Mlak, I.; Golebiowski, P.; Mazurek, M.; Ciesielka, M.; Malecka-Massalska, T. Polymorphism of regulatory region of APEH gene (c.-521G>C, rs4855883) as a relevant predictive factor for radiotherapy induced oral mucositis and overall survival in head neck cancer patients. Oncotarget 2018, 9, 29644–29653. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.; Li, G.; Hua, L.; Chen, S.; Huang, H. Association between XRCC1 single-nucleotide polymorphism and acute radiation reaction in patients with nasopharyngeal carcinoma: A cohort study. Medicine (Baltimore) 2017, 96, e8202. [Google Scholar] [CrossRef]
- Schack, L.M.H.; Naderi, E.; Fachal, L.; Dorling, L.; Luccarini, C.; Dunning, A.M.; Head; Neck Group of the Radiogenomics, C.; Danish, H.; Neck Cancer, G.; et al. A genome-wide association study of radiotherapy induced toxicity in head and neck cancer patients identifies a susceptibility locus associated with mucositis. Br J Cancer 2022, 126, 1082–1090. [CrossRef]
- Yang, D.W.; Wang, T.M.; Zhang, J.B.; Li, X.Z.; He, Y.Q.; Xiao, R.; Xue, W.Q.; Zheng, X.H.; Zhang, P.F.; Zhang, S.D.; et al. Genome-wide association study identifies genetic susceptibility loci and pathways of radiation-induced acute oral mucositis. J 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, R.; Tang, D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther 2021, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Jin, L. TMEM173 variants and potential importance to human biology and disease. Genes Immun 2019, 20, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 2019, 20, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: infection, inflammation and cancer. Nat Rev Immunol 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Dregalla, R.C.; Zhou, J.; Idate, R.R.; Battaglia, C.L.; Liber, H.L.; Bailey, S.M. Regulatory roles of tankyrase 1 at telomeres and in DNA repair: suppression of T-SCE and stabilization of DNA-PKcs. Aging (Albany NY) 2010, 2, 691–708. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, H.; Li, P.; Liu, H.; Zhou, H.; Yang, X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol 2018, 129, 44–51. [Google Scholar] [CrossRef]
- Subramaniam, N.; Muthukrishnan, A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J 2019, 10, e12454. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: a review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Enomoto, A.; Ota, S.; Sugimoto, M.; Uezono, Y. Time-Course of Salivary Metabolomic Profiles during Radiation Therapy for Head and Neck Cancer. J 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Feng, D.; Li, F.; Luo, B.; Zhu, J.; Yang, Q.; Zheng, L.; Dong, Q.; Chen, M.; Xu, Z.; et al. A randomized, controlled phase II trial of maxillofacial and oral massage in attenuating severe radiotherapy-induced oral mucositis and lipid metabolite changes in nasopharyngeal carcinoma. Radiother Oncol 2021, 163, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br J Pharmacol 2017, 174, 2358–2372. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.S.; Liu, R.J.; Shen, Z.B.; Wei, Q.; Zheng, Y.Y.; Jia, L.Q.; Wang, L.H.; Li, L.F.; Li, J.; Xue, W.H. Transcriptome sequencing and metabolome analysis reveal the mechanism of Shuanghua Baihe Tablet in the treatment of oral mucositis. Chin J Nat Med 2021, 19, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, C.; Jin, D.; Shi, Y.; Zhang, M.; Bo, M.; Qian, D.; Wang, M.; Li, G. Metabolomics Combined with Network Pharmacology-Based Strategy to Reveal the Underlying Mechanism of Zhenhuang Submicron Emulsion in Treating Oropharyngeal Mucositis Complications of Radiation Therapy for Head and Neck Cancer. Drug Des Devel Ther 2022, 16, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Yang, X.J.; Chao, Y.L.; Zheng, H.M.; Sheng, H.F.; Liu, H.Y.; He, Y.; Zhou, H.W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Almstahl, A.; Finizia, C.; Carlen, A.; Fagerberg-Mohlin, B.; Alstad, T. Mucosal microflora in head and neck cancer patients. Int 2018, 16, 459–466. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E., Jr.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch Oral Biol 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front 2021, 12, 687627. [Google Scholar] [CrossRef]
- Mlak, R.; Powrozek, T.; Brzozowska, A.; Homa-Mlak, I.; Mazurek, M.; Malecka-Massalska, T. RRM1 gene expression evaluated in the liquid biopsy (blood cfRNA) as a non-invasive, predictive factor for radiotherapy-induced oral mucositis and potential prognostic biomarker in head and neck cancer patients. Cancer Biomark 2018, 22, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Volker, U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J Proteomics 2015, 125, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Camino, A.; Oosterom, N.; den Hoed, M.A.H.; Lopez-Lopez, E.; Martin-Guerrero, I.; Pluijm, S.M.F.; Pieters, R.; de Jonge, R.; Tissing, W.J.E.; Heil, S.G.; et al. The miR-1206 microRNA variant is associated with methotrexate-induced oral mucositis in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics 2017, 27, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Fan, M.; Zhou, D.; Hong, Y.; Zhang, J.; Liu, H.; Sharma, S.; Wang, G.; Dong, Q. miR-200c Modulates the Pathogenesis of Radiation-Induced Oral Mucositis. Oxid Med Cell Longev 2019, 2019, 2352079. [Google Scholar] [CrossRef] [PubMed]
- Kiyomi, A.; Yoshida, K.; Arai, C.; Usuki, R.; Yamazaki, K.; Hoshino, N.; Kurokawa, A.; Imai, S.; Suzuki, N.; Toyama, A.; et al. Salivary inflammatory mediators as biomarkers for oral mucositis and oral mucosal dryness in cancer patients: A pilot study. PLoS ONE 2022, 17, e0267092. [Google Scholar] [CrossRef]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Volker, U. Proteome data of whole saliva which are associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. Data Brief 2016, 8, 501–505. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Wu, X.; Spitz, M.; Kurzrock, R.; Fisch, M.; Bruera, E.; Shete, S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol 2008, 9, 777–785. [Google Scholar] [CrossRef]
- Health, N.I.O. NIDCR Prospective Observational or Biomarker Validation Study Cooperative Agreement. Available online: https://grants.nih.gov/grants/guide/pa-files/PAR-20-060.html (accessed on 13 April 2023).

| Scale | Items |
|---|---|
| World Health Organization* [24] | 0=No changes; 1= Soreness/erythema; 2= Soreness/erythema; ulceration ability to eat solid food; 3= Soreness/erythema + ulceration + ability to use a liquid diet only; 4= Soreness/erythema + ulceration + no possible oral alimentation |
| Radiation Therapy Oncology Group [25,28] | Grade I: Erythema; Grade II: Patchy reaction (<1.5 cm, non-contiguous); Grade III: Confluent mucositis (>1.5 cm, contiguous); Grade IV: Ulceration, necrosis, bleeding. |
| National Cancer Institute [26] | Grade 0 (None) None; Grade 1 (Mild) Painless ulcers, erythema, or mild soreness in the absence of lesions; Grade 2 (Moderate) Painful erythema, oedema, or ulcers but eating or swallowing possible; Grade 3 (Severe) Painful erythema, oedema, or ulcers requiring IV hydration; Grade 4 (Life-threatening) Severe ulceration or requiring parenteral or enteral nutritional support or prophylactic intubation; Grade 5 (Death) Death related to toxicity |
| Western Consortium for Cancer Nursing Research [29] | Lesions: none; Color: pink; Bleeding: none; 1= Lesions: 1–4; Color: slight red; Bleeding: N/A; 2= Lesions: >4; Color: moderate red; Bleeding: with eating and oral hygiene; 3= Lesions: coalescing; Color: very red; Bleeding: spontaneous; |
| Oral Assessment Guide [30] | Eight categories of oral health (lips, tongue, gums and tissues, saliva, natural teeth, dentures, oral cleanliness and dental pain) are assessed as healthy, changes or unhealthy. |
| Oral Mucositis Assessment Scale (OMAS) [31] | Graded 0–3 for ulceration and 0–2 for erythema at nine sites within the oral cavity. |
| The Oral Mucositis Weekly Questionnaire | 8 items; Likert scale: Soreness/Pain, Activity limitation, Quality of Life |
| Oral mucositis daily questionnaire [32,33] | 10 items; Likert scale: Overall health, Severity , Functional limitations |
| Oral Health Impact Profile-14 (OHIP-14) [34] | 14 items; Likert scale : Functional limitation, Physical pain, Psychological discomfort, Psychological disability, Physical disability, Social disability, Handicap |
| MD Anderson Symptom Inventory- Head and Neck (MDASI-HN) [35] | 28 items: 0–10 scale Along with the core MDASI’s 13 symptom items and 6 interference items, the MDASI-HN also assesses 9 symptoms relevant to head and neck cancer |
| Patient-Reported Oral Mucositis Symptoms (PROMS) [36] | 10 items: Visual analogue scale : Pain, Oral functions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





