Submitted:
17 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theoretical section
2.1. Aluminum surface and its reaction with water
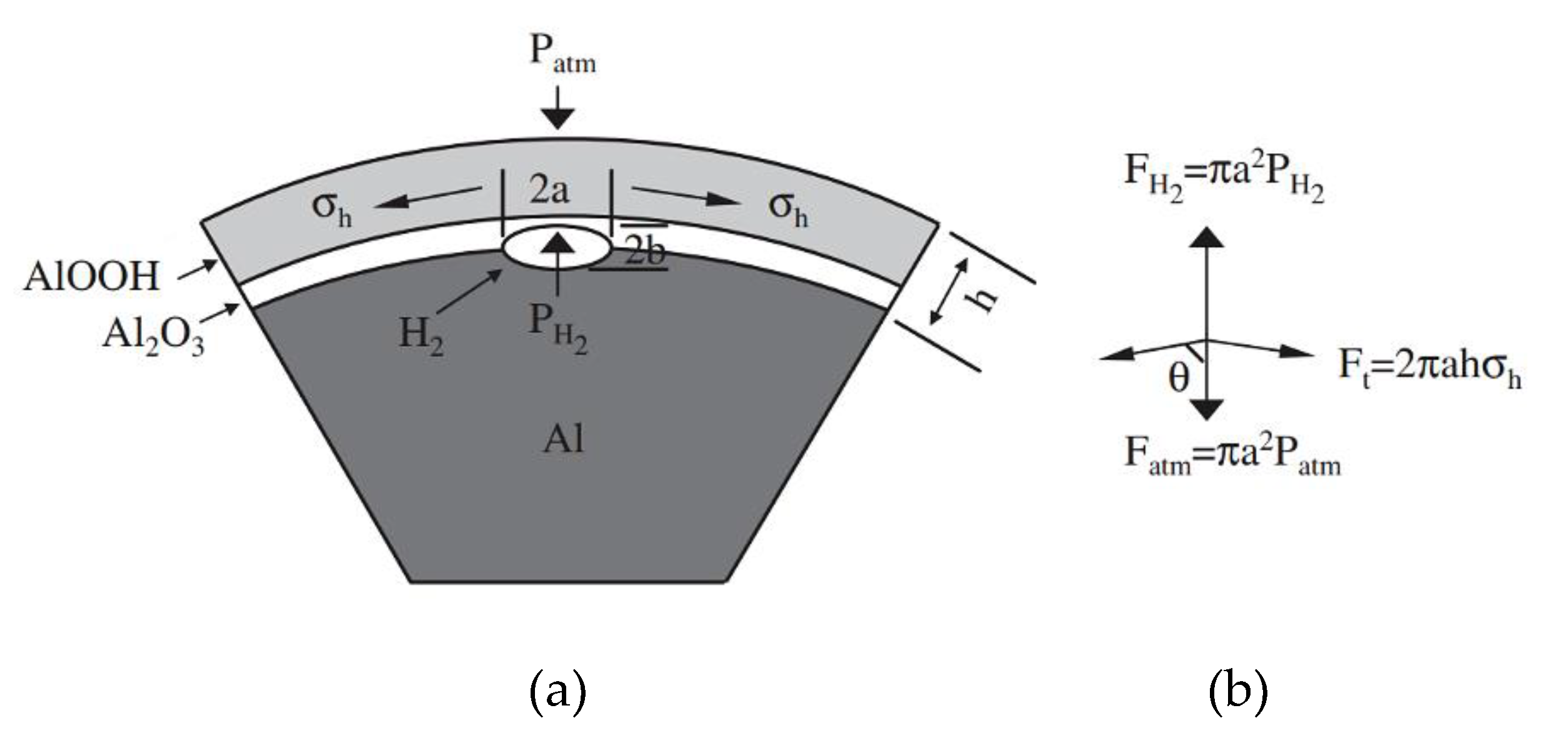
2.2. The role of the alkali promoter
2.3. Determining the activation energy
2.4. Reaction kinetics in two steps
2.4.1. Surface reaction rate step
2.4.2. Mass transfer rate step
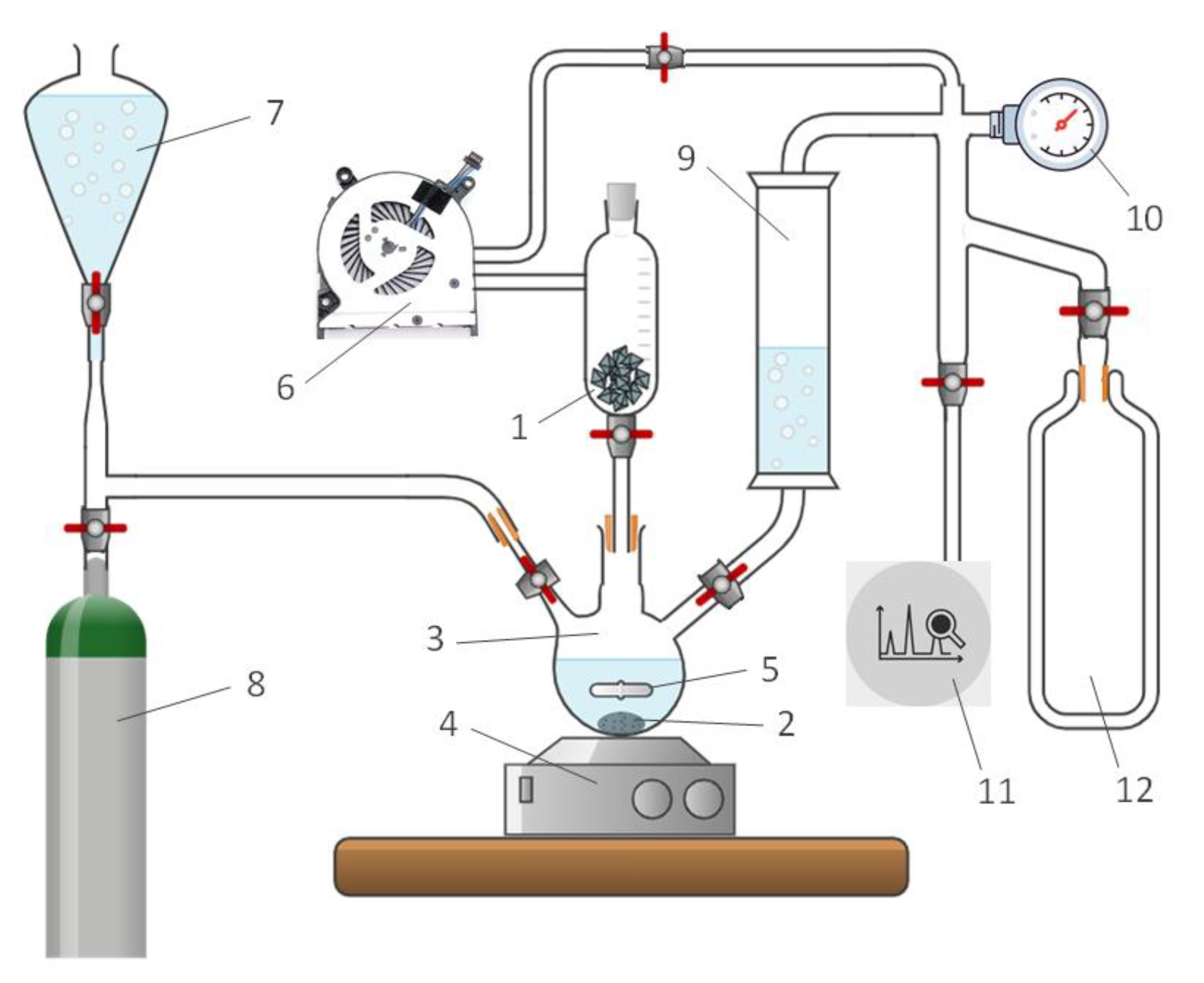
3. Materials and Methods
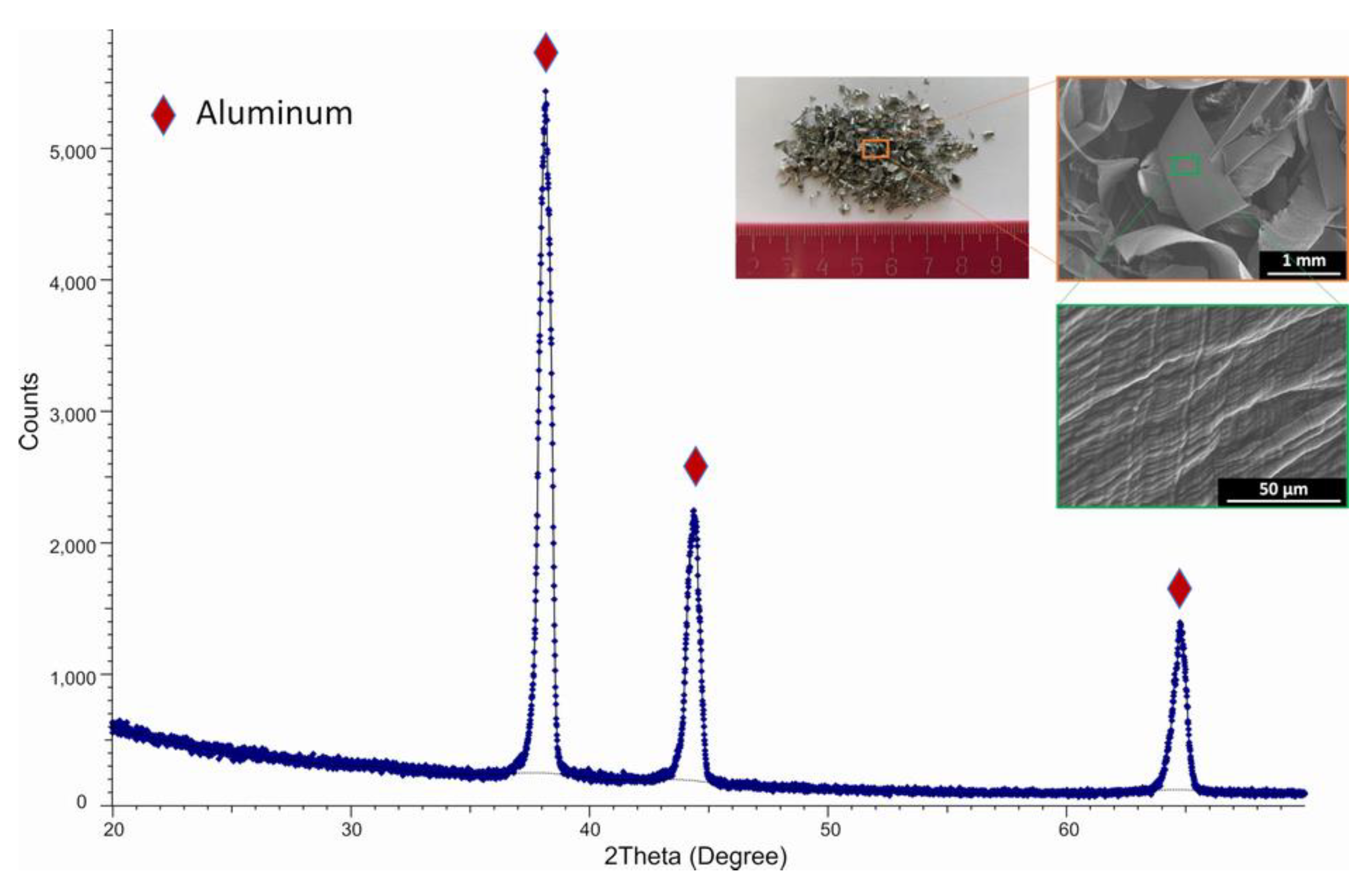
3.1. Experimental sample
3.2. Experimental setup
4. Results and Discussion
4.1. Stirring adjustment at the surface reaction rate step
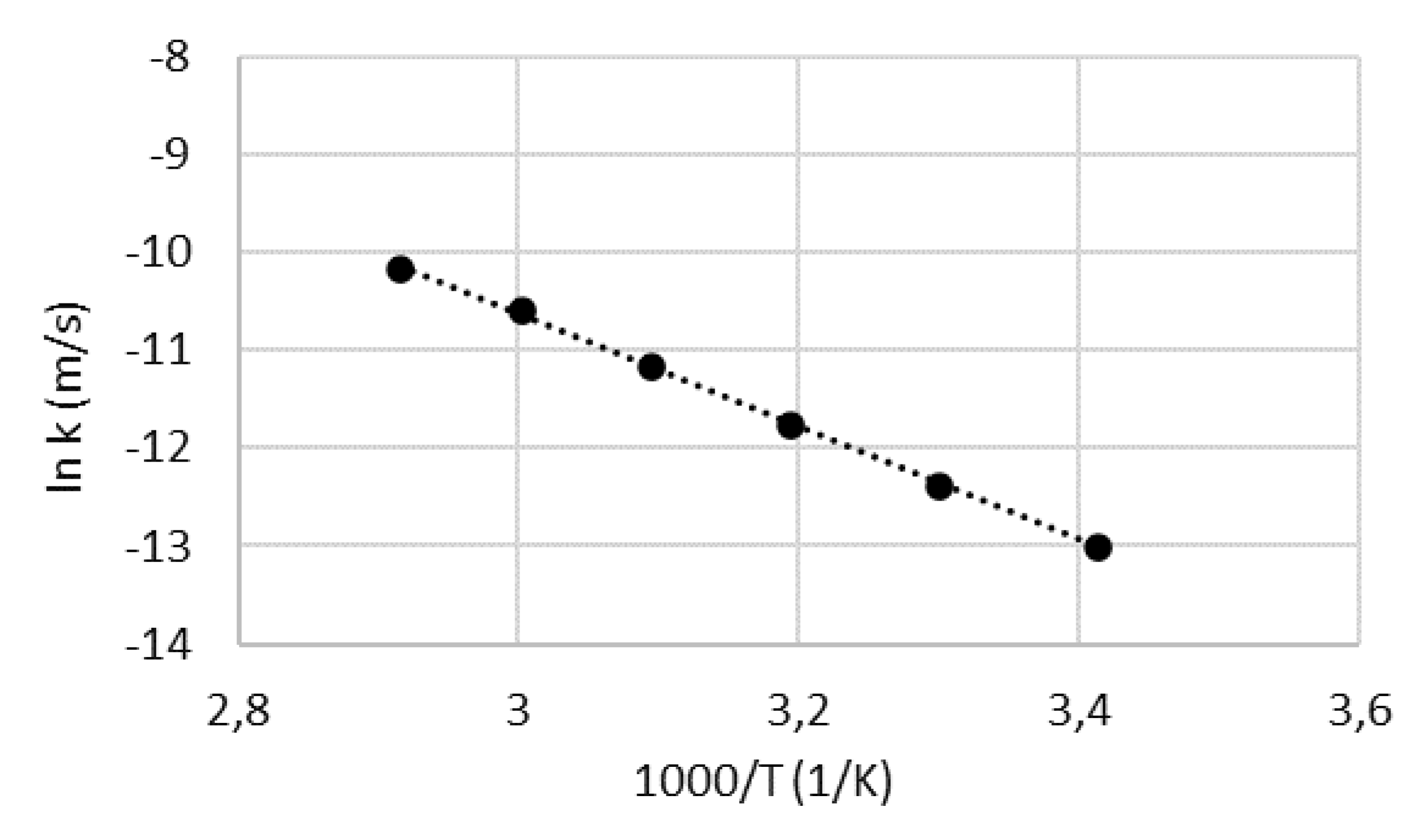
4.2. Activation energy
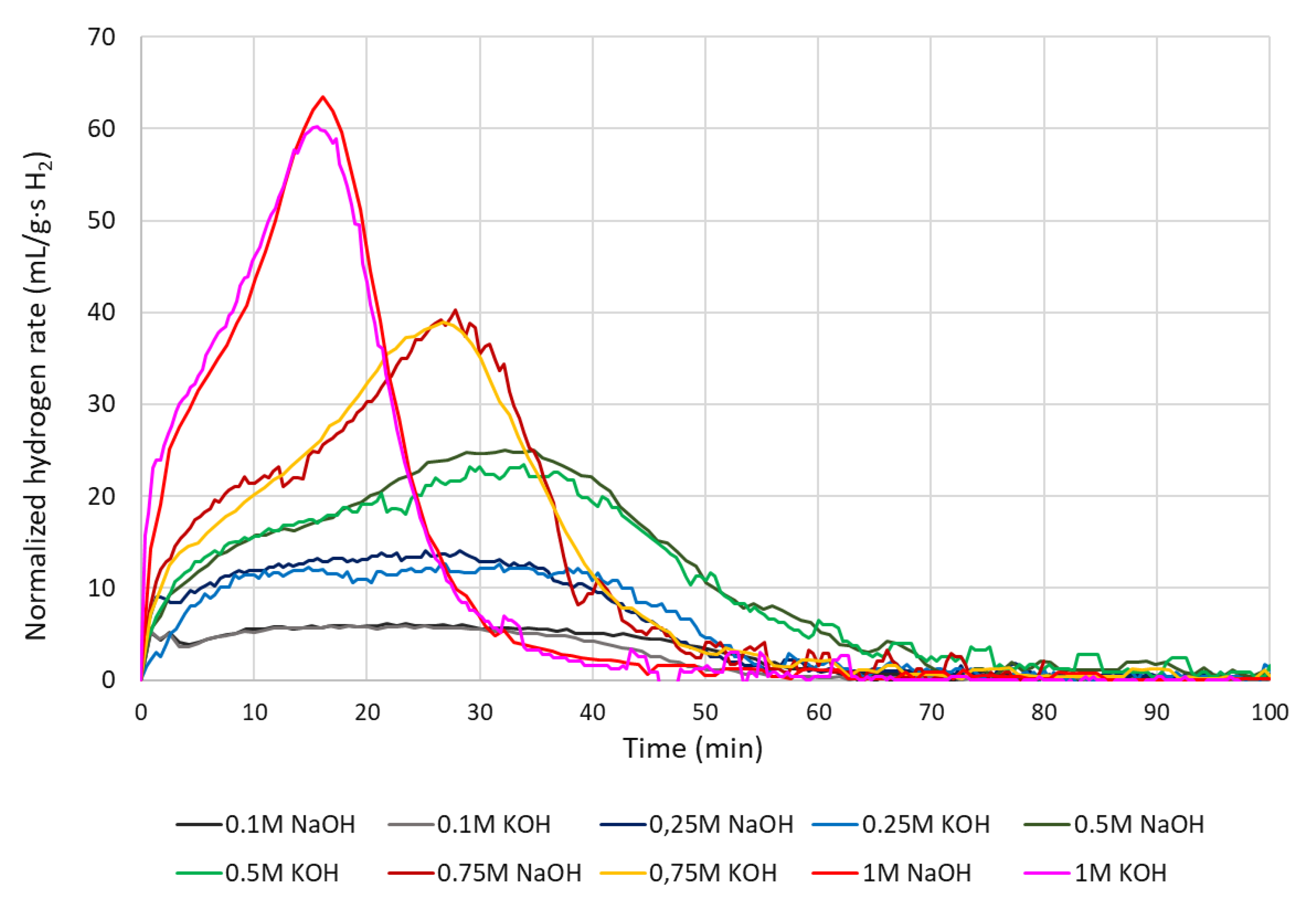
4.3. Normalized cumulative hydrogen yield
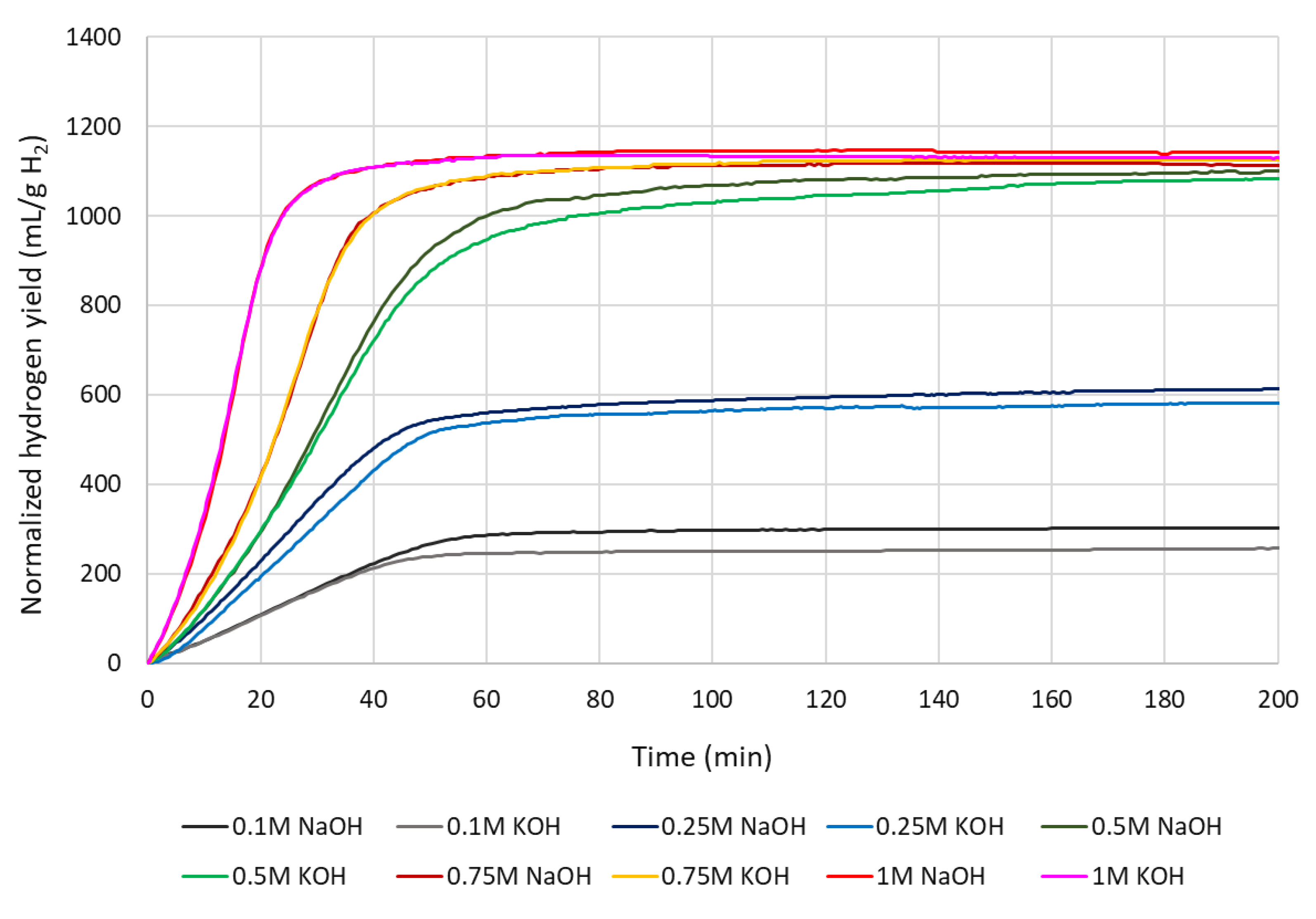
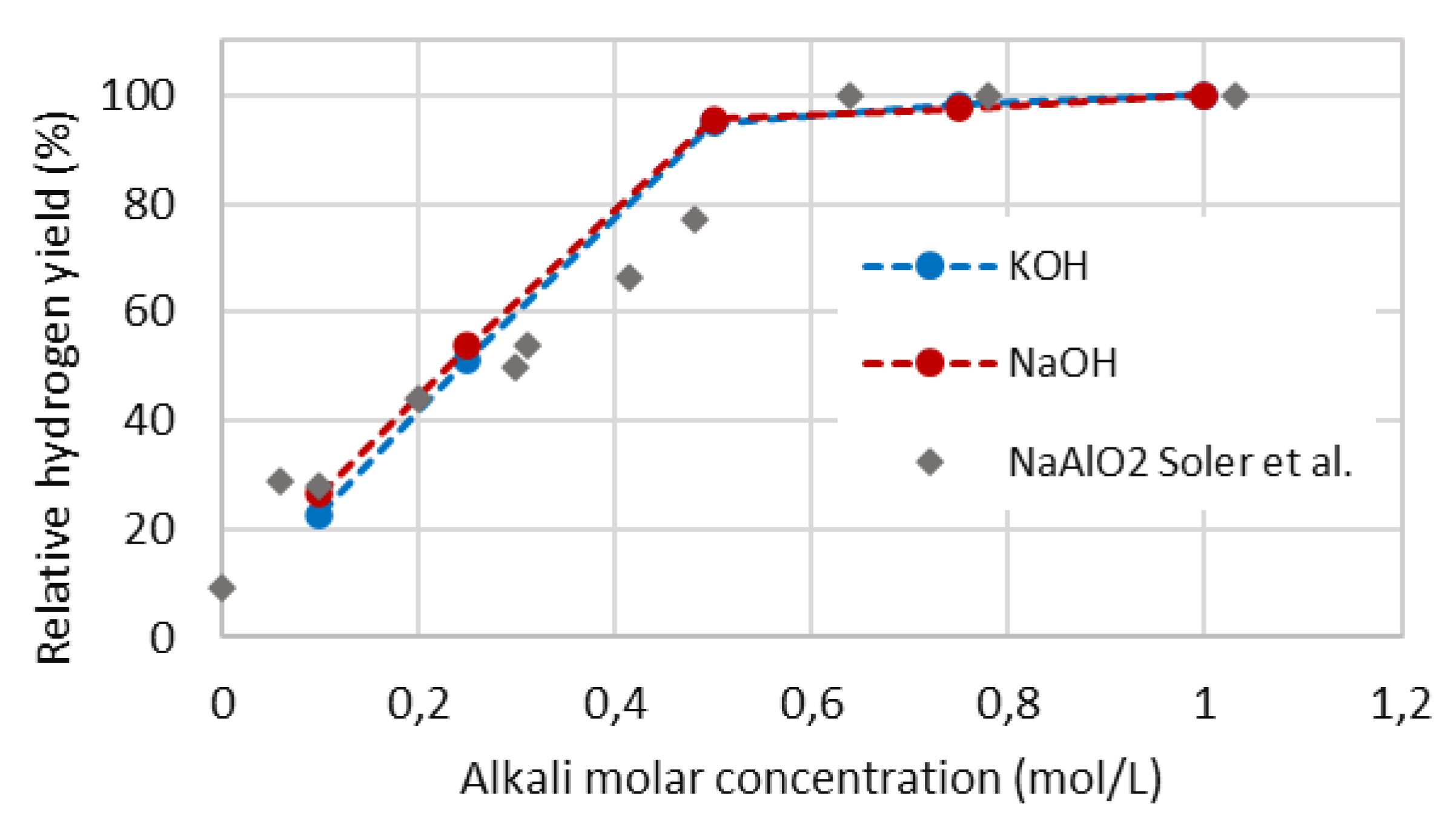
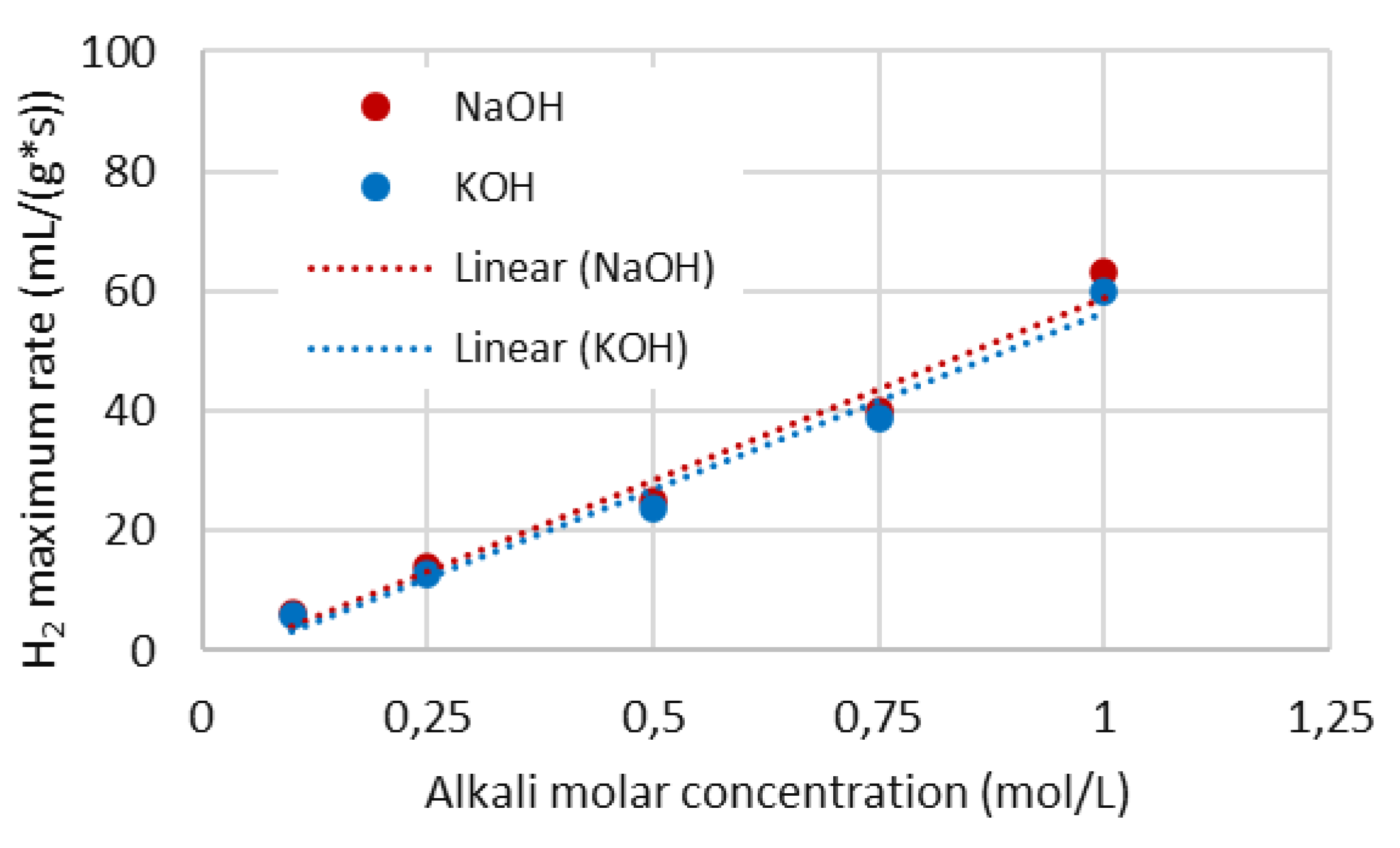
4.4. Hydrogen production rate
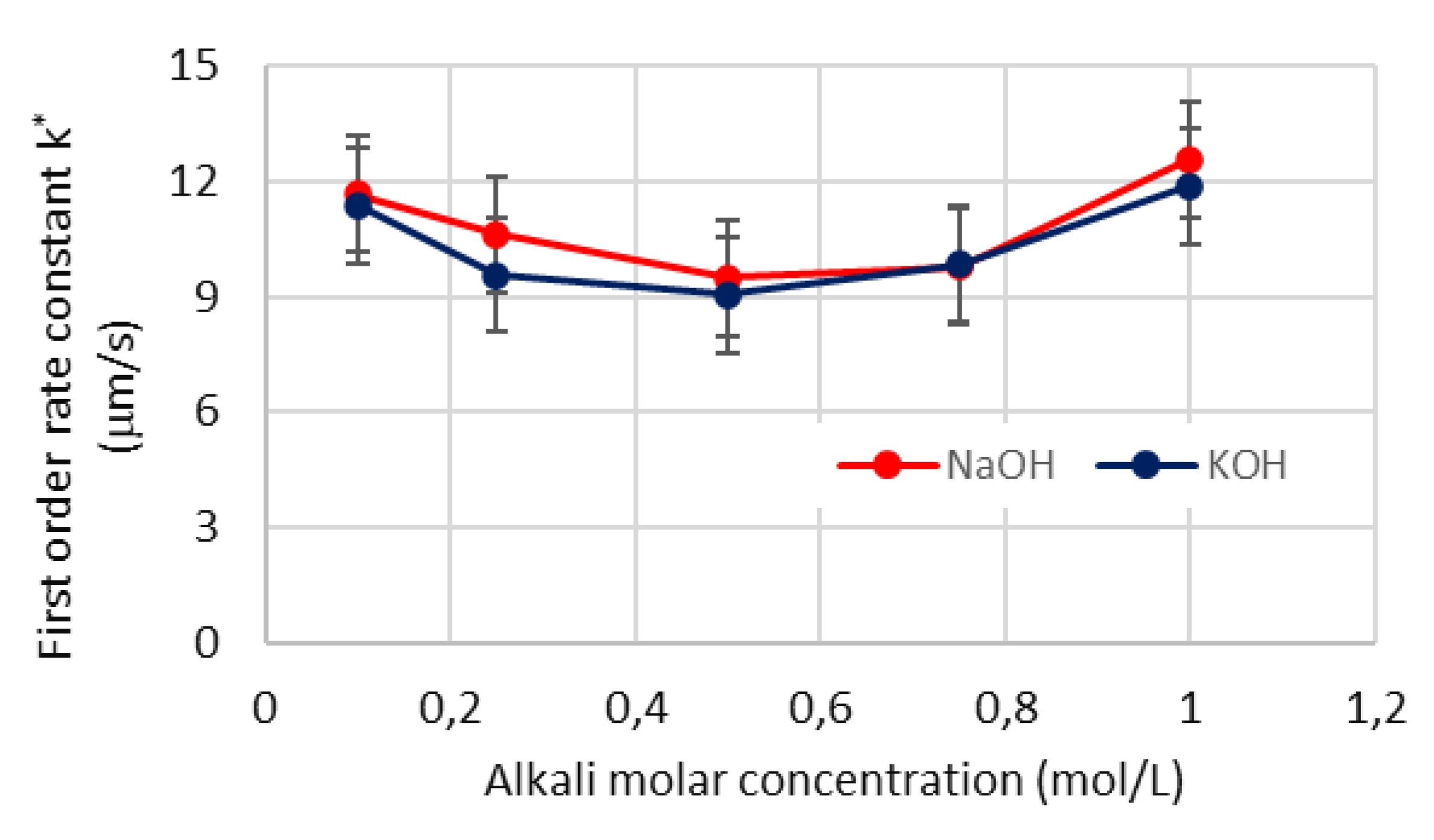
4.5. First order rate constant for surface reaction
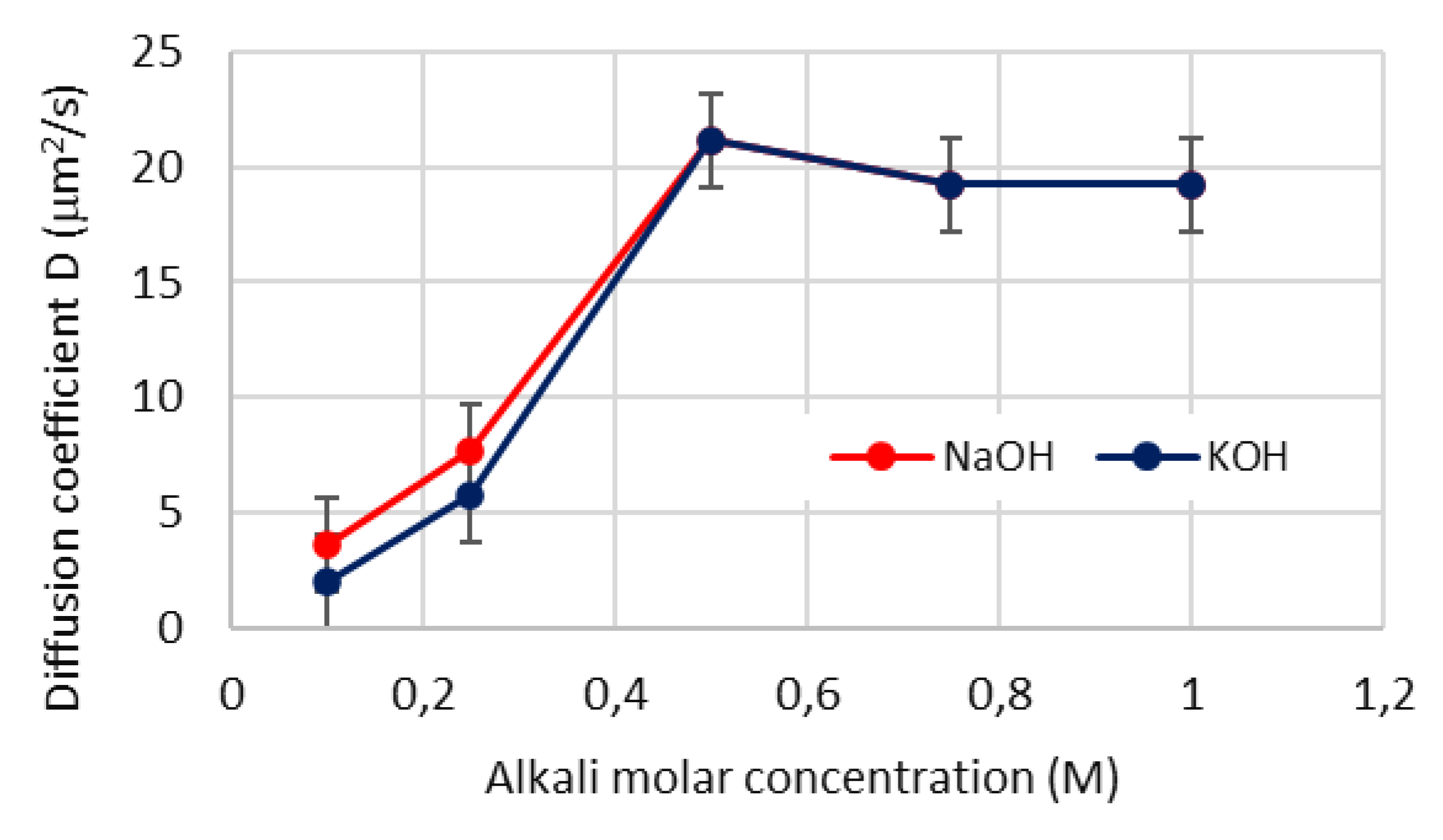
4.6. Diffusion coefficient of aqueous reactant in the byproduct layer
4.7. Economic Analysis
4.7.1. Profit estimation of performed experiments
4.7.2. Open problems of further treatment of aluminum hydroxide
- Hydroxide salt make-up required between the runs;
- Impurity levels of chemical elements within the solid aluminum hydroxide Al(OH)3 product and its general suitability for recycling/end-use.
5. Conclusions
Acknowledgements
References
- G. V. Calder and T. D. Stark, “Aluminum reactions and problems in municipal solid waste landfills,” Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, vol. 14, no. 4, pp. 258–265, Sep. 2010. [CrossRef]
- “Primary Aluminium Production - International Aluminium Institute.” https://international-aluminium.org/statistics/primary-aluminium-production/ (accessed Jan. 29, 2023).
- L. Soler, J. Macanás, M. Muñoz, and J. Casado, “Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications,” J Power Sources, vol. 169, no. 1, pp. 144–149, Jun. 2007. [CrossRef]
- T. Hiraki and T. Akiyama, “Exergetic life cycle assessment of new waste aluminium treatment system with co-production of pressurized hydrogen and aluminium hydroxide,” Int J Hydrogen Energy, vol. 34, no. 1, pp. 153–161, Jan. 2009. [CrossRef]
- S. S. Razavi-Tousi and J. A. Szpunar, “Mechanism of Corrosion of Activated Aluminum Particles by Hot Water,” Electrochim Acta, vol. 127, pp. 95–105, May 2014. [CrossRef]
- E. Elsarrag, A. Elhoweris, and Y. Alhorr, “The production of hydrogen as an alternative energy carrier from aluminium waste,” Energy Sustain Soc, vol. 7, no. 1, pp. 1–14, Dec. 2017.
- J. Xuan, M. K. H. Leung, D. Y. C. Leung, and M. Ni, “A review of biomass-derived fuel processors for fuel cell systems,” Renewable and Sustainable Energy Reviews, vol. 13, no. 6–7, pp. 1301–1313, 2009, Accessed: Jan. 29, 2023. [Online]. Available: https://ideas.repec.org/a/eee/rensus/v13y2009i6-7p1301-1313.html.
- S. C. Amendola et al., “A Safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst,” Int J Hydrogen Energy, vol. 25, no. 10, pp. 969–975, Oct. 2000. [CrossRef]
- E. D. , S. P. F. , D. C. Y. , W. X. R. Wanga, “A mini-type hydrogen generator fromaluminum for proton exchange membrane fuel cells,” J Power Sources, vol. 181, pp. 144–148, 2008.
- W. Z. Gai, W. H. Liu, Z. Y. Deng, and J. G. Zhou, “Reaction of Al powder with water for hydrogen generation under ambient condition,” Int J Hydrogen Energy, vol. 37, no. 17, pp. 13132–13140, Sep. 2012. [CrossRef]
- B. C. Bunker et al., “Hydration of Passive Oxide Films on Aluminum,” Journal of Physical Chemistry B, vol. 106, no. 18, pp. 4705–4713, May 2002. [CrossRef]
- Z. Y. Deng, J. M. F. Ferreira, Y. Tanaka, and J. Ye, “Physicochemical Mechanism for the Continuous Reaction of γ-Al2O3-Modified Aluminum Powder with Water,” Journal of the American Ceramic Society, vol. 90, no. 5, pp. 1521–1526, May 2007. [CrossRef]
- Z. Y. Deng, J. M. F. Ferreira, and Y. Sakka, “Hydrogen-generation materials for portable applications,” Journal of the American Ceramic Society, vol. 91, no. 12, pp. 3825–3834, Dec. 2008. [CrossRef]
- Levenspiel, “Chemical Reaction Engineering, 3rd Edition | Wiley,” New York, p. 704, 1998, Accessed: Jan. 29, 2023. [Online]. Available: https://www.wiley.com/en-us/Chemical+Reaction+Engineering%2C+3rd+Edition-p-9780471254249.
- T. Hiraki, M. Takeuchi, M. Hisa, and T. Akiyama, “Hydrogen production from waste aluminum at different temperatures, with LCA,” Mater Trans, vol. 46, no. 5, pp. 1052–1057, May 2005. [CrossRef]
- C. B. Porciúncula, N. R. Marcilio, I. C. Tessaro, and M. Gerchmann, “Production of hydrogen in the reaction between aluminum and water in the presence of NaOH and KOH,” Brazilian Journal of Chemical Engineering, vol. 29, no. 2, pp. 337–348, Apr. 2012. [CrossRef]
- L. Soler, A. M. Candela, J. Macanás, M. Muñoz, and J. Casado, “In situ generation of hydrogen from water by aluminum corrosion in solutions of sodium aluminate,” J Power Sources, vol. 192, no. 1, pp. 21–26, Jul. 2009. [CrossRef]
- S. S. Martínez, W. L. Benítes, A. A. Á. Gallegos, and P. J. Sebastián, “Recycling of aluminum to produce green energy,” Solar Energy Materials and Solar Cells, vol. 88, no. 2, pp. 237–243, Jul. 2005. [CrossRef]
- “(1) (PDF) Design of Hydrogen Generator for On-Board Hydrogen Generation from Waste Aluminum Chips.” https://www.researchgate.net/publication/303382386_Design_of_Hydrogen_Generator_for_On-Board_Hydrogen_Generation_from_Waste_Aluminum_Chips (accessed Jan. 29, 2023).
- “(1) Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen | Request PDF.” https://www.researchgate.net/publication/350299739_Analysis_of_Valorization_Process_of_Aluminum_Breakage_Scraps_to_Obtain_Green_Hydrogen (accessed Jan. 29, 2023).
- C. M. A. Brett, A. N. A. Maria, and O. Brett, “Principles , Methods , and Applications,” Electrochemistry, vol. 67, no. 2, p. 444, 1993, Accessed: Jan. 29, 2023. [Online]. Available: http://www.getcited.org/pub/102998784.
- “AlEnCycles - Aluminium-Redox-Cycles for the Production of Heat and Electricity for Buildings based on Renewable Energies | Zenodo.” https://zenodo.org/record/6393255#.ZBjQkMJBy70 (accessed Mar. 20, 2023).
- “Sodium Hydroxide / Caustic Soda Supplier & Distributor | Univar Solutions | Univar Solutions.” https://www.univarsolutions.com/product-categories/essential-chemicals-ingredients/liquid-caustic-soda?hasprice=cspweb (accessed Mar. 20, 2023).
- “Hydrogen cost and sales prices | H2Valleys.” https://h2v.eu/analysis/statistics/financing/hydrogen-cost-and-sales-prices (accessed Mar. 20, 2023).
- W. Z. Gai, L. Y. Wang, M. Y. Lu, and Z. Y. Deng, “Effect of low concentration hydroxides on Al hydrolysis for hydrogen production,” Energy, vol. 268, p. 126731, Apr. 2023. [CrossRef]
- S. Kanehira et al., “Controllable hydrogen release via aluminum powder corrosion in calcium hydroxide solutions,” Journal of Asian Ceramic Societies, vol. 1, no. 3, pp. 296–303, Sep. 2013. [CrossRef]
- Y. Zhu and D. R. Cooper, “An Optimal Reverse Material Supply Chain for U.S. Aluminum Scrap,” Procedia CIRP, vol. 80, pp. 677–682, Jan. 2019. [CrossRef]
- V. K. Soo, J. R. Peeters, P. Compston, M. Doolan, and J. R. Duflou, “Economic and Environmental Evaluation of Aluminium Recycling based on a Belgian Case Study,” Procedia Manuf, vol. 33, pp. 639–646, Jan. 2019. [CrossRef]
- R. Modaresi and D. B. Müller, “The role of automobiles for the future of aluminum recycling,” Environ Sci Technol, vol. 46, no. 16, pp. 8587–8594, Aug. 2012. https://doi.org/10.1021/ES300648W/SUPPL_FILE/ES300648W_SI_001.PDF.
- M. Y. Haller, D. Amstad, M. Dudita, A. Englert, and A. Häberle, “Combined heat and power production based on renewable aluminium-water reaction,” Renew Energy, vol. 174, pp. 879–893, Aug. 2021. [CrossRef]
- J. D. Gale, A. L. Rohl, V. Milman, and M. C. Warren, “An ab Initio Study of the Structure and Properties of Aluminum Hydroxide:Gibbsite and Bayerite,” Journal of Physical Chemistry B, vol. 105, no. 42, pp. 10236–10242, Oct. 2001. [CrossRef]
- “Oxides and hydroxides of aluminum | WorldCat.org.” https://www.worldcat.org/title/oxides-and-hydroxides-of-aluminum/oclc/18997314 (accessed Mar. 18, 2023).
| Al chips 9 kg |
Green H2 sale 1 kg |
Gibbsite sale 26 kg |
NaOH price 26.6 kg 50wt.% (aq) |
NaOH loss of 1.33% (with 1M NaOH) | Profit of Al hydrolysis process |
|---|---|---|---|---|---|
| 0.5 €/kg⋅9 kg = 4.5 € |
6 € | 0.2 €/kg⋅26 kg = 5.2 € |
chemical: 547 € | 7.3 € | - 0.6 € |
| technical: 76 € | 1.0 € | 5.7 € |
| NaOH price 26.6 kg 50wt.% (aq) |
NaOH loss of 1.33% (with 1M NaOH) | Profit of Al hydrolysis process with 1M | NaOH loss of 1% (with 0.75M NaOH) | Profit of Al hydrolysis process with 0.75M | “Over-profit” of decreased NaOH molarity vs. overtime |
|---|---|---|---|---|---|
| chemical: 547 € | 7.3 € | - 0.6 € | 5.5 € | 1.2 € | 3.6 €/h |
| technical: 76 € | 1.0 € | 5.7 € | 0.76 € | 5.9 € | 0.4 €/h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





