Submitted:
28 March 2023
Posted:
29 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Maretials and Methods
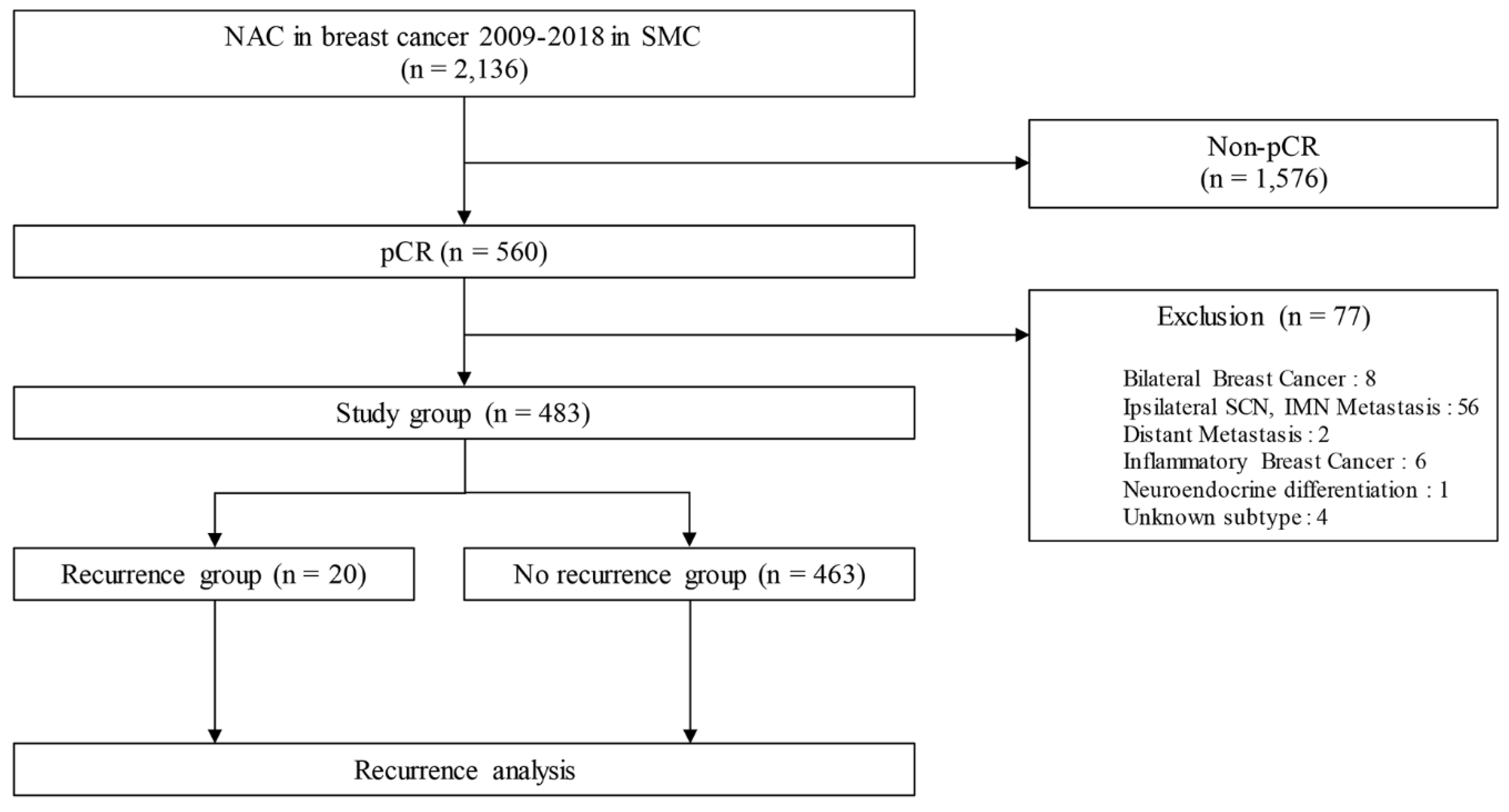
2.1. Patients’ selection
2.2. Definition
2.3. Statistical analyses
2.4. IRB number
3. Results
3.1. Patients’ and tumor characteristics
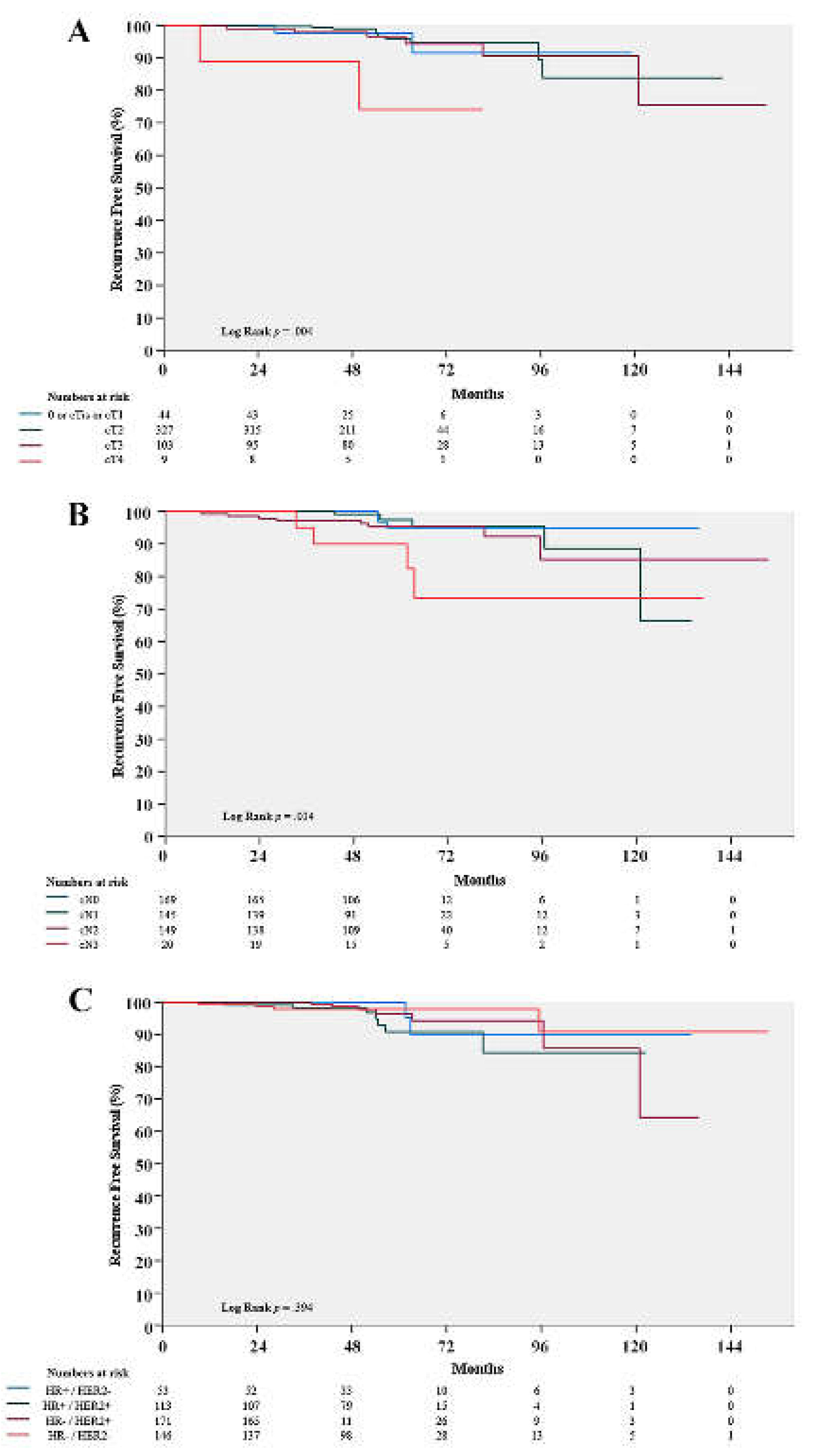
3.2. Clinicopathologic factors associated with RFS
3.3. Prognosis of pCR patients with clinicopathologic factors
3.4. Clinicopathologic characteristics of patients with a recurrence and metastases
4. Discussion
Author Contributions
Conflicts of Interest
References
- Caudle, A.S., et al., Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res, 2012. 14(3): p. R83. [CrossRef]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol, 2018. 19(1): p. 27-39.
- Ikeda, T., et al., The role of neoadjuvant chemotherapy for breast cancer treatment. Breast Cancer, 2002. 9(1): p. 8-14. [CrossRef]
- Haque, W., et al., Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat, 2018. 170(3): p. 559-567. [CrossRef]
- Gianni, L., et al., 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol, 2016. 17(6): p. 791-800. [CrossRef]
- Schneeweiss, A., et al., Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol, 2013. 24(9): p. 2278-84. [CrossRef]
- Cortazar, P., et al., Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet, 2014. 384(9938): p. 164-72. [CrossRef]
- Orsaria, P., et al., Clinical Outcomes Among Major Breast Cancer Subtypes After Neoadjuvant Chemotherapy: Impact on Breast Cancer Recurrence and Survival. Anticancer Res, 2021. 41(5): p. 2697-2709. [CrossRef]
- Fisher, B., et al., Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol, 1998. 16(8): p. 2672-85. [CrossRef]
- Wolmark, N., et al., Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr, 2001(30): p. 96-102. [CrossRef]
- Mittendorf, E.A., et al., Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet, 2020. 396(10257): p. 1090-1100. [CrossRef]
- Schmid, P., et al., Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med, 2020. 382(9): p. 810-821. [CrossRef]
- Hammond, M.E., et al., American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol, 2010. 28(16): p. 2784-95. [CrossRef]
- Wolff, A.C., et al., Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol, 2013. 31(31): p. 3997-4013. [CrossRef]
- Coates, A.S., et al., Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol, 2015. 26(8): p. 1533-46. [CrossRef]
- Fei, F., et al., Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer, 2015. 51(3): p. 301-9.
- Gonzalez-Angulo, A.M., et al., Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol, 2005. 23(28): p. 7098-104. [CrossRef]
- O'Shaughnessy, J., et al., Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: a real-world evidence study. Breast Cancer Res Treat, 2021. 187(3): p. 903-913. [CrossRef]
- Broglio, K.R., et al., Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol, 2016. 2(6): p. 751-60.
- Tanioka, M., et al., Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer, 2010. 103(3): p. 297-302.
- Chou, H.H., et al., Factors affecting locoregional recurrence in breast cancer patients undergoing surgery following neoadjuvant treatment. BMC Surg, 2021. 21(1): p. 160. [CrossRef]
- Liedtke, C., et al., Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol, 2008. 26(8): p. 1275-81. [CrossRef]
- Wang-Lopez, Q., et al., Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol, 2015. 95(1): p. 88-104.
- Chica-Parrado, M.R., et al., Resistance to Neoadjuvant Treatment in Breast Cancer: Clinicopathological and Molecular Predictors. Cancers (Basel), 2020. 12(8). [CrossRef]
- Ishitobi, M., et al., Risk Factors for Ipsilateral Breast Tumor Recurrence in Triple-Negative or HER2-Positive Breast Cancer Patients Who Achieve a Pathologic Complete Response After Neoadjuvant Chemotherapy. Ann Surg Oncol, 2021. 28(5): p. 2545-2552. [CrossRef]
- Werutsky, G., et al., Locoregional recurrence risk after neoadjuvant chemotherapy: A pooled analysis of nine prospective neoadjuvant breast cancer trials. Eur J Cancer, 2020. 130: p. 92-101. [CrossRef]
- Swisher, S.K., et al., Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy. Ann Surg Oncol, 2016. 23(3): p. 749-56. [CrossRef]
- Chaudry, M., et al., Recurrence and survival among breast cancer patients achieving a pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat, 2015. 153(2): p. 417-23. [CrossRef]
- Spring, L.M., et al., Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res, 2020. 26(12): p. 2838-2848. [CrossRef]
- Liu, H., et al., Pathologic Complete Response and Its Impact on Breast Cancer Recurrence and Patient's Survival after Neoadjuvant Therapy: A Comprehensive Meta-Analysis. Comput Math Methods Med, 2021. 2021: p. 7545091. [CrossRef]
- Xie, L.Y., et al., Markers Associated With Tumor Recurrence in Patients With Breast Cancer Achieving a Pathologic Complete Response After Neoadjuvant Chemotherapy. Front Oncol, 2022. 12: p. 860475. [CrossRef]
- Chan, A., et al., Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin Breast Cancer, 2021. 21(1): p. 80-91.e7. [CrossRef]
- Poggio, F., et al., Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol, 2018. 29(7): p. 1497-1508. [CrossRef]
- Li, X., et al., Tailoring neoadjuvant chemotherapy for patients with breast cancer who have achieved pathologic complete response. Transl Cancer Res, 2020. 9(2): p. 1205-1214. [CrossRef]
- Hassett, M.J., et al., Neoadjuvant treatment strategies for HER2-positive breast cancer: cost-effectiveness and quality of life outcomes. Breast Cancer Res Treat, 2020. 181(1): p. 43-51. [CrossRef]
| Characteristics | Recurrence No. (%) N = 20 (4.1) |
No Recurrence No. (%) N = 463 (95.9) |
% | p-value | |
|---|---|---|---|---|---|
| Mean F/U duration (month) | 59.0 (0.5 - 153.3) | ||||
| Age at diagnosis (years) | 0.162 | ||||
| ≤ 35 | 2 (10.0) | 58 (12.5) | 12.4 | ||
| 36 - 50 | 6 (30.0) | 226 (48.8) | 48.0 | ||
| > 50 | 12 (60.0) | 179 (38.7) | 39.5 | ||
| Clinical T stage | 0.045 | ||||
| cT0 or Tis or T1 | 2 (10.0) | 42 (9.1) | 9.1 | ||
| cT2 | 10 (50.0) | 317 (68.5) | 67.7 | ||
| cT3 | 6 (30.0) | 97 (21.0) | 21.3 | ||
| cT4 | 2 (10.0) | 7 (1.5) | 1.9 | ||
| Clinical N stage | 0.002 | ||||
| cN0 | 3 (15.0) | 166 (35.9) | 35.0 | ||
| cN1 | 5 (25.0) | 140 (30.2) | 30.0 | ||
| cN2 | 8 (40.0) | 141 (30.5) | 30.8 | ||
| cN3 | 4 (20.0) | 16 (3.5) | 4.1 | ||
| FNA of metastatic lymph node | 0.450 | ||||
| negative by proven Bx | 4 (20.0) | 106 (22.9) | 22.8 | ||
| positive by proven Bx | 13 (65.0) | 235 (50.8) | 51.3 | ||
| Did not Bx | 3 (15.0) | 122 (26.3) | 25.9 | ||
| Molecular subtype at diagnosis | 0.573 | ||||
| HR+ / HER2- | 2 (10.0) | 51 (11.0) | 11.0 | ||
| HR+ / HER2+ | 7 (35.0) | 106 (22.9) | 23.4 | ||
| HR- / HER2+ | 7 (35.0) | 164 (35.4) | 35.4 | ||
| HR- / HER2- (TNBC) | 4 (20.0) | 142 (30.7) | 30.2 | ||
| Ki67 at diagnosis | 1.000 | ||||
| < 20% | 2 (10.0) | 51 (11.0) | 11.0 | ||
| ≥ 20% | 18 (90.0) | 407 (87.9) | 88.0 | ||
| Unknown | 0 (0.0) | 5 (1.1) | 1.0 | ||
| Breast surgery | 0.574 | ||||
| Mastectomy | 5 (25.0) | 93 (20.1) | 20.3 | ||
| BCS | 15 (75.0) | 370 (79.9) | 79.7 | ||
| Axillary surgery | 0.189 | ||||
| SLNB only | 12 (60.0) | 346 (74.7) | 74.1 | ||
| ALND | 8 (40.0) | 117 (25.3) | 25.9 | ||
| Adjuvant RT | 0.665 | ||||
| Yes | 18 (90.0) | 427 (92.2) | 92.1 | ||
| No | 2 (10.0) | 36 (7.8) | 7.9 | ||
| NAC regimen | 0.020 | ||||
| AC | 0 (0.0) | 13 (2.8) | 2.7 | ||
| T | 0 (0.0) | 4 (0.9) | 0.8 | ||
| AC+T | 9 (45.0) | 240 (51.8) | 51.6 | ||
| ACTH | 5 (25.0) | 19 (4.1) | 5.0 | ||
| TCHP | 6 (30.0) | 139 (30.0) | 30.0 | ||
| Others | 0 (0.0) | 48 (10.4) | 9.9 | ||
| Characteristics | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 0.141 | ||||||
| ≤ 35 | 1.19 | 024 - 5.90 | 0.836 | ||||
| 36 - 50 | 1 | ||||||
| > 50 | 2.57 | 0.96 - 6.85 | 0.060 | ||||
| Clinical T stage | 0.049 | 0.320 | |||||
| cT0 / cTis / cT1 | 1 | 1 | |||||
| cT2 | 0.66 | 0.14 - 3.02 | 0.594 | 0.83 | 0.18 - 3.84 | 0.807 | |
| cT3 | 1.2 | 0.24 - 5.95 | 0.826 | 1.19 | 0.24 - 5.91 | 0.830 | |
| cT4 | 5.64 | 0.79 - 40.11 | 0.084 | 4.14 | 0.54 - 31.8 | 0.172 | |
| Clinical N stage | 0.010 | 0.073 | |||||
| cN0 | 1 | 1 | |||||
| cN1 | 1.9 | 0.45 - 7.97 | 0.379 | 1.75 | 0.41 - 7.38 | 0.449 | |
| cN2 | 2.9 | 0.77 - 10.94 | 0.117 | 2.89 | 0.76 - 10.93 | 0.119 | |
| cN3 | 10.93 | 2.44 - 48.94 | 0.002 | 7.35 | 1.47 - 36.82 | 0.015 | |
| Molecular subtype at diagnosis | 0.584 | ||||||
| HR+ / HER2- | 1 | ||||||
| HR+ / HER2+ | 1.75 | 0.36 - 8.45 | 0.488 | ||||
| HR- / HER2+ | 1.15 | 0.24 - 5.56 | 0.861 | ||||
| HR- / HER2- (TNBC) | 0.75 | 0.14 - 4.09 | 0.738 | ||||
| Ki67 at diagnosis | |||||||
| < 20% | 1 | ||||||
| ≥ 20% | 1.14 | 0.27 - 4.93 | 0.857 | ||||
| Breast surgery | |||||||
| Mastectomy | 1 | ||||||
| BCS | 0.76 | 0.28 - 2.10 | 0.603 | ||||
| Axillary surgery | |||||||
| SLNB only | 1 | ||||||
| ALND | 1.66 | 0.67 - 4.13 | 0.273 | ||||
| Initial diagnosis | State at recurrence | Initial treatment information | Expire | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) | HR | HER2 | cT | cN | Ki67 | LRR | Distant metastasis | RFS (months) | Breast OP | Axilla OP | Adjuvant RT | |
| 1 | 32 | + | - | cT2 | cN1 | ≥ 20% | sternum, lung | 16.6 | mastectomy | SLNB only | - | - | |
| 2 | 50 | + | - | cT3 | cN3 | < 20% | sternum, lung, Rt paratracheal LN | 37.8 | mastectomy | ALND | + | + | |
| 3 | 52 | + | + | cT3 | cN2 | ≥ 20% | brain | 13.3 | mastectomy | SLNB only | + | - | |
| 4 | 55 | + | + | cT3 | cN2 | ≥ 20% | ipsilateral SCN | C6-spine, vertebral cervical LN | 47.5 | BCS | ALND | + | - |
| 5 | 45 | + | + | cT3 | cN2 | ≥ 20% | brain | 6.3 | BCS | SLNB only | + | - | |
| 6 | 56 | + | + | cT2 | cN0 | ≥ 20% | ipsilateral breast | contralateral ALN | 46.8 | BCS | SLNB only | + | + |
| 7 | 50 | + | + | cT2 | cN1 | ≥ 20% | ipsilateral breast | 8.9 | BCS | SLNB only | + | - | |
| 8 | 58 | + | + | cT2 | cN0 | < 20% | ipsilateral SCN | 45.0 | BCS | ALND | + | - | |
| 9 | 56 | + | + | cT3 | cN3 | ≥ 20% | brain | 6.0 | BCS | SLNB only | + | + | |
| 10 | 45 | - | + | cT2 | cN1 | ≥ 20% | brain | 20.5 | BCS | ALND | + | - | |
| 11 | 38 | - | + | cT2 | cN1 | ≥ 20% | ipsilateral breast | 12.3 | BCS | SLNB only | + | - | |
| 12 | 53 | - | + | cT4 | cN2 | ≥ 20% | ipsilateral breast | contralateral ALN | 30.8 | BCS | ALND | + | - |
| 13 | 44 | - | + | cT3 | cN1 | ≥ 20% | ipsilateral breast | 9.1 | mastectomy | ALND | + | - | |
| 14 | 60 | - | + | cT2 | cN3 | ≥ 20% | lung, mediastinum | 20.5 | BCS | SLNB only | + | - | |
| 15 | 52 | - | + | cT1 | cN3 | ≥ 20% | brain | 29.1 | BCS | ALND | + | + | |
| 16 | 61 | - | + | cT2 | cN0 | ≥ 20% | ipsilateral breast | 49.2 | BCS | SLNB only | + | - | |
| 17 | 55 | - | - | cT4 | cN2 | ≥ 20% | brain | 8.2 | mastectomy | ALND | - | - | |
| 18 | 32 | - | - | cT2 | cN2 | ≥ 20% | ipsilateral breast | 92.1 | BCS | SLNB only | + | + | |
| 19 | 42 | - | - | cT1 | cN2 | ≥ 20% | ipsilateral breast/ALN | brain, paratracheal LN | 12.8 | BCS | SLNB only | + | - |
| 20 | 60 | - | - | cT2 | cN2 | ≥ 20% | brain | 17.0 | BCS | SLNB only | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




