Submitted:
10 March 2023
Posted:
13 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Organic Substrates from Permafrost Peatlands Used for Aqueous Leachate Preparation
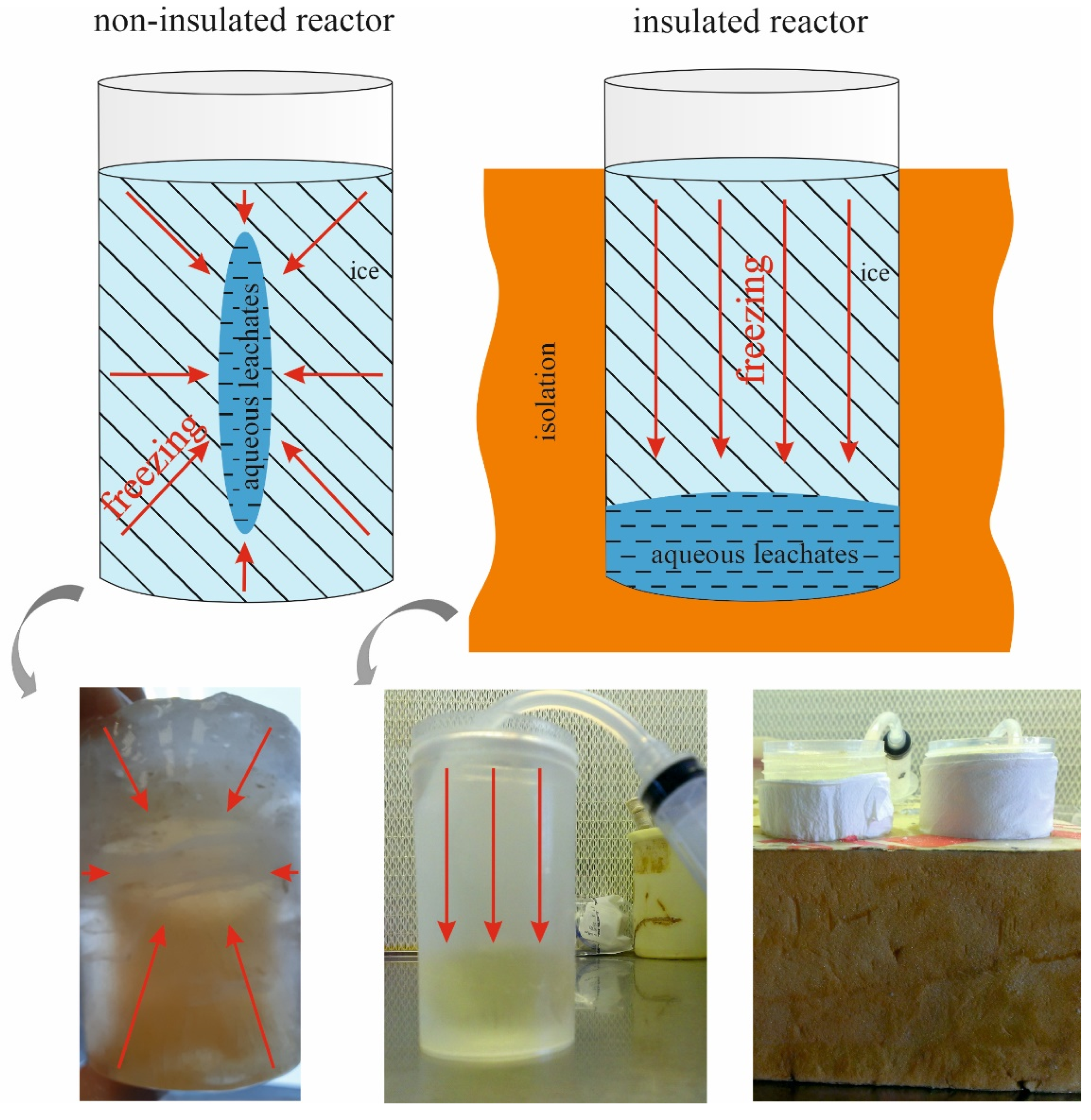
2.2. Freezing Experiments of Aqueous Leachates
2.3. Chemical Analyses
2.4. Data Interpretation
3. Results
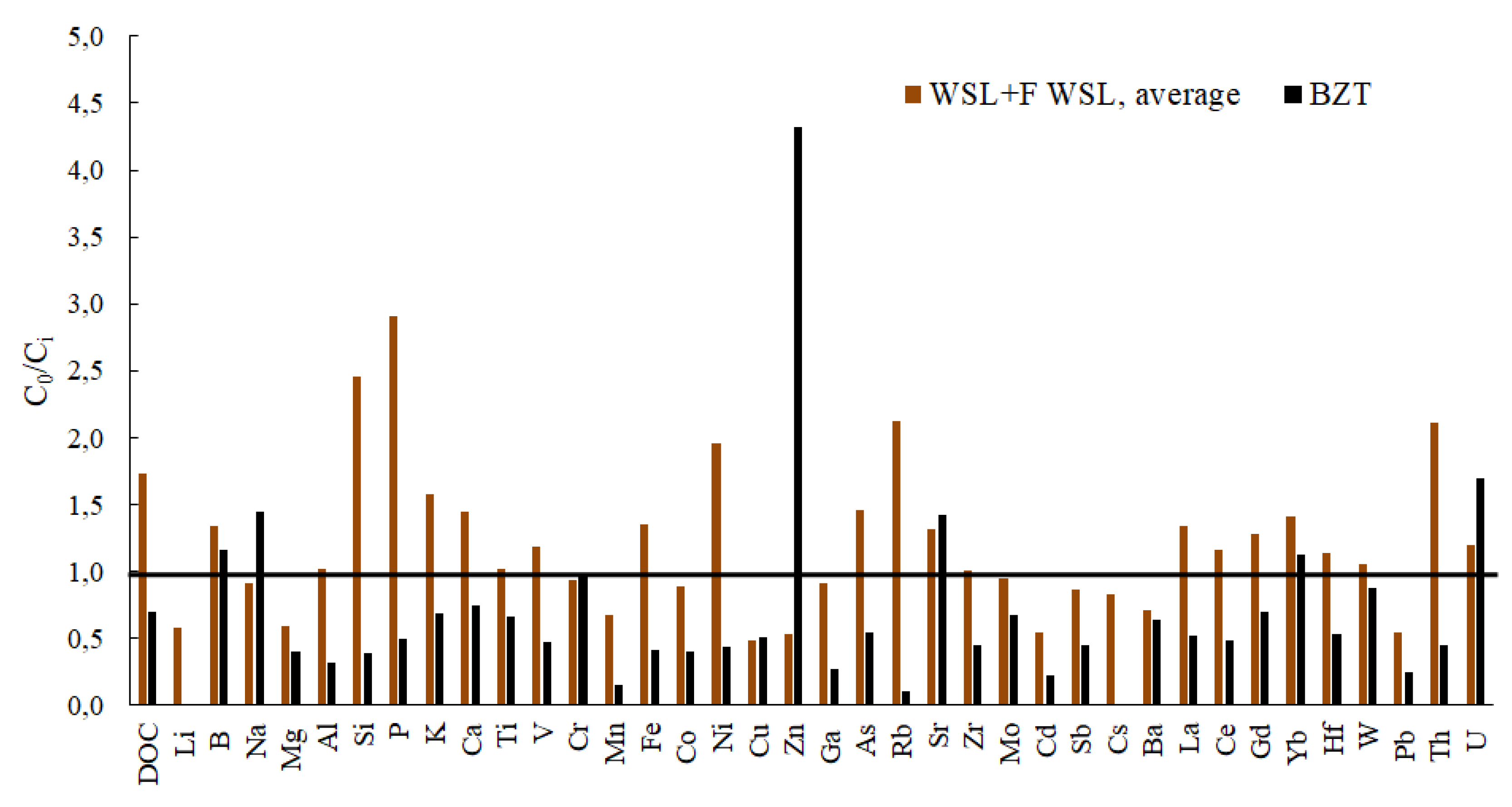
3.1. Initial Leachate Composition and the Impact of Reactor Design and Freezing Mode on Element Concentration in the Remaining Fluid
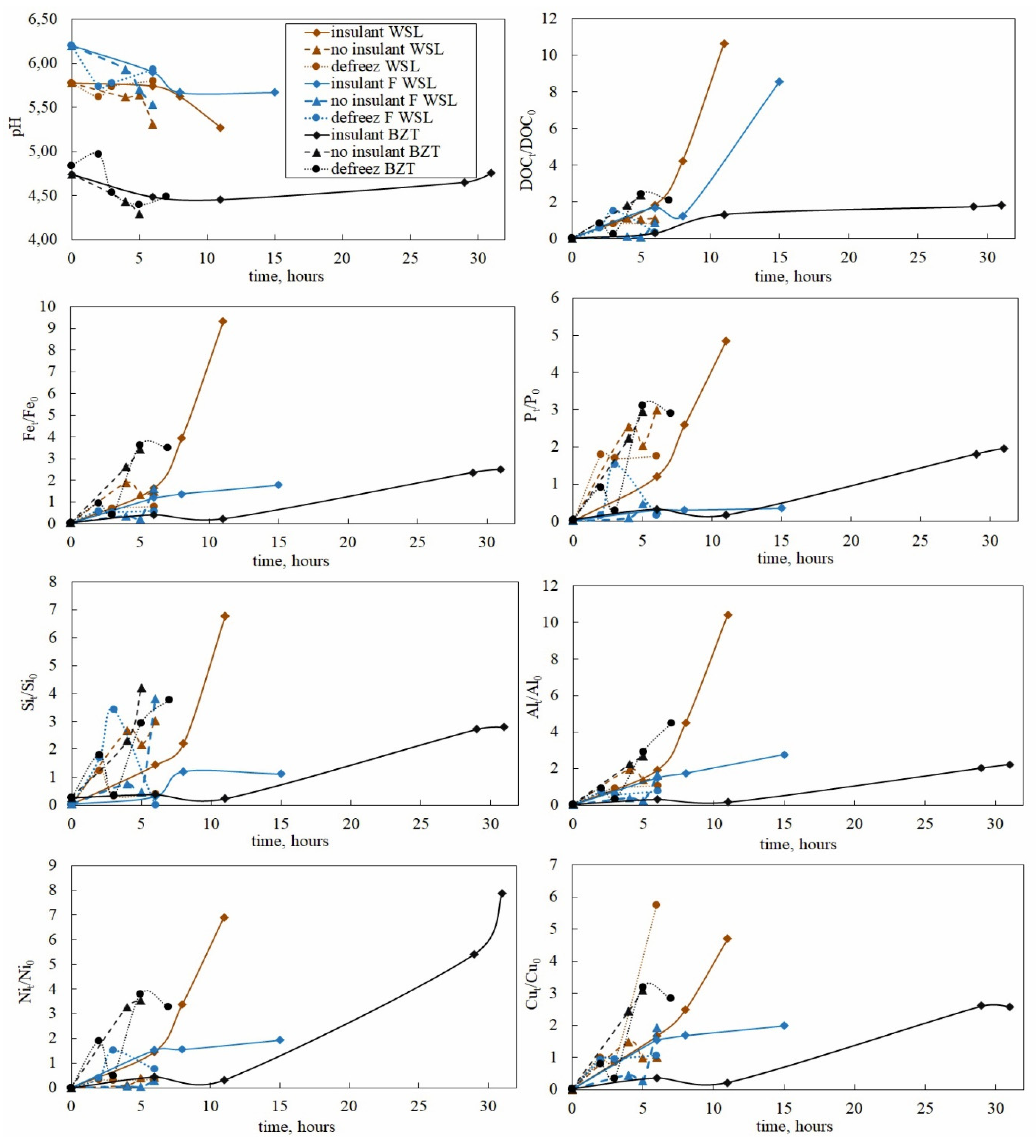
3.2. Evolution of pH, DOC and Metal Concentration in the Fluid Phase during Freezing and Thawing of Peat Leachates
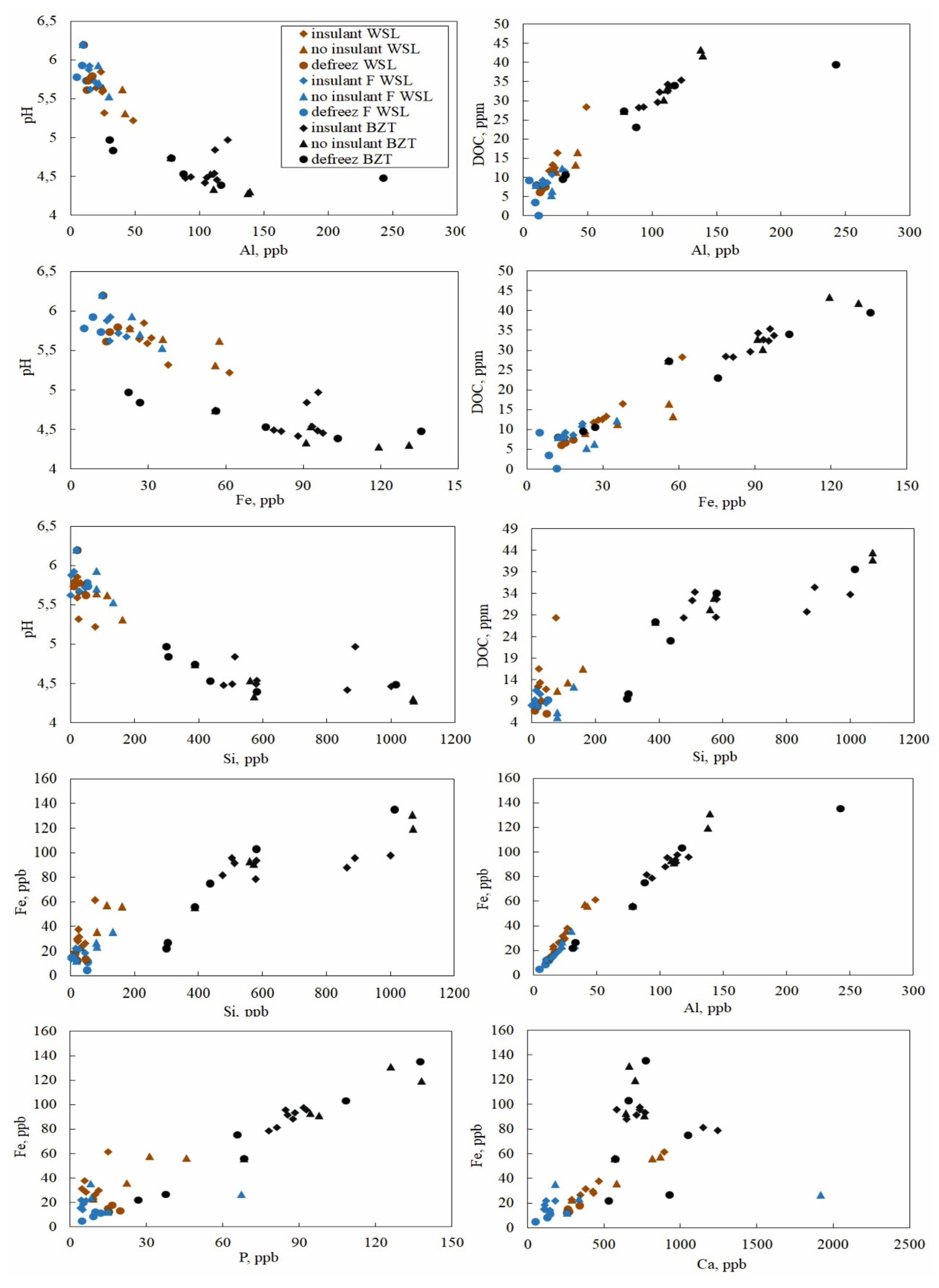
3.3. Group of Elements Depending on Their Conservative and Non-Conservative Behavior during Freezing Revealed via Correlation Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henry, H.A.L. Climate change and soil freezing dynamics: Historical trends and projected changes. Clim. Change 2008, 87, 421–434. [Google Scholar] [CrossRef]
- Hayashi, M. The Cold Vadose Zone: Hydrological and Ecological Significance of Frozen-Soil Processes. Vadose Zone J. 2013, 12, 1–8. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, Q.; Zhang, W.; He, H.; Wang, L. Water Migration and Segregated Ice Formation in Frozen Ground: Current Advances and Future Perspectives. Front. Earth Sci. 2022, 10. [Google Scholar] [CrossRef]
- Reeve, A.S.; Siegel, D.I.; Glaser, P.H. Geochemical controls on peatland pore water from the Hudson Bay Lowland: A multivariate statistical approach. J. Hydrol. 1996, 181, 285–304. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dissolved organic carbon and major and trace elements in peat porewater of sporadic, discontinuous, and continuous permafrost zones of western Siberia. Biogeosciences 2017, 14, 3561–3584. [Google Scholar] [CrossRef]
- Ma, Q.; Jin, H.; Yu, C.; Bense, V.F. Dissolved organic carbon in permafrost regions: A review. Sci. China Earth Sci. 2019, 62, 349–364. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Kopysov, S.G.; Krickov, I.V.; Shirokova, L.S.; Loiko, S.V.; Lim, A.G.; Kolesnichenko, L.G.; Vorobyev, S.N.; Kirpotin, S.N. Impact of Permafrost Thaw and Climate Warming on Riverine Export Fluxes of Carbon, Nutrients and Metals in Western Siberia. Water 2020, 12, 1817. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Loiko, S.V.; Shirokova, L.S. Organic and organo-mineral colloids in discontinuous permafrost zone. Geochim. et Cosmochim. Acta 2016, 188, 1–20. [Google Scholar] [CrossRef]
- Lim, A.G.; Loiko, S.V.; Kuzmina, D.M.; Krickov, I.V.; Shirokova, L.S.; Kulizhsky, S.P.; Pokrovsky, O.S. Organic carbon, and major and trace elements reside in labile low-molecular form in the ground ice of permafrost peatlands: A case study of colloids in peat ice of Western Siberia. Environ. Sci. Process. Impacts 2022, 24, 1443–1459. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Kuzmina, D.M.; Shirokova, L.S.; Kulizhskiy, S.P.; Golovatskaya, E.A.; Pokrovsky, O.S. Colloidal organic carbon and trace elements in peat porewaters across a permafrost gradient in Western Siberia. Geoderma 2021, 390, 114971. [Google Scholar] [CrossRef]
- Manasypov, R.M.; Vorobyev, S.N.; Loiko, S.V.; Kritzkov, I.V.; Shirokova, L.S.; Shevchenko, V.P.; Kirpotin, S.N.; Kulizhsky, S.P.; Kolesnichenko, L.G.; Zemtzov, V.A.; et al. Seasonal dynamics of organic carbon and metals in thermokarst lakes from the discontinuous permafrost zone of western Siberia. Biogeosciences 2015, 12, 3009–3028. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Keeney, D.R.; McCarty, G.W. Effect of freeze-thaw events on mineralization of soil nitrogen. Biol. Fertil. Soils 1992, 14, 116–120. [Google Scholar] [CrossRef]
- Dietzel, M. Impact of cycling freezing on precipitation of silica in Me-SiO2-H2O systems and geochemical implications for cryosoils and sediments. Chem. Geol. 2005, 216, 79–88. [Google Scholar] [CrossRef]
- Du, L.; Dyck, M.; Shotyk, W.; He, H.; Lv, J.; Cuss, C.W.; Bie, J. Lead immobilization processes in soils subjected to freeze-thaw cycles. Ecotoxicol. Environ. Saf. 2020, 192, 110288. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, R.D.; Driscoll, C.T.; Groffman, P.M.; Tierney, G.L.; Fahey, T.J.; Hardy, J.P. Soil Freezing and the Acid-Base Chemistry of Soil Solutions in a Northern Hardwood Forest. Soil Sci. Soc. Am. J. 2003, 67, 1897–1908. [Google Scholar] [CrossRef]
- Hentschel, K.; Borken, W.; Matzner, E. Repeated freeze–thaw events affect leaching losses of nitrogen and dissolved organic matter in a forest soil. J. Plant Nutr. Soil Sci. 2008, 171, 699–706. [Google Scholar] [CrossRef]
- Kim, E.-A.; Lee, H.K.; Choi, J.H. Effects of a controlled freeze-thaw event on dissolved and colloidal soil organic matter. Environ. Sci. Pollut. Res. 2016, 24, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.S.; Jonasson, S.; Michelsen, A. Repeated freeze–thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl. Soil Ecol. 2002, 21, 187–195. [Google Scholar] [CrossRef]
- Leuther, F.; Schlüter, S. Impact of freeze–thaw cycles on soil structure and soil hydraulic properties. SOIL 2021, 7, 179–191. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Saiers, J.E.; Ryan, J.N. Colloid-Facilitated Mobilization of Metals by Freeze–Thaw Cycles. Environ. Sci. Technol. 2013, 48, 977–984. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Saiers, J.E.; Ryan, J.N. Colloid Mobilization in a Fractured Soil during Dry–Wet Cycles: Role of Drying Duration and Flow Path Permeability. Environ. Sci. Technol. 2015, 49, 9100–9106. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Vanapalli, S.K. Effect of freeze-thaw cycling on the soil-freezing characteristic curve of five Canadian soils. Vadose Zone J. 2020, 19. [Google Scholar] [CrossRef]
- Semenov, V.M.; Kogut, B.M.; Lukin, S.M. Effect of repeated drying-wetting-freezing-thawing cycles on the active soil organic carbon pool. Eurasian Soil Sci. 2014, 47, 276–286. [Google Scholar] [CrossRef]
- Schimel, J.P.; Clein, J.S. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol. Biochem. 1996, 28, 1061–1066. [Google Scholar] [CrossRef]
- Vestgarden, L.S.; Austnes, K. Effects of freeze–thaw on C and N release from soils below different vegetation in a montane system: A laboratory experiment. Glob. Chang. Biol. 2009, 15, 876–887. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Li, P.; Xu, G.; Shi, P.; Zhang, Y. Effects of freeze-thaw cycles on aggregate-associated organic carbon and glomalin-related soil protein in natural-succession grassland and Chinese pine forest on the Loess Plateau. Geoderma 2018, 334, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, W.; Feng, W.; Xiao, D.; Hou, X. Reconstruction of Soil Particle Composition During Freeze-Thaw Cycling: A Review. Pedosphere 2016, 26, 167–179. [Google Scholar] [CrossRef]
- Nagare, R.M.; Schincariol, R.A.; Quinton, W.L.; Hayashi, M. Effects of freezing on soil temperature, freezing front propagation and moisture redistribution in peat: Laboratory investigations. Hydrol. Earth Syst. Sci. 2012, 16, 501–515. [Google Scholar] [CrossRef]
- Nagare, R.M.; Schincariol, R.A.; Quinton, W.L.; Hayashi, M. Moving the Field into the Lab: Simulation of Water and Heat Transport in Subarctic Peat. Permafr. Periglac. Process. 2012, 23, 237–243. [Google Scholar] [CrossRef]
- Smerdon, B.D.; Mendoza, C.A. Hysteretic freezing characteristics of riparian peatlands in the Western Boreal Forest of Canada. Hydrol. Process. 2009, 24, 1027–1038. [Google Scholar] [CrossRef]
- McCarter, C.; Rezanezhad, F.; Quinton, W.; Gharedaghloo, B.; Lennartz, B.; Price, J.; Connon, R.; Van Cappellen, P. Pore-scale controls on hydrological and geochemical processes in peat: Implications on interacting processes. Earth-Science Rev. 2020, 207, 103227. [Google Scholar] [CrossRef]
- Schwamborn, G.; Schirrmeister, L.; Frütsch, F.; Diekmann, B. Quartz weathering in freeze–thaw cycles: Experiment and application to the el'gygytgyn crater lake record for tracing siberian permafrost history. Geogr. Ann. Ser. A, Phys. Geogr. 2012, 94, 481–499. [Google Scholar] [CrossRef]
- Wang, J.Y.; Song, C.C.; Hou, A.X.; Miao, Y.Q.; Yang, G.S.; Zhang, J. Effects of freezing thawing cycle on peatland active organic carbon fractions and enzyme activities in the Da Xing’anling Mountains, Northeast China. Environ. Earth Sci. 2014, 72, 1853–1860. [Google Scholar] [CrossRef]
- Chen, J.; Xue, S.; Lin, Y.; Wang, C.; Wang, Q.; Han, Q. Effect of freezing–thawing on dissolved organic matter in water. Desalination Water Treat. 2015, 57, 17230–17240. [Google Scholar] [CrossRef]
- Fellman, J.B.; D'Amore, D.V.; Hood, E. An evaluation of freezing as a preservation technique for analyzing dissolved organic C, N and P in surface water samples. Sci. Total. Environ. 2008, 392, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Juan, Y.; Tian, L.; Chen, X.; Sun, W.; Chen, L. Modification of the composition of dissolved nitrogen forms, nitrogen transformation processes, and diversity of bacterial communities by freeze-thaw events in temperate soils. Pedobiologia 2018, 71, 41–49. [Google Scholar] [CrossRef]
- Kim, E.-A.; Choi, J.H. Changes in the mineral element compositions of soil colloidal matter caused by a controlled freeze-thaw event. Geoderma 2018, 318, 160–166. [Google Scholar] [CrossRef]
- Chung, H.Y..; Jung, J.; Lee, D.H.; Kim, S.; Lee, M.K.; Lee, J.I.; Yoo, K.-C.; Lee, Y.I.; Kim. K. Chemical weathering of granite in ice and its implication for weathering in polar regions. Minerals. 2020, 10, 185. [CrossRef]
- Savenko, A.V.; Savenko, V.S.; Pokrovsky, O.S. Phase Fractionation of Chemical Elements During the Formation of Ice in Fresh Surface Waters. Doklady Acad. Sci. Ser. Earth Sci. Geography 2020, 492, 327–332. [Google Scholar] [CrossRef]
- Morgalev, S.Y.; Morgaleva, T.G.; Morgalev, Y.N.; Loiko, S.V.; Manasypov, R.M.; Lim, A.G.; Pokrovsky, O.S. Experimental modeling of the bacterial community translocation during freezing and thawing of peat permafrost soils of Western Siberia. IOP Conference Series: Earth and Environmental Science 2019, 40, 012017. [CrossRef]
- Morgalev, S.Y.; Lim, A.G.; Morgaleva, T.G.; Morgalev, Y.N.; Manasypov, R.M.; Kuzmina, D.; Shirokova, L.S.; Orgogozo, L.; Loiko, S.V.; Pokrovsky, O.S. Fractionation of organic C, nutrients, metals and bacteria in peat porewater and ice after freezing and thawing. Environ. Sci. Pollut. Res. 2022, 30, 823–836. [Google Scholar] [CrossRef]
- Payandi-Rolland, D.; Shirokova, L.S.; Labonne, F.; Bénézeth, P.; Pokrovsky, O.S. Impact of freeze-thaw cycles on organic carbon and metals in waters of permafrost peatlands. Chemosphere 2021, 279, 130510. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, L.S.; Chupakov, A.V.; Zabelina, S.A.; Neverova, N.V.; Payandi-Rolland, D.; Causserand, C.; Karlsson, J.; Pokrovsky, O.S. Humic surface waters of frozen peat bogs (permafrost zone) are highly resistant to bio- and photodegradation. Biogeosciences 2019, 16, 2511–2526. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Chupakov, A.V.; Ivanova, I.S.; Moreva, O.Y.; Zabelina, S.A.; Shutskiy, N.A.; Loiko, S.V.; Pokrovsky, O.S. Lichen, moss and peat control of C, nutrient and trace metal regime in lakes of permafrost peatlands. Sci. Total. Environ. 2021, 782, 146737. [Google Scholar] [CrossRef] [PubMed]
- Morgalev, Y.N.; Lushchaeva, I.V.; Morgaleva, T.G.; Kolesnichenko, L.G.; Loiko, S.V.; Krickov, I.V.; Lim, A.; Raudina, T.V.; Volkova, I.I.; Shirokova, L.S.; et al. Bacteria primarily metabolize at the active layer/permafrost border in the peat core from a permafrost region in western Siberia. Polar Biol. 2017, 40, 1645–1659. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Shirokova, L.S.; Kisil, O.Y.; Kolesova, S.N.; Lim, A.G.; Kuzmina, D.; Pouillé, S.; Alexis, M.A.; Castrec-Rouelle, M.; Loiko, S.V.; et al. Bacterial Number and Genetic Diversity in a Permafrost Peatland (Western Siberia): Testing a Link with Organic Matter Quality and Elementary Composition of a Peat Soil Profile. Diversity 2021, 13, 328. [Google Scholar] [CrossRef]
- Heimburger, A.; Tharaud, M.; Monna, F.; Losno, R.; Desboeufs, K.; Nguyen, E.B. SLRS-5 Elemental Concentrations of Thirty-Three Uncertified Elements Deduced from SLRS-5/SLRS-4 Ratios. Geostand. Geoanalytical Res. 2012, 37, 77–85. [Google Scholar] [CrossRef]
- Yeghicheyan, D.; Bossy, C.; Le Coz, M.B.; Douchet, C.; Granier, G.; Heimburger, A.; Lacan, F.; Lanzanova, A.; Rousseau, T.C.C.; Seidel, J.; et al. A Compilation of Silicon, Rare Earth Element and Twenty-One other Trace Element Concentrations in the Natural River Water Reference Material SLRS-5 (NRC-CNRC). Geostand. Geoanalytical Res. 2013, 37, 449–467. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Karlsson, J.; Giesler, R. Freeze-thaw cycles of Arctic thaw ponds remove colloidal metals and generate low-molecular-weight organic matter. Biogeochemistry 2018, 137, 321–336. [Google Scholar] [CrossRef]
- Lim, A.G.; Loiko, S.V.; Kuzmina, D.M.; Krickov, I.V.; Shirokova, L.S.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dispersed ground ice of permafrost peatlands: a non-accounted for source of C, nutrients and metals. Chemosphere 2020, 266, 128953. [Google Scholar] [CrossRef]
- Kokelj, S.V.; Burn, C.R. Geochemistry of the active layer and near-surface permafrost, Mackenzie delta region, Northwest Territories, Canada. Can. J. Earth Sci. 2005, 42, 37–48. [Google Scholar] [CrossRef]
- French, H.; Shur, Y. The principles of cryostratigraphy. Earth-Science Rev. 2010, 101, 190–206. [Google Scholar] [CrossRef]
- Lamhonwah, D.; Lafrenière, M.J.; Lamoureux, S.F.; Wolfe, B.B. Multi-year impacts of permafrost disturbance and thermal perturbation on High Arctic stream chemistry. Arct. Sci. 2017, 3, 254–276. [Google Scholar] [CrossRef]
- Lamhonwah, D.; Lafrenière, M.J.; Lamoureux, S.F.; Wolfe, B.B. Evaluating the hydrological and hydrochemical responses of a High Arctic catchment during an exceptionally warm summer. Hydrol. Process. 2017, 31, 2296–2313. [Google Scholar] [CrossRef]
- Ewing, S.A.; O'Donnell, J.A.; Aiken, G.R.; Butler, K.; Butman, D.; Windham-Myers, L.; Kanevskiy, M.Z. Long-term anoxia and release of ancient, labile carbon upon thaw of Pleistocene permafrost. Geophys. Res. Lett. 2015, 42, 10–730. [Google Scholar] [CrossRef]
- Ostroumov, V.; Hoover, R.; Ostroumova, N.; Van Vliet-Lanoë, B.; Siegert, C.; Sorokovikov, V. Redistribution of soluble components during ice segregation in freezing ground. Cold Reg. Sci. Technol. 2001, 32, 175–182. [Google Scholar] [CrossRef]
- Shafique, U.; Anwar, J.; Uz-Zaman, W.; Rehman, R.; Salman, M.; Dar, A.; Jamil, N. Forced migration of soluble and suspended materials by freezing front in aqueous systems. J. Hydro-environment Res. 2012, 6, 221–226. [Google Scholar] [CrossRef]
- Takenaka, N.; Bandow, H. Chemical Kinetics of Reactions in the Unfrozen Solution of Ice. J. Phys. Chem. A 2007, 111, 8780–8786. [Google Scholar] [CrossRef]
- Elliott, A.C.; Henry, H.A.L. Freeze–thaw cycle amplitude and freezing rate effects on extractable nitrogen in a temperate old field soil. Biol. Fertil. Soils 2009, 45, 469–476. [Google Scholar] [CrossRef]
- Xue, S.; Wen, Y.; Hui, X.; Zhang, L.; Zhang, Z.; Wang, J.; Zhang, Y. The migration and transformation of dissolved organic matter during the freezing processes of water. J. Environ. Sci. 2015, 27, 168–178. [Google Scholar] [CrossRef]
- Petrich, C.; Eicken, H. Growth, Structure and Properties of Sea Ice. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley Blackwell: Oxford, UK, 2010; Volume 2. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Mo, X.; Xue, H.; Liu, M.; Chen, H. Insights into the kinetic processes of solute migration by unidirectional freezing in porous media with micromodel visualization at the pore-scale. Sci. Total. Environ. 2021, 784, 147178. [Google Scholar] [CrossRef]
- Ju, Z.; Du, Z.; Guo, K.; Liu, X. Irrigation with freezing saline water for 6 years alters salt ion distribution within soil aggregates. J. Soils Sediments 2018, 19, 97–105. [Google Scholar] [CrossRef]
- Giesy, J.P.; Briese, L.A. Particulate formation due to freezing humic waters. Water Resour. Res. 1978, 14, 542–544. [Google Scholar] [CrossRef]
- McCarthy, J.F.; Zachara, J.M. Subsurface transport of contaminants. Environ. Sci. Technol. 1989, 23, 496–502. [Google Scholar] [CrossRef]
- Murphy, E.; Zachara, J. The role of sorbed humic substances on the distribution of organic and inorganic contaminants in groundwater. Geoderma 1995, 67, 103–124. [Google Scholar] [CrossRef]
- Fariña, A.O.; Peacock, C.L.; Fiol, S.; Antelo, J.; Carvin, B. A universal adsorption behaviour for Cu uptake by iron (hydr)oxide organo-mineral composites. Chem. Geol. 2018, 479, 22–35. [Google Scholar] [CrossRef]
- Krickov, I.V.; Pokrovsky, O.S.; Manasypov, R.; Lim, A.G.; Shirokova, L.S.; Viers, J. Colloidal transport of carbon and metals by western Siberian rivers during different seasons across a permafrost gradient. Geochim. Cosmochim. Acta 2019, 265, 221–241. [Google Scholar] [CrossRef]
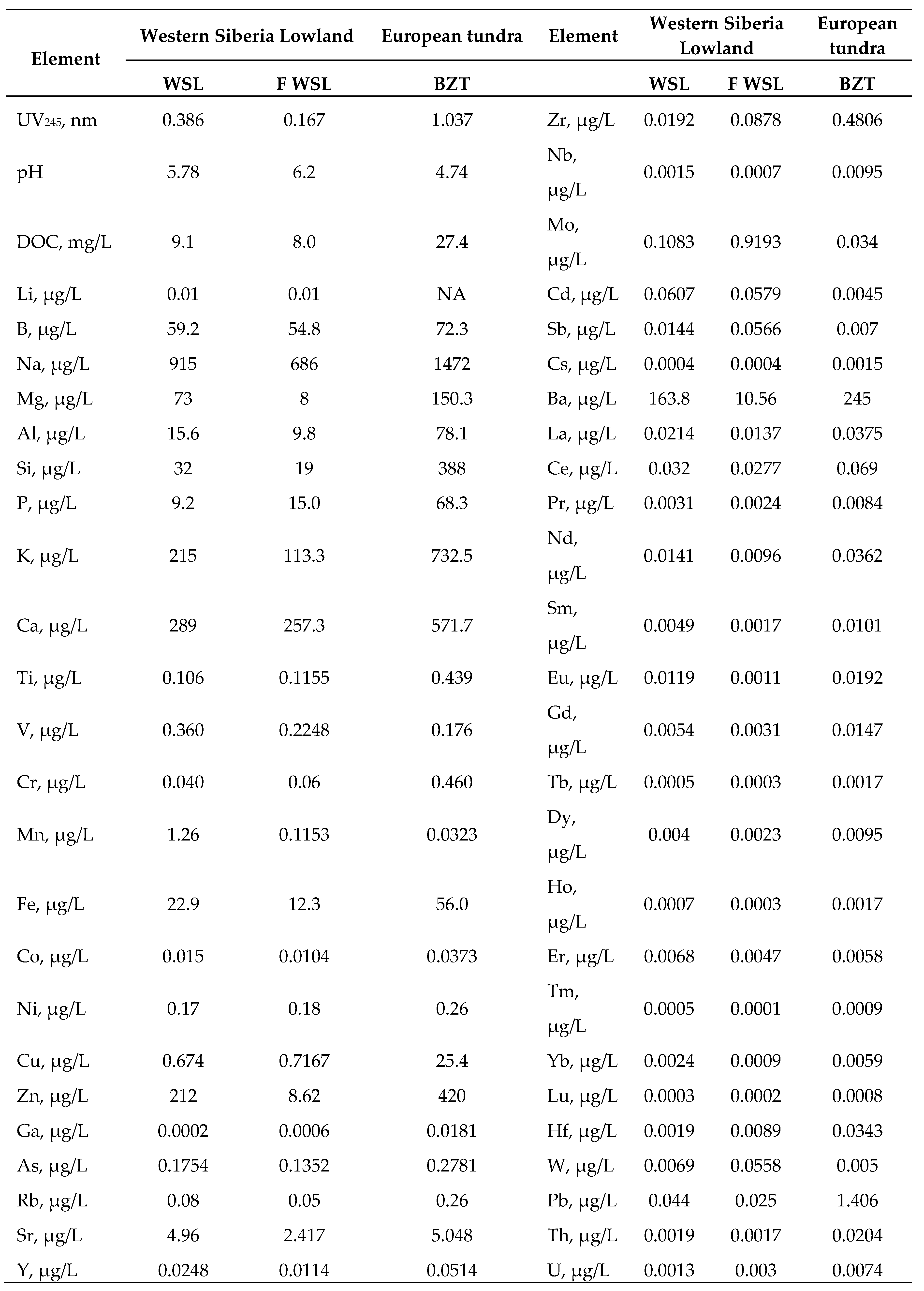 |
| WSL | F WSL | BZT |
|---|---|---|
| С0/Сfinal <0.5 | ||
| Li, Cu, Pb , Th, U | Mg, Mn, Zn, Cd, Ba | Mg, Al, Si, V, Mn, Fe, Co, Ni, Ga, Rb, Zr, Cd, Sb, Cs, Ce, Pb, Th |
| С0/Сfinal 0.5–1.5 | ||
| DOC, B, Na, Mg, Al, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Zn, Ga, As, Rb, Sr, Zr, Mo, Cd, Sb, Cs, Ba, REEs, Hf | Li, B, Na, Al, Ti, V, Fe, Co, Ni, Cu, Ga, Zr, Mo, Sb, Cs, Ba, REEs, Hf, Pb, U | DOC, B, Na, P, K, Ca, Ti, Cr, Cu, As, Sr, Mo, Ba, REEs, Hf, W |
| С0/Сfinal 1.5–3 | ||
| Si, Ni, Yb | DOC, K, Ca, As, Sr | U |
| С0/Сfinal >3 | ||
| P, Rb, Th | Zn | |
| freezing WSL+F WSL+BZT | thawing WSL+F WSL+BZT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DOC | Na | Al | Fe | DOC | Na | Al | Fe | ||
| DOC | 1 | -0.0584 | 0.9752* | 0.9537* | DOC | 1 | 0.1815 | 0.9320* | 0.9695* |
| Li | 0.2841 | 0.6957 | 0.4028 | 0.4249 | Li | 0.9149* | 0.3597 | 0.9141* | 0.9396* |
| B | 0.0137 | 0.9399* | 0.0984 | 0.1099 | B | 0.2050 | 0.9889* | 0.1702 | 0.2581 |
| Na | -0.0584 | 1 | 0.0585 | 0.0556 | Na | -0.4262 | 1 | 0.1483 | 0.2255 |
| Mg | 0.2708 | 0.7249* | 0.3661 | 0.3465 | Mg | 0.6507 | 0.5771 | 0.6468 | 0.6935 |
| Al | 0.9752* | 0.0585 | 1 | 0.9714* | Al | 0.9320* | 0.1483 | 1 | 0.9684* |
| Si | 0.9433* | 0.0777 | 0.9784* | 0.9533* | Si | 0.9302* | 0.2209 | 0.9686* | 0.9516* |
| P | 0.8843* | 0.2769 | 0.9342* | 0.9110* | P | 0.9797* | 0.1920 | 0.9584* | 0.9802* |
| K | 0.8286* | 0.0625 | 0.8723* | 0.8240* | K | 0.8810* | 0.1027 | 0.9076* | 0.8650* |
| Ca | 0.2949 | 0.7511* | 0.3536 | 0.4123 | Ca | 0.6472 | 0.7605* | 0.6221 | 0.6901 |
| Ti | 0.9329* | 0.1386 | 0.9767* | 0.9448* | Ti | 0.9463* | 0.3545 | 0.8872* | 0.9503* |
| V | -0.0965 | -0.0566 | -0.1673 | 0.0424 | V | 0.2062 | -0.0739 | 0.2954 | 0.3317 |
| Cr | 0.7382* | -0.0688 | 0.7430* | 0.6332 | Cr | 0.8028* | 0.5378 | 0.7283* | 0.7761* |
| Mn | -0.3950 | -0.2971 | -0.4310 | -0.3443 | Mn | -0.4973 | -0.2645 | -0.3969 | -0.4086 |
| Fe | 0.9537* | 0.0556 | 0.9714* | 1 | Fe | 0.9695* | 0.2254 | 0.9684* | 1 |
| Co | 0.5802 | 0.2920 | 0.6675 | 0.6908 | Co | 0.8884* | 0.1750 | 0.9240* | 0.9248* |
| Ni | 0.7402* | -0.1376 | 0.7681* | 0.7031* | Ni | 0.9220* | 0.2549 | 0.9012* | 0.9375* |
| Cu | 0.9422* | 0.0668 | 0.9787* | 0.9200* | Cu | 0.9824* | 0.2509 | 0.9490* | 0.9864* |
| Zn | 0.3588 | 0.3950 | 0.3647 | 0.3455 | Zn | 0.2217 | 0.8124* | 0.1310 | 0.2148 |
| Ga | 0.9447* | -0.0082 | 0.9617* | 0.8982* | Ga | 0.8970* | 0.1903 | 0.9884* | 0.9363* |
| As | 0.7844* | -0.0093 | 0.7550* | 0.8698* | As | 0.9210* | 0.2233 | 0.9128* | 0.9598* |
| Rb | 0.7832* | -0.1507 | 0.8052* | 0.7907* | Rb | 0.7769* | -0.1060 | 0.9331* | 0.8367* |
| Sr | -0.1877 | 0.5511 | -0.1361 | -0.0676 | Sr | -0.0097 | -0.1975 | -0.0006 | -0.0246 |
| Y | 0.8549* | 0.0521 | 0.8609* | 0.8934* | Y | 0.9522* | 0.3111 | 0.9301* | 0.9605* |
| Zr | 0.8053* | 0.0260 | 0.8602* | 0.7922* | Zr | 0.9661* | 0.2834 | 0.9565* | 0.9804* |
| Nb | 0.9546* | 0.1243 | 0.9925* | 0.9635* | Nb | 0.9577* | 0.3725 | 0.8911* | 0.9628* |
| Mo | -0.5612 | 0.2892 | -0.4798 | -0.5046 | Mo | -0.4110 | -0.2568 | -0.3710 | -0.4251 |
| Cd | -0.3985 | -0.1202 | -0.3732 | -0.3835 | Cd | -0.5188 | -0.3213 | -0.4304 | -0.4827 |
| Sb | -0.5286 | 0.1542 | -0.4595 | -0.4747 | Sb | -0.3971 | -0.2852 | -0.3401 | -0.3979 |
| Cs | 0.6519 | -0.1790 | 0.6329 | 0.6351 | Cs | 0.6829 | -0.0942 | 0.8846* | 0.7569* |
| Ba | 0.4225 | -0.1811 | 0.4279 | 0.4552 | Ba | 0.7095* | -0.0895 | 0.7918* | 0.7180* |
| La | 0.6586 | -0.0468 | 0.6731 | 0.6531 | La | 0.9526* | 0.1168 | 0.9755* | 0.9722* |
| Ce | 0.6706 | -0.0138 | 0.6699 | 0.6963 | Ce | 0.9631* | 0.1622 | 0.9812* | 0.9906* |
| Nd | 0.9474* | 0.0619 | 0.9602* | 0.9655* | Nd | 0.9643* | 0.2661 | 0.9659* | 0.9818* |
| Hf | 0.7619* | 0.0501 | 0.8238* | 0.7434* | Hf | 0.9608* | 0.3444 | 0.9306* | 0.9613* |
| Pb | 0.9462* | 0.0543 | 0.9799* | 0.9318* | Pb | 0.9271* | 0.1359 | 0.9964* | 0.9687* |
| Th | 0.9580* | 0.0672 | 0.9842* | 0.9479* | Th | 0.9781* | 0.1818 | 0.9742* | 0.9873* |
| U | 0.3306 | 0.4467 | 0.3885 | 0.3045 | U | 0.6095 | 0.5018 | 0.4967 | 0.5296 |
| freezing WSL+F WSL+BZT (insulant+no insulant) | thawing WSL+F WSL+ BZT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOC | Na | Al | Ca | Fe | DOC | Na | Al | Ca | Fe | ||
| DOC | 1 | -0.4156 | 0.5072 | 0.0843 | 0.5100 | DOC | 1 | -0.3016 | 0.5712 | 0.1688 | 0.6304 |
| Li | -0.1530 | 0.7967* | 0.4767 | 0.7761* | 0.4696 | Li | 0.2761 | -0.0676 | 0.4645 | 0.3155 | 0.3766 |
| B | -0.0739 | 0.6382 | 0.0415 | 0.6214 | 0.1158 | B | -0.0679 | 0.9436* | -0.0395 | 0.7411* | 0.1240 |
| Na | -0.4156 | 1 | 0.1041 | 0.7425* | 0.1953 | Na | -0.3016 | 1 | -0.1697 | 0.5711 | -0.0609 |
| Mg | -0.2679 | 0.8074* | 0.1838 | 0.7995* | 0.1953 | Mg | -0.2314 | -0.0126 | -0.1526 | -0.7061 | -0.2045 |
| Al | 0.5072 | 0.1041 | 1 | 0.2329 | 0.7560* | Al | 0.5712 | -0.1697 | 1 | 0.2742 | 0.9317* |
| Si | 0.1082 | 0.4472 | 0.5676 | 0.3854 | 0.8156* | Si | 0.2021 | -0.1342 | 0.4378 | -0.4496 | 0.3752 |
| P | 0.1482 | 0.4320 | 0.2525 | 0.7756* | 0.5143 | P | 0.7269* | -0.3039 | 0.4626 | 0.2122 | 0.4140 |
| K | -0.0056 | 0.3047 | 0.6187 | 0.2150 | 0.4870 | K | -0.2479 | -0.0247 | 0.3868 | -0.2146 | 0.2404 |
| Ca | 0.0843 | 0.7425* | 0.2329 | 1 | 0.3776 | Ca | 0.1688 | 0.5711 | 0.2742 | 1 | 0.4163 |
| Ti | 0.5325 | 0.1927 | 0.7393* | 0.3701 | 0.8923* | Ti | 0.4915 | -0.0464 | 0.7842* | 0.4037 | 0.8648* |
| V | 0.7352* | -0.1233 | 0.4677 | 0.3208 | 0.7364* | V | 0.5676 | 0.0204 | 0.8124* | 0.6671 | 0.9248* |
| Cr | 0.3214 | -0.2685 | 0.3539 | -0.1761 | -0.1111 | Cr | 0.1342 | 0.0799 | 0.0982 | 0.2944 | 0.0721 |
| Mn | -0.0527 | -0.1320 | 0.4663 | -0.3944 | 0.1574 | Mn | -0.2300 | 0.4363 | 0.4854 | 0.2417 | 0.5073 |
| Fe | 0.5100 | 0.1953 | 0.7560* | 0.3776 | 1 | Fe | 0.6304 | -0.0609 | 0.9317* | 0.4163 | 1 |
| Co | 0.0272 | 0.4662 | 0.7655* | 0.2426 | 0.5674 | Co | -0.1855 | 0.0481 | 0.5476 | -0.0876 | 0.4929 |
| Ni | 0.0342 | -0.3260 | -0.2851 | -0.2410 | -0.0968 | Ni | 0.6660 | -0.0068 | 0.6953 | 0.3143 | 0.8425* |
| Cu | -0.1651 | 0.3935 | 0.5024 | 0.1072 | 0.6297 | Cu | 0.2010 | -0.1413 | 0.2787 | 0.1113 | 0.1933 |
| Zn | -0.1390 | 0.0970 | 0.2811 | -0.2016 | 0.1828 | Zn | -0.4330 | 0.1063 | -0.1833 | -0.6541 | -0.2768 |
| Ga | 0.5550 | -0.2729 | 0.4173 | 0.0082 | 0.0492 | Ga | 0.5962 | -0.2789 | 0.5572 | -0.0347 | 0.4060 |
| As | 0.7066* | -0.0685 | 0.3519 | 0.3874 | 0.6971 | As | 0.5922 | -0.0274 | 0.8507* | 0.5036 | 0.8904* |
| Rb | 0.2731 | -0.1322 | 0.6126 | -0.0054 | 0.3259 | Rb | 0.5634 | -0.3121 | 0.9508* | 0.1782 | 0.8402* |
| Sr | -0.1735 | 0.7653* | 0.2376 | 0.8512* | 0.2824 | Sr | -0.2950 | -0.0756 | 0.1700 | -0.3811 | -0.0417 |
| Y | 0.1877 | 0.1517 | 0.5959 | 0.1345 | 0.6976 | Y | 0.5229 | 0.0566 | 0.9368* | 0.3604 | 0.9358* |
| Zr | 0.3338 | -0.0874 | 0.4281 | 0.0913 | 0.3792 | Zr | 0.6300 | -0.0906 | 0.9480* | 0.4148 | 0.9705* |
| Nb | 0.0318 | 0.5789 | 0.7029* | 0.4167 | 0.7073* | Nb | 0.0933 | 0.1119 | 0.6551 | 0.0888 | 0.7199* |
| Mo | 0.5238 | 0.2488 | 0.9165* | 0.4235 | 0.8317* | Mo | 0.0401 | 0.1440 | 0.6823 | 0.0976 | 0.6416 |
| Cd | -0.0607 | -0.1219 | 0.4597 | -0.3656 | 0.2252 | Cd | 0.2183 | -0.1742 | 0.8165* | 0.0156 | 0.8099* |
| Sb | 0.3598 | -0.0489 | 0.4976 | 0.1477 | 0.5904 | Sb | 0.1665 | -0.1549 | 0.5969 | -0.0309 | 0.4949 |
| Cs | 0.5773 | -0.2419 | 0.5398 | 0.0382 | 0.3247 | Cs | 0.5899 | -0.2591 | 0.9123* | 0.2353 | 0.7915* |
| Ba | -0.2300 | 0.0473 | 0.1627 | -0.2749 | 0.0073 | Ba | -0.3542 | -0.0635 | 0.0718 | -0.6617 | -0.0606 |
| La | -0.0399 | -0.1035 | -0.2501 | -0.1054 | -0.1870 | La | 0.5587 | -0.1475 | 0.9793* | 0.2546 | 0.9591* |
| Ce | 0.1158 | -0.0381 | 0.0226 | 0.0197 | -0.0057 | Ce | 0.6383 | -0.1569 | 0.9644* | 0.3963 | 0.9670* |
| Pr | 0.2602 | -0.0751 | 0.1179 | 0.0857 | 0.1968 | Pr | 0.6663 | -0.0715 | 0.9459* | 0.4638 | 0.9769* |
| Nd | 0.3362 | 0.0534 | 0.2615 | 0.2513 | 0.4887 | Nd | 0.6476 | -0.0423 | 0.9222* | 0.5276 | 0.9306* |
| Sm | 0.4565 | -0.1096 | 0.7472* | -0.0797 | 0.6653 | Sm | 0.5272 | 0.0892 | 0.8231* | 0.3027 | 0.8126* |
| Gd | 0.2701 | -0.0438 | 0.4191 | 0.0736 | 0.4928 | Gd | 0.5253 | -0.2441 | 0.9277* | 0.2001 | 0.8383* |
| Dy | 0.3761 | 0.0351 | 0.4851 | 0.1995 | 0.7265* | Dy | 0.4651 | 0.0003 | 0.8763* | 0.2797 | 0.9322* |
| Yb | 0.2059 | 0.1526 | 0.5809 | 0.1827 | 0.5640 | Yb | 0.2427 | 0.2509 | 0.6487 | 0.2005 | 0.6722 |
| Hf | 0.2560 | -0.0663 | 0.4668 | 0.0583 | 0.2919 | Hf | 0.5711 | 0.0393 | 0.8259* | 0.3117 | 0.8759* |
| W | -0.0986 | 0.4389 | -0.0700 | 0.4516 | 0.0428 | W | -0.3747 | 0.7092* | 0.1301 | 0.3248 | 0.1942 |
| Pb | -0.1740 | 0.1610 | 0.4887 | -0.1852 | 0.2997 | Pb | 0.4775 | -0.2282 | 0.8482* | 0.1417 | 0.7263* |
| Th | 0.6065 | 0.1494 | 0.5774 | 0.5807 | 0.7874* | Th | 0.5951 | -0.1594 | 0.9138* | 0.4679 | 0.9234* |
| U | 0.2473 | 0.2469 | 0.6766 | 0.3174 | 0.5078 | U | -0.2065 | 0.3369 | 0.1920 | -0.0993 | 0.0739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




