Submitted:
05 February 2023
Posted:
06 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Carbon dioxide utilization principle
2.1. Reaction mechanism
2.2. CO2 dephosphorization analysis
2.3. CO2 denitrification analysis
2.4. Analysis of soot production in steelmaking
3. Industrial tests
3.1. Material conditions
3.2. Injection mode
3.3. Experimental statistics and analysis
4. Analysis and discussion of laboratory research and industrial test results
4.1. Dephosphorization efficiency
4.2. Slag composition and carbon and oxygen deposition of molten steel
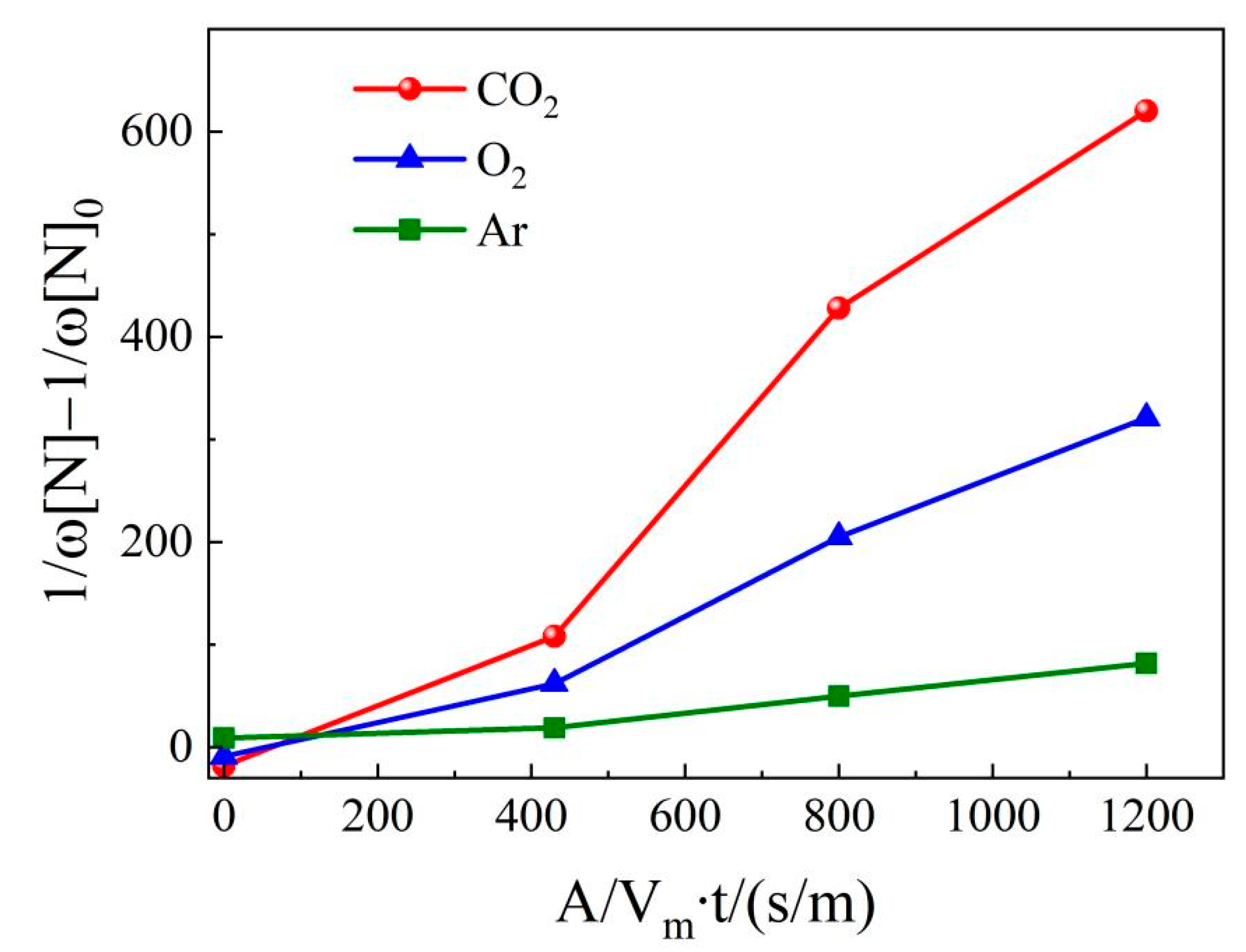
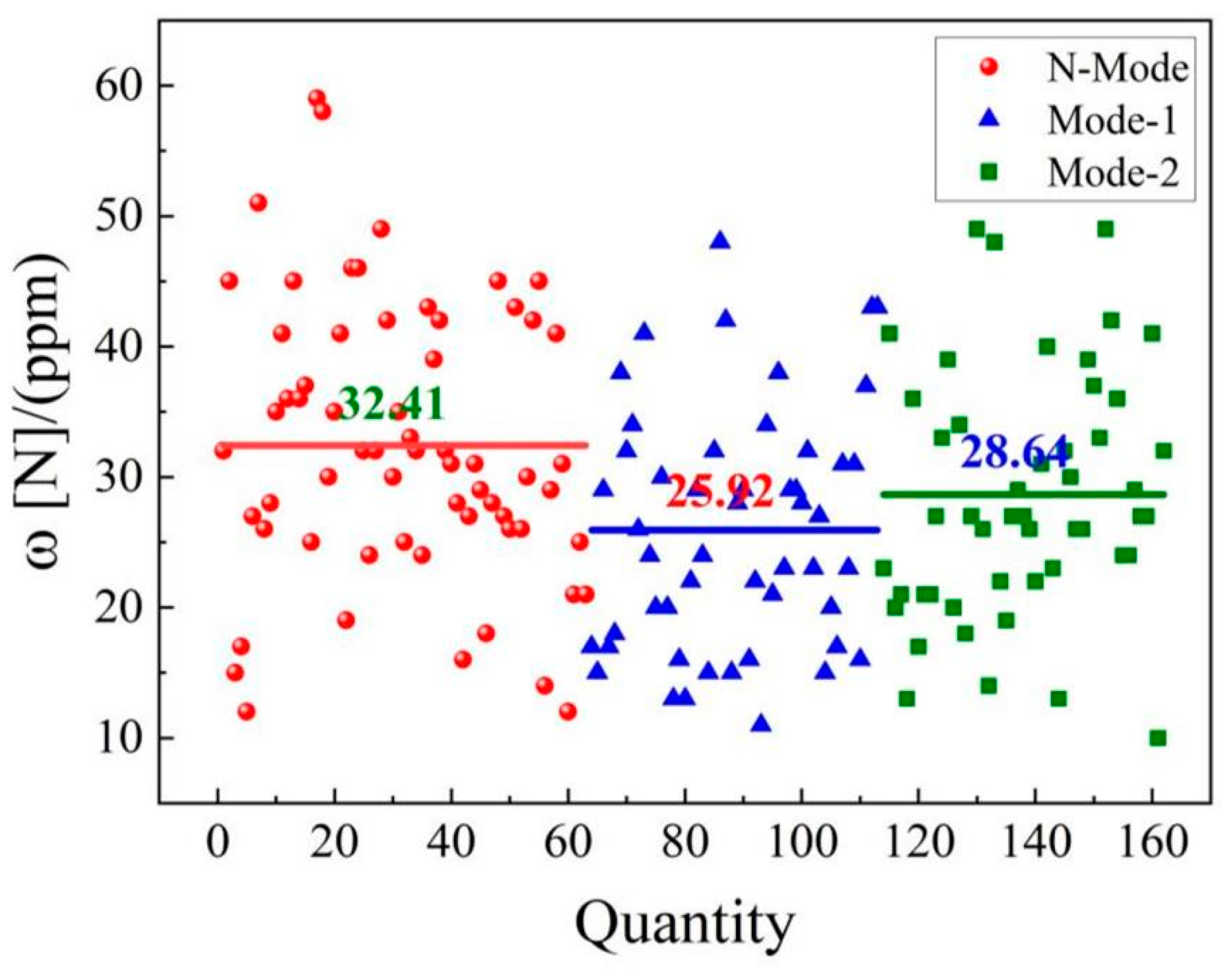
4.3. Nitrogen content in molten steel
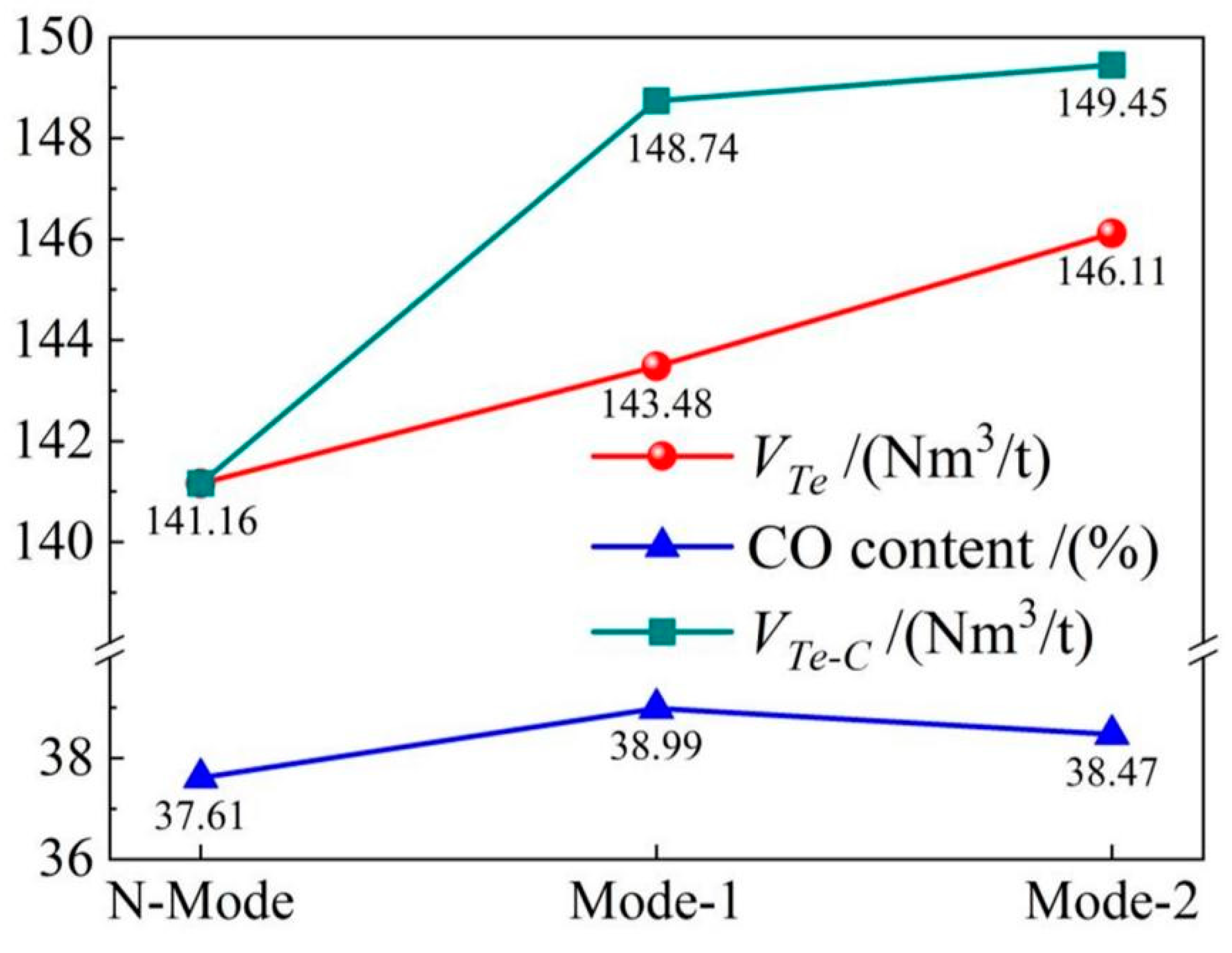
4.4. Gas recovery
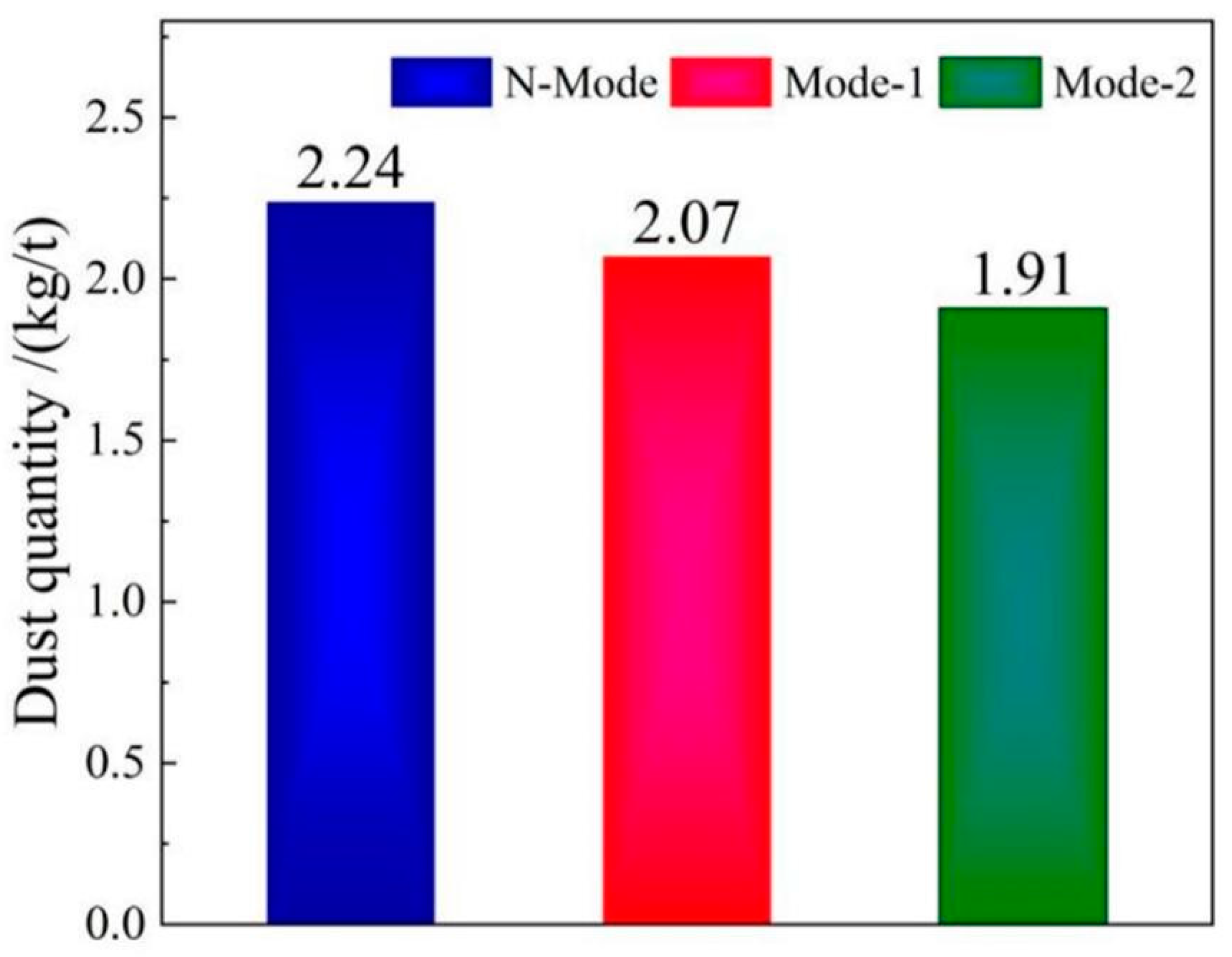
4.5. Soot production
5. Conclusion
- The COMI-B technology helps to improve the converter dephosphorization efficiency, the bubble proliferation effect, and cooling characteristics of top and bottom combined blowing CO2, and the dephosphorization rate is increased by 4.2%.
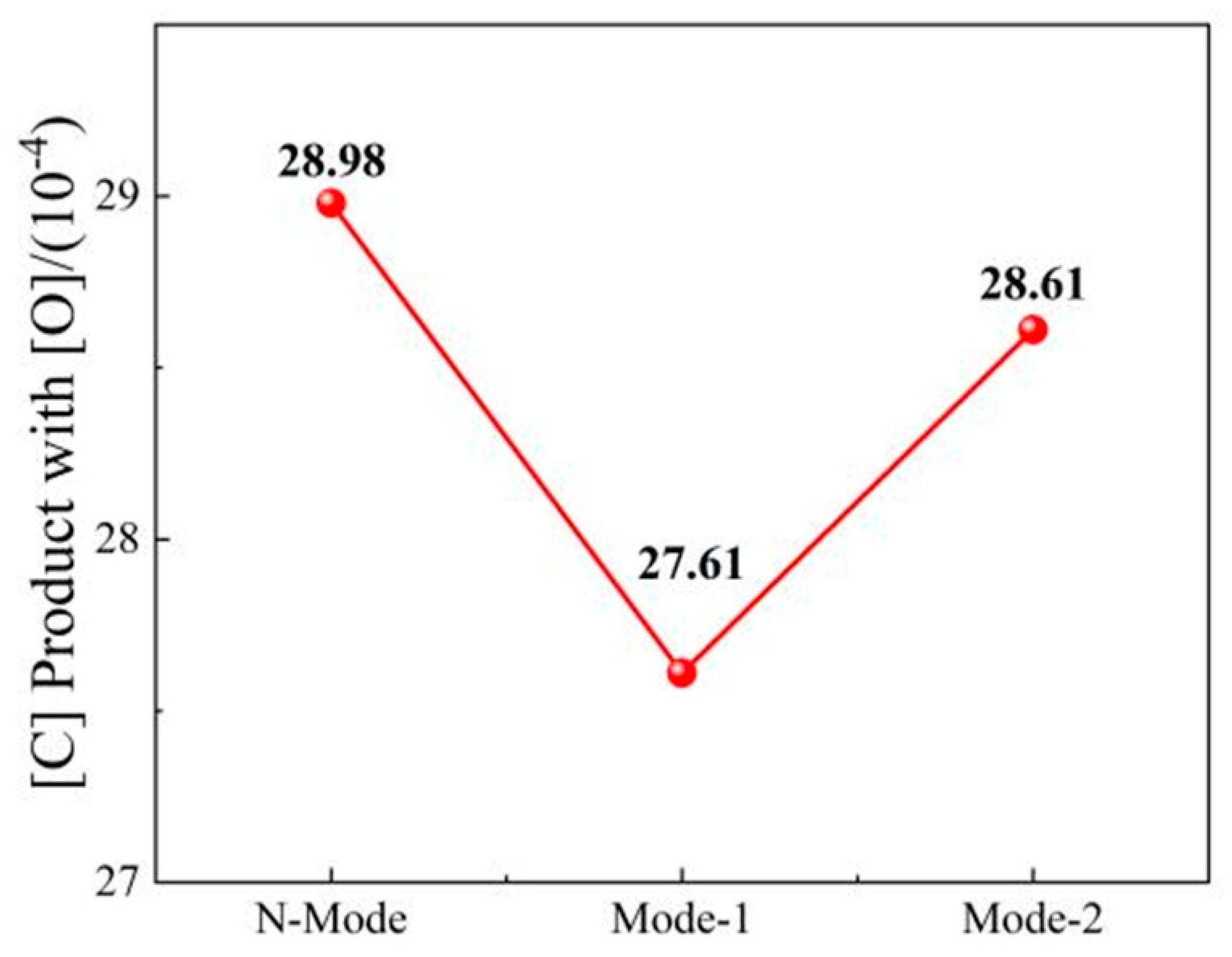
- COMI-B technology helps to reduce the oxidizability of slag, oxidizability and N content of molten steel. The bubble proliferation effect of bottom blowing CO2 increases the rate of deoxidation and denitrification and reduces the carbon-oxygen product and nitrogen content of slag (FeO), molten steel and nitrogen by 2.04%, 1.37 × 10−4 and 20%, respectively.
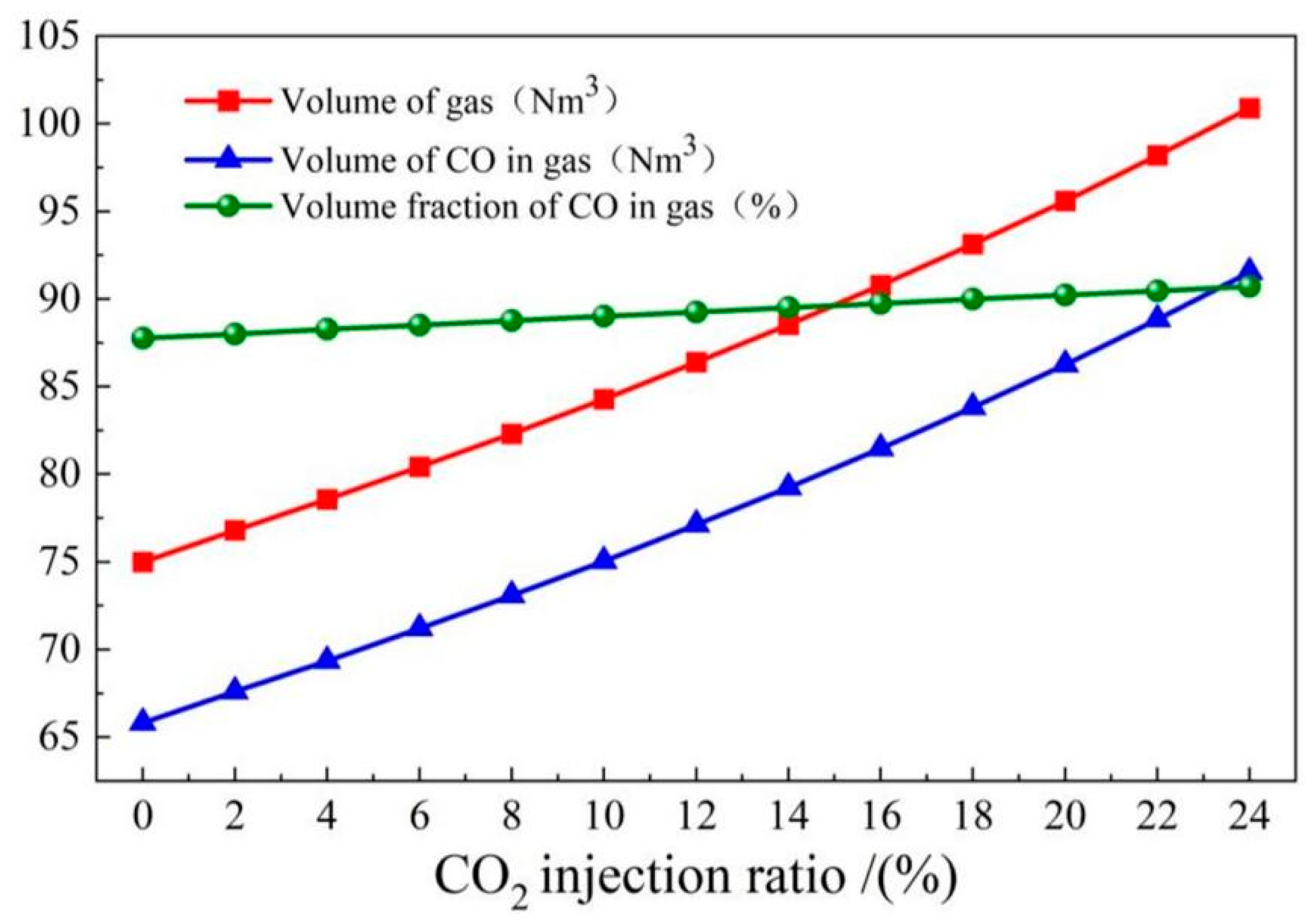
- The COMI-B technology helps to increase gas recovery and gas calorific value, top and bottom combined blowing CO2 participates in the reaction to generate a large amount of CO gas, and 1 mol CO2 produces 2 mol CO, which increases the recovery amount of gas with the same calorific value by 5.9%.
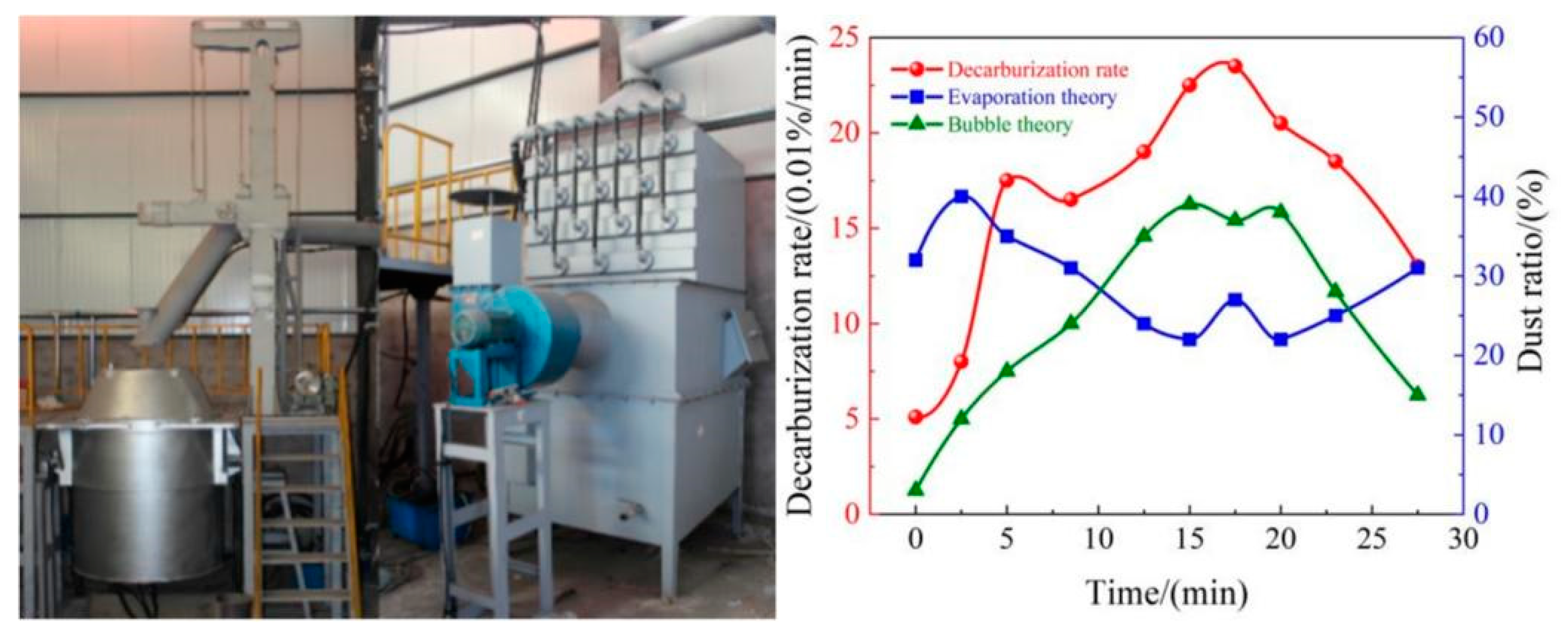
- The COMI-B technology helps to reduce the soot production in the smelting process, the top blowing CO2 reduces the high temperature fire zone of the molten pool, reduces the amount of soot formed by the evaporation of molten iron, and reduces the large size soot content by 14.7%.
Funding
Glossary
| COMI-B | Carbon dioxide oxygen mixed injection - bottom blowing |
| CCS | Carbon capture and storage |
| CCU | Carbon dioxide oxygen mixed injection - bottom blowing |
| CCUS | Carbon Capture, Utilization and Storage |
| ES | Early smelting stage |
| MS | Middle smelting stage |
| LS | Late smelting stage |
References
- Duan, Y.; Han, Z.L.; Zhang, H.; Wang, H.Y. Research on the applicability and impact of CO2 emission reduction policies on China’s steel industry. International Journal of Climate Change Strategies and Management 2021, 13, 352–374. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, S.; Peng, T.D. ; OXM A review of CO2 emissions reduction technologies and low-carbon development in the iron and steel industry focusing on China. Renewable and Sustainable Energy Reviews 2021, 143, 1–23. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renewable & Sustainable Energy Reviews. 2016, 55, 537–549. [Google Scholar]
- Chen, Y.; Zeng, J.H.; Zhang, M.; Pan, H. Research on key technologies of 38CrMoAl steel produced by BOF-LF-RH-CC processp. Advanced Materials Research 2011, 284-286, 1031–1038. [Google Scholar] [CrossRef]
- Zhao, P.; Dong, P.L. Carbon emission cannot be ignored in future of Chinese steel industry. Iron and Steel. 2018, 53, 1–7. [Google Scholar]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 utilization in the perspective of industrial ecology, an overview. Journal of CO2 Utilization. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Shangguan, F.Q.; Li, X.P.; Zhang, C.X. Main energy-saving measures in steel production and the potential analysis of CO2 emission reduction. Energy for Metallurgical Ndusiry. 2009, 28, 3–7. [Google Scholar]
- Prokhorov, E.S. Analysis of equilibrium states of reacting carbon-oxygen thermodynamic system. Journal of Physics: Conference Series 2018, 1105, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhou, L.F.; Li, Y. Development status of CO2 marine storage. Geological Science and Technology Information. 2018, 37, 218–224. [Google Scholar]
- Burton, E.A.; Birkinshaw, K.; Myer, L.; Myhre, R.; Coombs, M.J. Informing policy development for geologic carbon sequestration in California. Energy Procedia. 2009, 1, 4617–4624. [Google Scholar] [CrossRef]
- Nocitoa, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: carbon capture utilization and storage (CCUS). Current Opinion in Green and Sustainable Chemistry. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Yang, G.D.; Li, Y.L.; Atrens, A.; Liu, D.Q.; Wang, Y.S.; Jia, L.; et al. Reactive transport modeling of long-term CO2 sequestration mechanisms at the Shenhua CCS demonstration project, China. Journal of Earth Science. 2017, 28, 457–472. [Google Scholar] [CrossRef]
- Peters, M.; Kçhler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical technologies for exploiting and recycling carbon dioxide into the value chain. Chem Sus Chem. 2011, 4, 1216–1240. [Google Scholar] [CrossRef]
- Voorhees, V.; Robert, F. Crediting carbon dioxide storage associated with enhanced oil recovery. Energy Procedia. 2017, 114, 7659–7666. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. Journal of CO2 Utilization 2013, 3-4, 65–73. [Google Scholar] [CrossRef]
- Stuardi, F.M.; MacPherson, F.; Leclaire, J. Integrated CO2 capture and utilization: A priority. Current Opinion in Green and Sustainable Chemistry. 2019, 16, 71–76. [Google Scholar] [CrossRef]
- Vishal, V.; Chandra, D.; Singh, U.; Verma, Y. Understanding initial opportunities and key challenges for CCUS deployment in India at scale. 2021, 175, 1–19.
- Jiang, K.; Peta, A. "The development of Carbon Capture Utilization and Storage (CCUS) research in China: A bibliometric perspective. ". Renewable and Sustainable Energy Reviews 2021, 138, 110521. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P.; Zhang, S.; Liang, X.; Sun, Y.; Angus, D. China's carbon capture, utilization and storage (CCUS) policy: A critical review. Renewable and Sustainable Energy Reviews 2020. [CrossRef]
- Hao, T.; Zhang, S.; Chen, W.Y. "Assessing representative CCUS layouts for China’s power sector toward carbon neutrality. ". Environmental Science & Technology 2021, 16, 11225–11235. [Google Scholar]
- Steeneveldt, R.; Berger, B.; Torp, T.A. CO2 capture and storage closing the knowing-doing gap capture and storage closing the knowing–doing gap. Chemical Engineering Research and Design 2006, 84, 739–763. [Google Scholar] [CrossRef]
- Ravento’s, M.; Duarte, S.; Alarco’n, R. Application and Possibilities of Supercritical CO2 Extraction in Food Processing Industry: An Overview. Food Science and Technology International 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Xu, Z.C.; Chen, X.Z.; Liu, J.G.; Zhang, Y.; Chau, S.; Bhattarai, N.; Wang, Y.; Li, Y.J.; Connor, T.; Li, Y.K. Impacts of irrigated agriculture on food–energy–water–CO2 nexus across metacoupled systems. Nature Communications 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hsieh, T.L.; Pottimurthy, Y.; Shah, V.; Xu, D.K.; Chen, Y.Y.; Fan, L.S.; Tong, A. "Design and operations of a 15 kWth subpilot unit for the methane-to-syngas chemical looping process with CO2 utilization. ". Industrial & Engineering Chemistry Research 2019, 59, 6886–6899. [Google Scholar]
- Kim, Y.; Hyun, K.; Ahn, D.; Kim, R.; Park, M.H.; Kim, Y. "Efficient aluminum catalysts for the chemical conversion of CO2 into cyclic carbonates at room temperature and atmospheric CO2 pressure. ". Chem Sus Chem 2019, 12, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Buelens, L.C.; Poelman, H.; Saeys, M.; Marin, G.B. ; GalvitaVV. "Upcycling the carbon emissions from the steel industry into chemicals using three metal oxide loops.". Energy Advances 2022, 1, 367–384. [Google Scholar]
- Valderramaa, M.; Puttenb, R.; Gruter, G. The potential of oxalic-and glycolic acid based polyesters (review). Towards CO2 as a feedstock (Carbon Capture and Utilization-CCU). European Polymer Journal. 2019, 119, 445–468. [Google Scholar]
- Zhang, J.J.; Qian, Q.L.; Wang, Y.; Bediako, B.B.; Yan, J.; Han, B.X. Synthesis of ethanol from aryl methyl ether/lignin, CO2 and H2. Chemical Science. 2019, 10, 10640–10646. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lee, M.S.; Den, W. Simultaneous carbon capture, biomass production, and diary wastewater purification by spirulina maxima photobioreaction. Industrial & Engineering Chemistry Research. 2013, 52, 2046–2055. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology-the U. S. department of energy's carbon sequestration program. International Journal of Greenhouse Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Bu, X.P. CO2 capture technologies and application. Clean Coal Technology. 2014, 20, 9–13. [Google Scholar]
- Klemeš, J.; Bulatov, I.; Cockerill, T. Techno-economic modelling and cost functions of CO2 capture processes. Computers & Chemical Engineering 2007, 31, 45–455. [Google Scholar]
- Wei, G.S.; Zhu, R.; Wu, X.T.; Dong, K.; Yang, L.Z.; Liu, R.Z. Technological innovations of carbon dioxide injection in EAF-LF steelmaking. Jom. 2018, 70, 969–976. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, R.; Wei, X.Y.; Wang, H.; Bi, X.R. Research on top and bottom mixed blowing CO2 in converter steelmaking process. Steel Research International. 2012, 83, 11–15. [Google Scholar] [CrossRef]
- Gu, Y.L.; Wang, H.J.; Zhu, R.; Wang, J.; Lv, M.; Wang, H. Study on experiment and mechanism of bottom blowing CO2 during the LF refining process. Steel Research International. 2014, 85, 589–598. [Google Scholar] [CrossRef]
- Wei, G.S.; Zhu, R.; Tang, T.P.; Dong, K.; Wu, X.T. Study on the impact characteristics of submerged CO2 and O2 mixed injection (S-COMI) in EAF steelmaking. Metallurgical & Materials Transactions B 2019, 50B, 1077–1090. [Google Scholar]
- Wei, G.S.; Zhu, R.; Wu, X.T.; Yang, L.Z.; Dong, K.; Cheng, T.; et al. Study on the fluid flow characteristics of coherent jets with CO2 and O2 mixed injection in electric arc furnace steelmaking processes. Metallurgical & Materials Transactions B 2018, 49B, 1405–1420. [Google Scholar]
- Wei, G.S.; Zhu, R.; Cheng, T.; Dong, K.; Yang, L.Z.; Tang, T.P.; et al. Effect of main gas composition on flow field characteristics of supersonic coherent Jets with CO2 and O2 mixed injection (COMI) at steelmaking temperature. ISIJ International. 2018, 58, 842–851. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Q.X.; Gao, Q.; Zeng, J.Q. Effects of calcium ferrite slag on dephosphorization of hot metal during pretreatment in the BOF converter. Journal of Materials Research and Technology 2020, 1–8. [Google Scholar] [CrossRef]
- Assis, A.N.; Tayeb, M.A.; Sridhar, S.M.; Fruehan, R.J. Phosphorus equilibrium between liquid iron and CaO-SiO2-MgO-Al2O3-FeO-P2O5 slags: EAF slags, the effect of alumina and new correlation. Metals. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, R.; Yang, L.Z. High efficiency dephosphorization by mixed injection during steelmaking process. Steel Research International, 2019; 1–7. [Google Scholar]
- Xue, Y.K.; Zhao, D.G.; Wang, S.H.; Li, C.X.; Guo, R.H. Phosphorus vaporization behaviour from converter slag. Ironmaking & Steelmaking. 2019, 48, 1–8. [Google Scholar]
- Wu, H.J.; Li, Q.Q.; Wang, Z.; Jiang, F.J. Vacuum denitrification and nitrogen absorption of molten steel under ultra-low nitrogen conditions. Materials Science & Technology. 2019, 35, 240–246. [Google Scholar]
- Diao, W.C.; Zhang, M.; Xu, T. Study on nitrogen control process during converter steelmaking process. Science & Technology of Baotou Steel. 2017, 43, 20–22. [Google Scholar]
- Fruehan, R.J. Fundamentals and practice for producing low nitrogen steels. ISIJ International. 1996, 36, 58–S61. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, Z.; Zhang, B.; Peng, Q.C.; Qiu, S.T.; Gan, Y. Behaviors of denitrogenation in RH-MFB. Journal of Iron & Steel Research International. 2013, 20, 40–44. [Google Scholar]
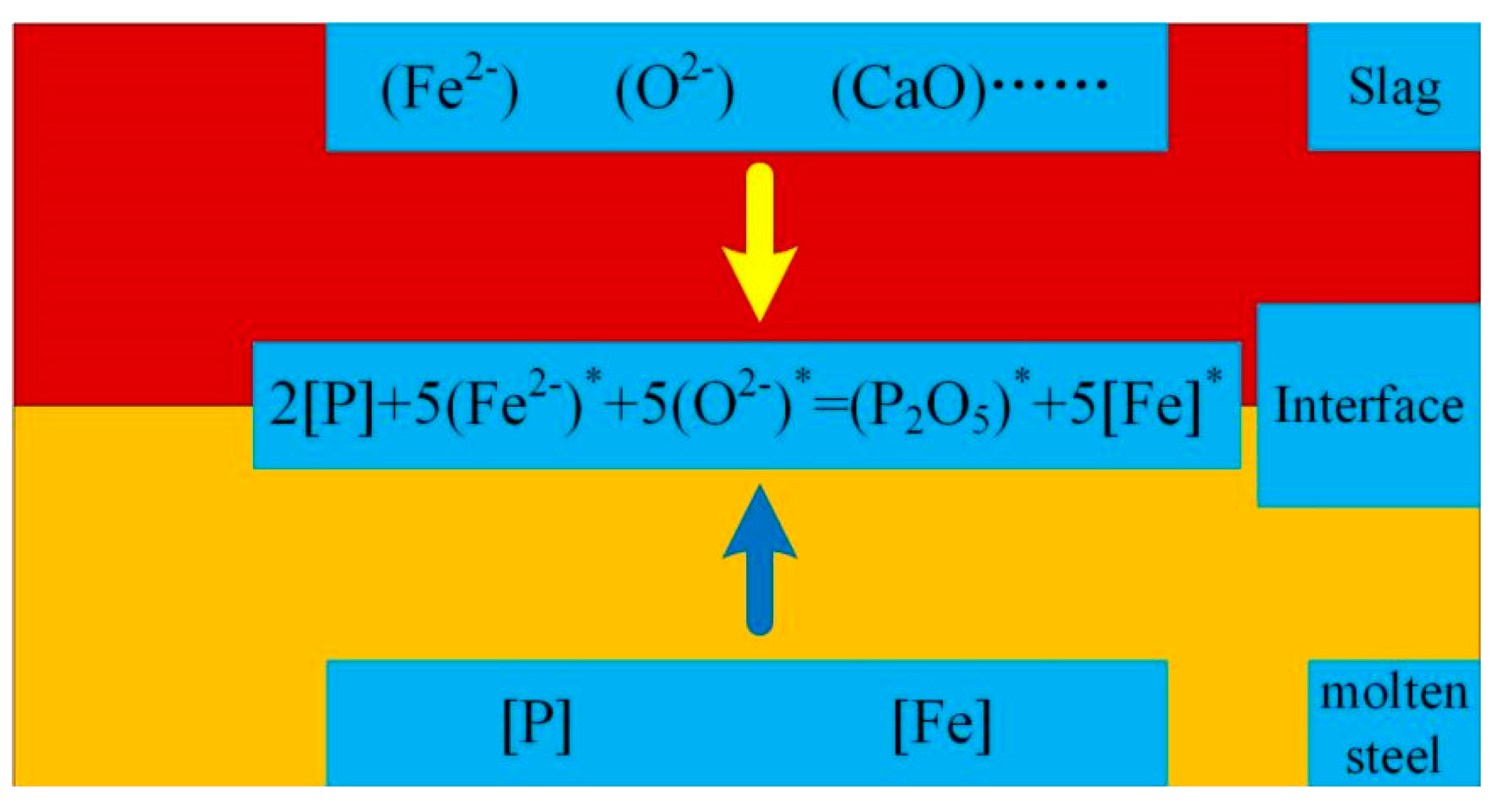
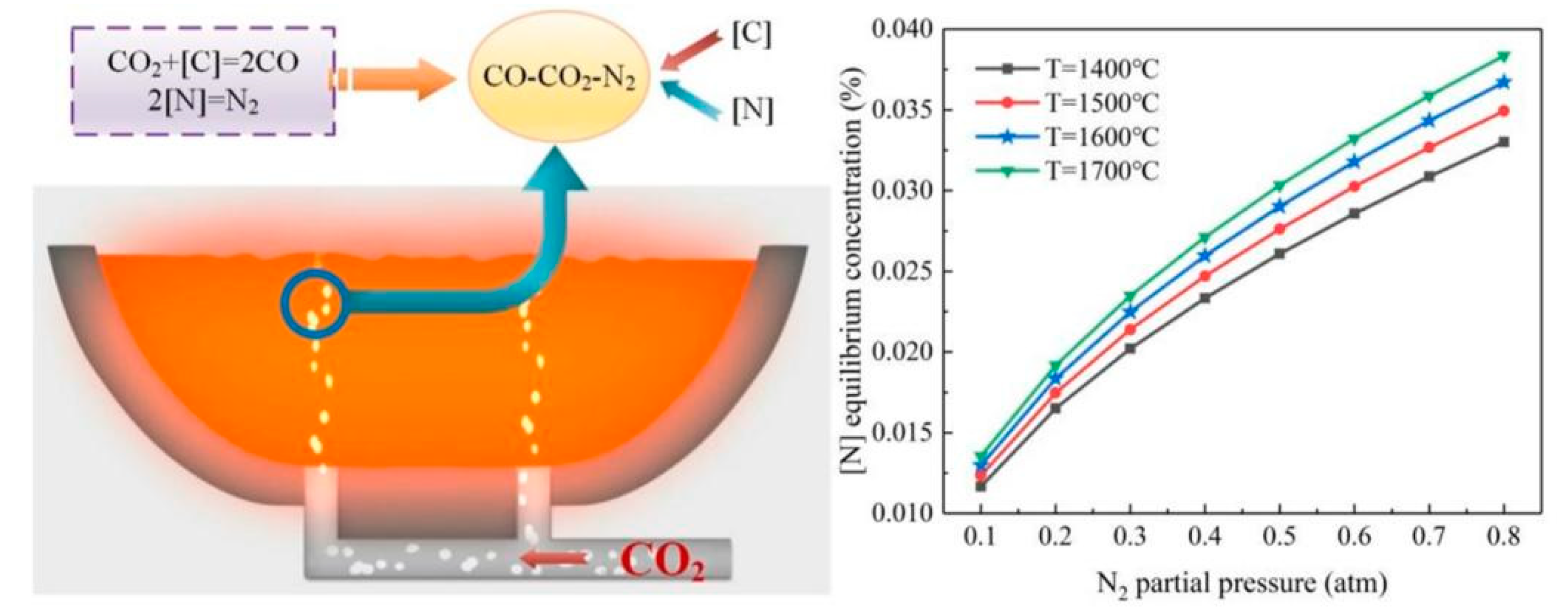
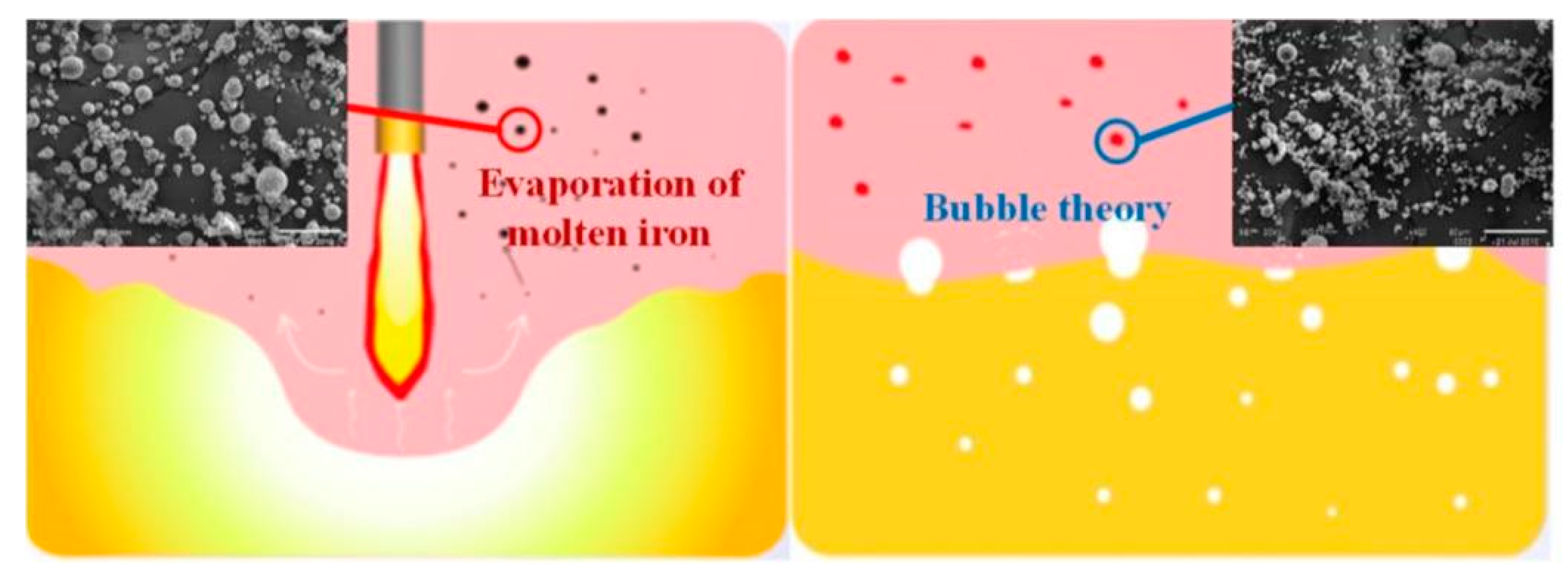
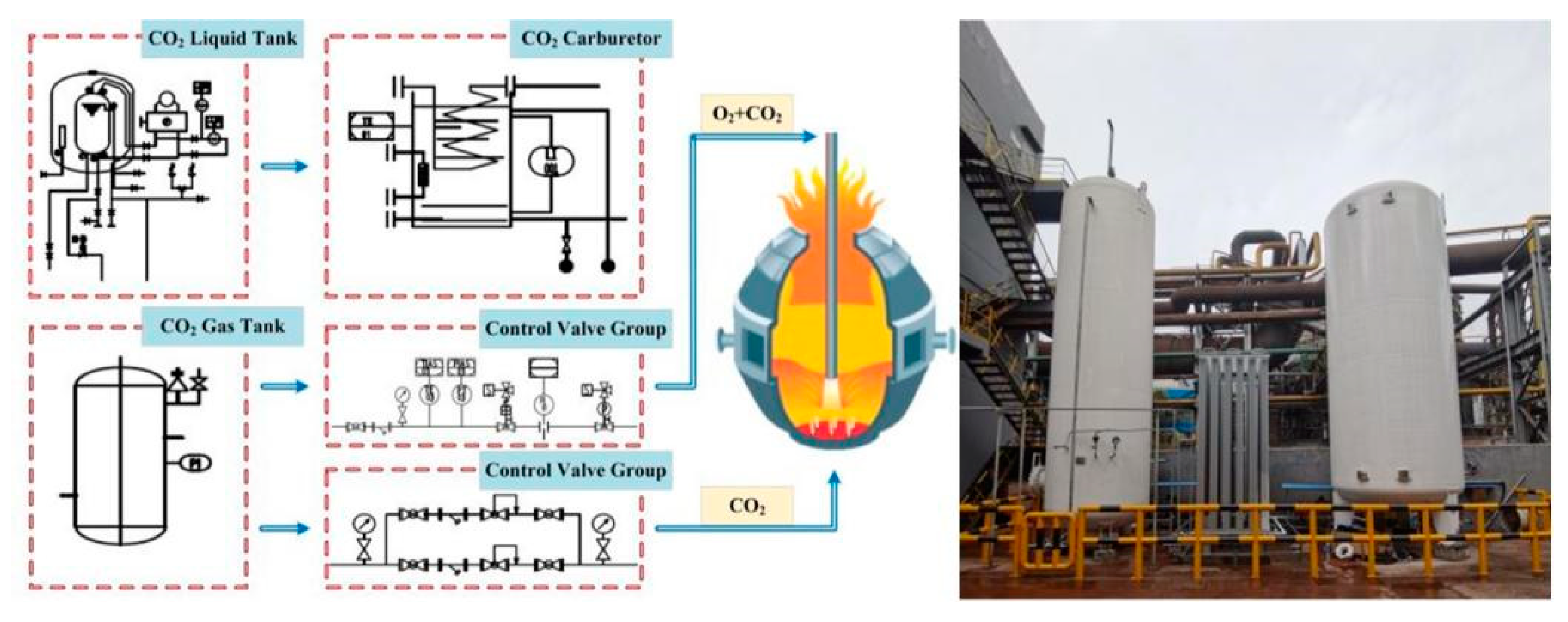
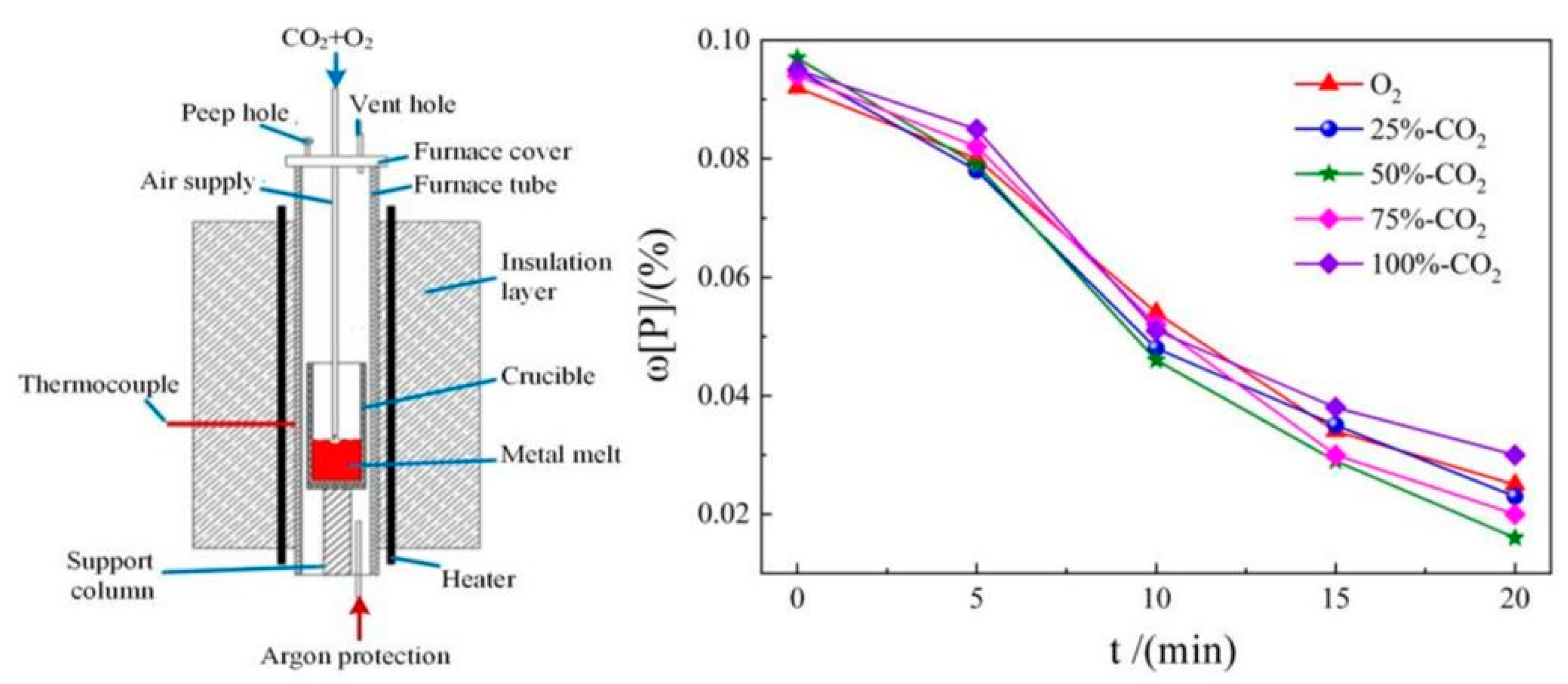
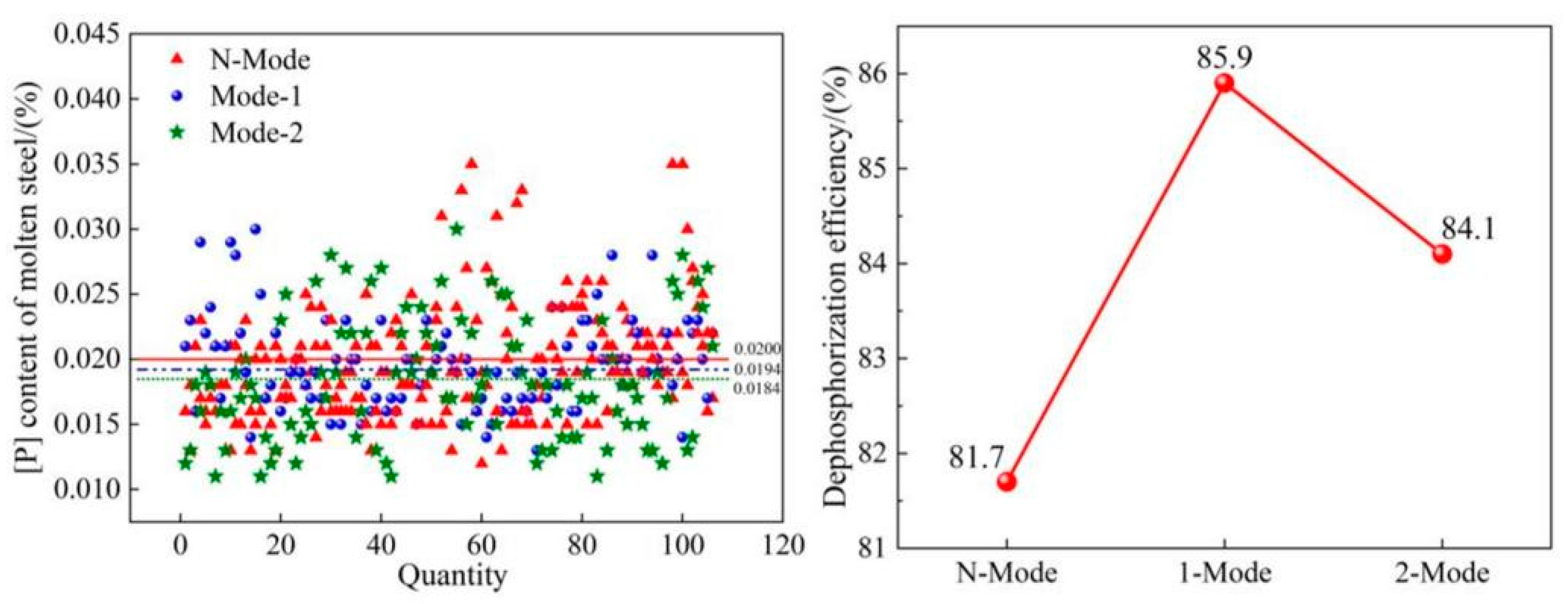
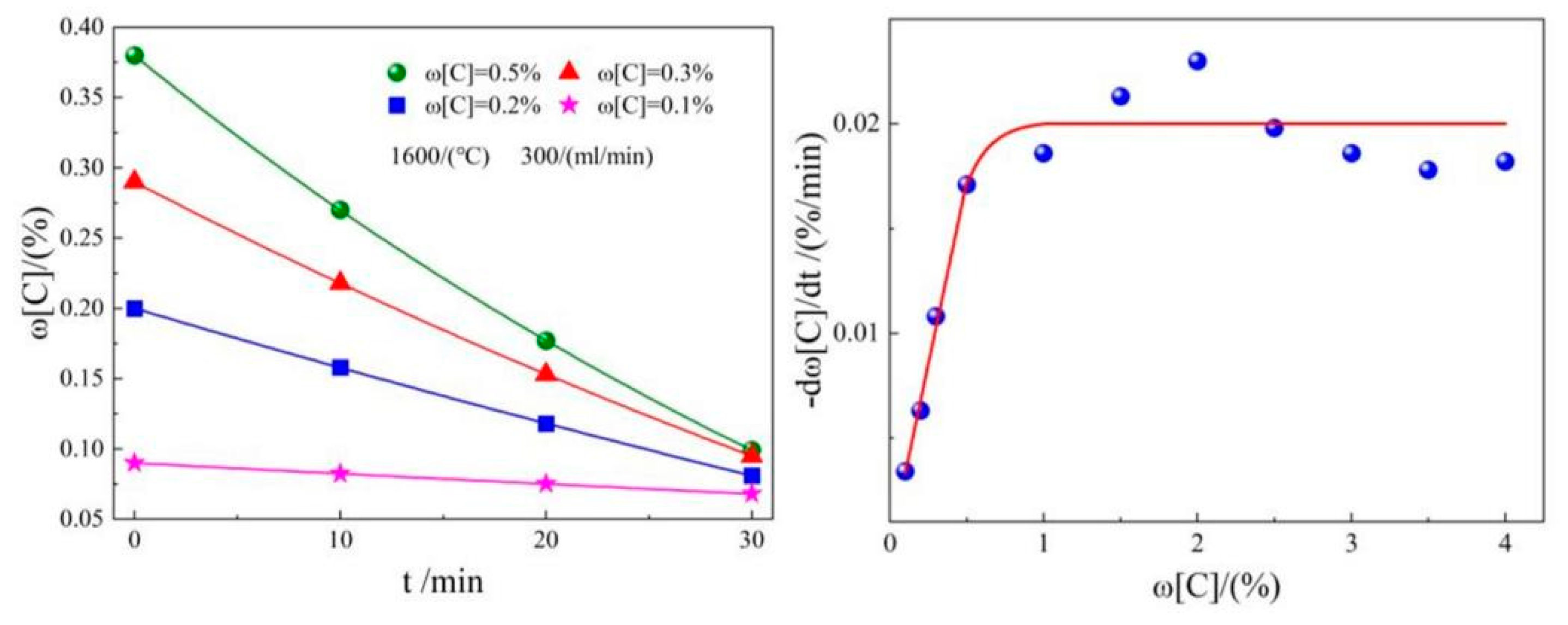
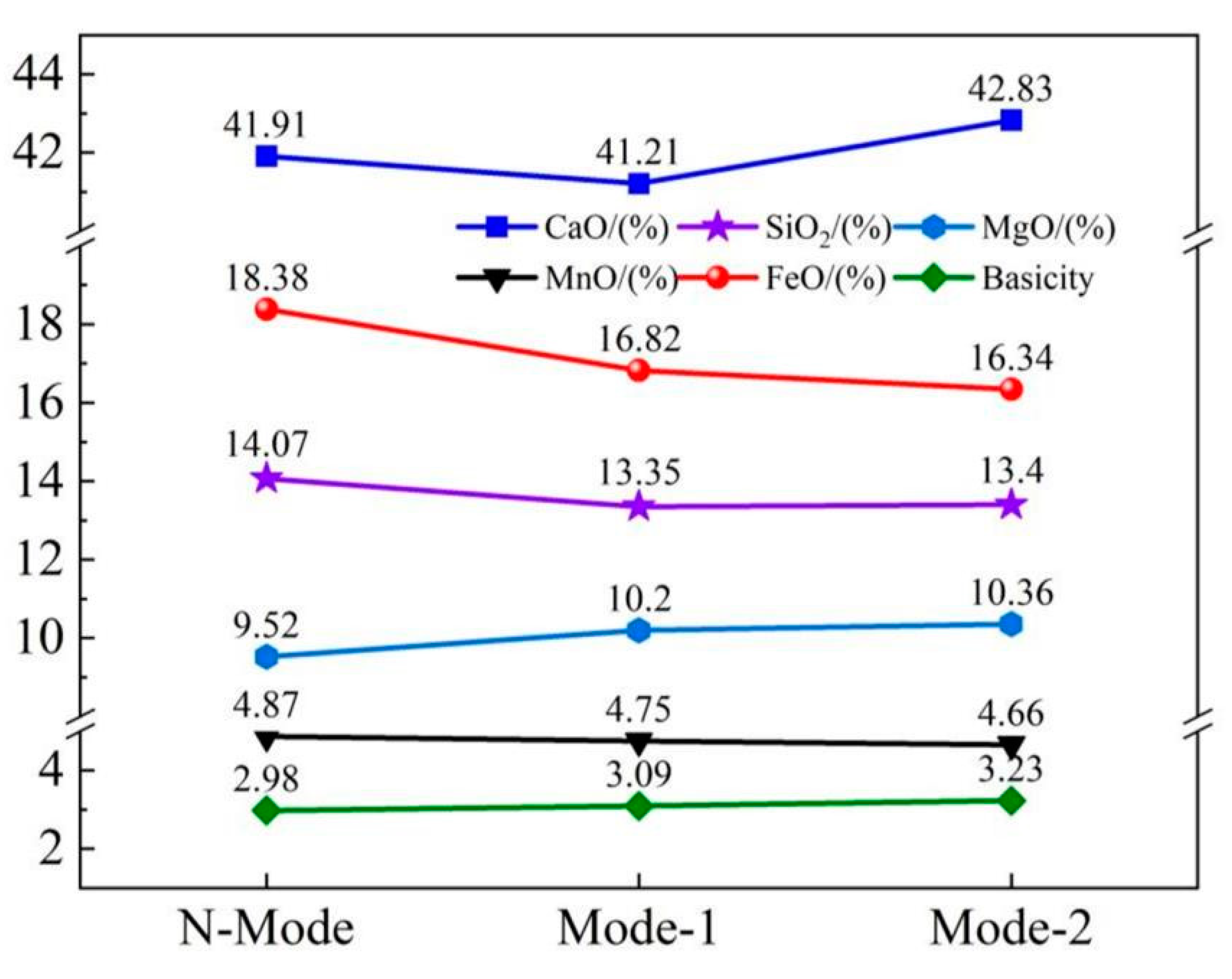
| Element | Reaction | ΔGθ/(J/mol) | ΔGθ1573K/ (kJ/mol) |
ΔGθ1873K/ (kJ/mol) |
|
|---|---|---|---|---|---|
| C | O2 + [C]=CO2(g) | -419050+42.34T | -352.45 | -339.75 | |
| O2 + 2 [C]=2CO(g) | -281160-84.18T | -413.58 | -438.83 | ||
| CO2(g) + [C]=2CO(g) | 137890-126.52T | -61.13 | -99.08 | ||
| Fe | O2(g) +2Fe(l)=2(FeO) | -458980+87.62T | -321.15 | -294.87 | |
| CO2(g) + Fe(l)=(FeO)+CO(g) | 48980-40.62T | -14.92 | -27.10 | ||
| Si | O2 + [Si]=(SiO2) | -804880+210.04T | -474.49 | -411.48 | |
| CO2(g) + 1/2 [Si]=1/2(SiO2)+CO(g) | -123970+20.59T | -91.58 | -85.40 | ||
| Mn | O2 + 2 [Mn]=2(MnO) | -824460+253.88T | -425.11 | -348.94 | |
| CO2(g) + [Mn]=(MnO)+CO(g) | -133760+42.51T | -66.89 | -54.14 | ||
| P | O2+4/5 [P]+8/5(CaO)= 2/5(4CaO·P2O5) |
-845832+255.3T | -444.25 | -367.66 | |
| CO2(g)+2/5 [P]=1/5(P2O5)+CO(g) | 13245+19.753T | 44.32 | 50.24 | ||
| CO2(g)+2/5 [P]+4/5(CaO)= 1/5(4CaO·P2O5)+CO(g) |
-144446+43.22T | -76.46 | -63.49 |
| Category | C/(%) | Si/(%) | Mn/(%) | P/(%) | S/(%) |
|---|---|---|---|---|---|
| Molten iron | 3.80~4.78 | 0.36~0.85 | 0.34~0.79 | 0.085~0.151 | 0.025~0.132 |
| Average value | 4.51 | 0.58 | 0.55 | 0.125 | 0.054 |
| Steel scrap | 0.14~0.25 | 0.18~0.62 | 0.35~1.50 | 0.013~0.048 | 0.018~0.052 |
| Average value | 0.21 | 0.33 | 0.76 | 0.026 | 0.031 |
| Pig iron | 4.08~4.85 | 0.47~1.14 | 0.41~0.85 | 0.101~0.178 | 0.052 ~0.152 |
| Average value | 4.28 | 0.62 | 0.64 | 0.146 | 0.062 |
| Lime/ (kg/t) |
Light burned dolomite/(kg/t) | Limestone /(kg/t) | Raw dolomite/(kg/t) | Mineral/ (kg/t) |
Sludge ball/(kg/t) |
|---|---|---|---|---|---|
| 21.8 | 18.0 | 10.9 | 3.8 | 10.7 | 10.6 |
| ES/(min) | MS/(min) | LS/(min) |
|---|---|---|
| <4.5 | 4.5≤t ≤10.5 | >10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




