1. Introduction
In the past two decades, the world has experienced the wrath of several disease outbreaks, particularly those resulting from re-emerging viruses like the severe acute respiratory syndrome virus 2 (SARS-CoV-2), monkeypox virus (MPXV), Zika virus, and Nipah virus (Mourya et al., 2019). These have been attributed to the spillovers from various animal species due to increased contact (with humans), genomic mutations eventually leading to the emergence of variants, and crossing of species barrier (Chadha et al., 2022a). With the prolonged coronavirus disease 2019 (COVID-19) pandemic and global monkeypox outbreak, several Asian countries are experiencing an unprecedented outbreak of the lumpy skin disease (LSD). It is a highly contagious viral infection of cattle that is caused by the lumpy skin disease virus (LSDV). This bovine virus is a member of the Capripovirus genus and subfamily Chordopoxviriniae, one of the most prominent animal poxviruses because of its serious complications in cattle, buffaloes, and other large ruminants, which directly hits the economics of the livestock sector (Tuppurainen et al., 2017). The disease is known as 'Neethling virus disease,' 'knopvelsiekte,' 'pseudo-urticaria,' and 'exanthema nodularis bovis' (Tuppurainen et al., 2017; Khan et al., 2021). Moreover, LSDV is closely related to the goat poxvirus (GTPV) and sheep poxvirus (SPPV), as it shares high sequence similarity and antigenic relationships with them (Diallo and Viljoen, 2007). LSD is a vector-borne, non-zoonotic, and transboundary disease that has emerged over the decades as a global catastrophic threat to livestock. Although LSD is a bovine ailment and does not affect or pose any threat to humans directly, it has indirect repercussions on human life. These include economic and financial losses and disruption of livestock, dairy, and meat industries, which ultimately lowers the gross domestic product (GDP) in countries that fundamentally depend on agricultural and dairy-related sectors (Gupta et al., 2020). The virus can infect all cattle, regardless of age and breed, but young calves and lactating mothers are reported to be more vulnerable to high infection rates (Tuppurainen et al., 2011). The LSDV finds easy access to susceptible animals through contaminated food (feed), water, and milk. Common arthropod vectors like biting flies, lice, ticks, mosquitoes, and wasps also play a critical role in transmitting LSD (Gupta et al., 2020). Although there are no references to natural LSD infection in goats and sheep despite close interactions with infected cattle, experimental infections characterized by the development of skin lesions and nodules have been reported in giant gazelles, giraffes, goats, impalas, sheep, and wildebeest (Liang et al., 2022). Considering its potentially high transmissibility across territorial borders and its negative economic impact, the World Organization for Animal Health (WOAH) has listed LSD as an important notifiable cross-border disease (Namazi and Khodakaram, 2021). The LSDV has also been considered a potent agro-terrorism agent due to its recent global emergence, transboundary spread, and notably high transmission rates (Khan et al., 2021). Moreover, high morbidity and low mortality rates are attributed to LSD. The viral infection extends both short- and long-term symptoms, depending on the immunological state of the infected animal (Liang et al., 2022). Short-term symptoms include lymphadenitis, anorexia, rhinorrhea, and bilateral epiphora (Liang et al., 2022), while prolonged illness results in ailments like mastitis, pneumonia, and deep holes in the body (Selim et al., 2021). Infected animals may also experience temporary or permanent infertility. Eventually, LSD reduces the economic value of the livestock in terms of milk yield and meat production, quality of animal hide, reproductive fitness, and long-term health. Earlier, LSDV was known to be restricted only to African nations; but it was subsequently reported across distinct geographical locations (non-endemic) worldwide. Recently, LSDV has been reported across regions of Asian and Middle-Eastern countries like India, Pakistan, Israel, Kuwait, Oman, and Yemen (Ayelet et al., 2014; Anwar et al., 2022). Moreover, multiple cluster outbreaks of LSD have erupted in the past five years in the Asian subcontinent itself, creating havoc and resulting in the death of cattle on a large scale. As per recent estimates from India, a country that relies heavily on its agricultural and livestock sectors, over 155,000 cattle deaths were reported in 2022 alone, making this animal disease a serious concern among epidemiologists, researchers, and veterinary scientists. In light of the recent events and considering the expanding geographical footprint of this viral disease, it becomes increasingly important for the scientific community to bridge the existing gaps in the biology of LSDV, its etiology, changing epidemiology, transmissibility, pathogenesis, diagnosis, and disease control and management. Hence, this review aims to augment latest knowledge on the complex biology of LSDV and its economic repercussions. In an attempt to gain more insights, this review also examines the recent LSD outbreaks and speculates the probable reasons behind the epidemiological shift and sudden resurgence of LSDV.
2. Etiology of LSDV: A Brief Overview of Virus Structure
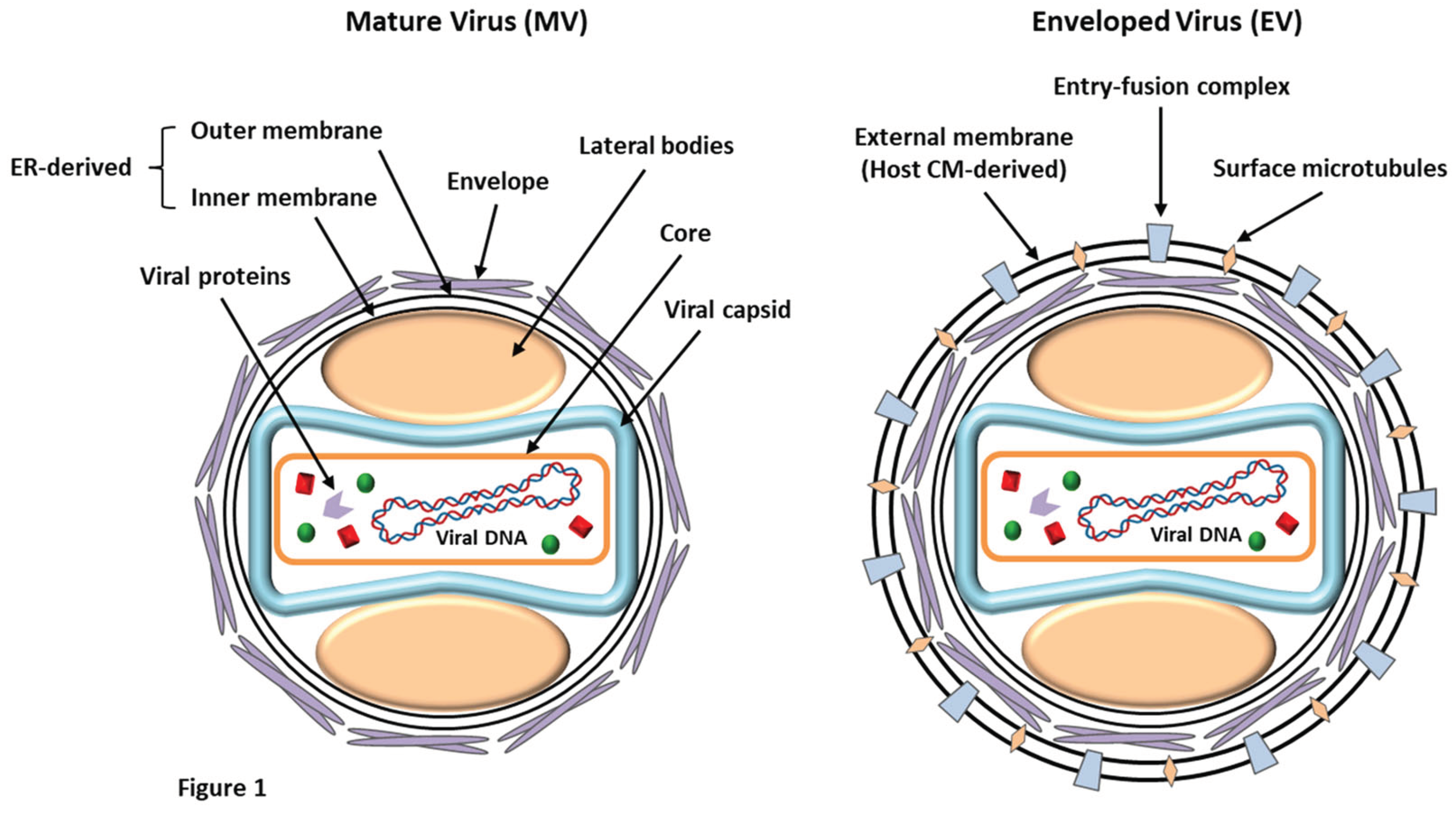
LSDV is an enveloped virus with a brick-shaped structure of roughly 320 x 260 nm in size, which belongs to the
Poxviridae (EFSA, 2015). Under an electron microscope, the structure of LSDV closely resembles that of the vaccinia virus, displaying a characteristic dumbbell-shaped core with lateral bodies (
Figure 1). The virus belongs to the genus
Capripoxvirus of the
Chordopoxvirinae subfamily along with two other species, i.e., the GTPV and SPPV (Bhanuprakash
et al., 2011). The virus harbors a double-stranded DNA genome, roughly about 151 kb in size, and comprises a chief coding region at the center, surrounded by identical terminals of 2.4 kb inverted repeats. The covalently-linked DNA strands contain palindromic hairpins at their terminals. The genome codes for nearly 156 putative genes, of which 30 structural and non-structural genes share about 97% sequence similarity with GTPV and SPPV (Tulman
et al., 2002). The viral DNA contains roughly 146 conserved genes that are critical in driving molecular processes like DNA replication, transcription, virion production, and assembly. However, LSDV completely depends on the host cellular machinery to translate viral mRNA. Also, the LSDV genome contains homologous genes such as G protein-coupled CC chemokine receptor (GPCR), interleukin-10 (IL-10), IL-1 binding proteins, and epidermal growth factor-like protein, which are commonly observed in related poxviruses (Tulman
et al., 2001). The viral DNA also contains an exclusive gene, LSDV132, which differs from other members of
Capripoxvirus.
LSDV exists in two forms: the enveloped virion (EV) and the mature virion (MV) (
Figure 1). These infectious forms have been categorized based on the existence of different surface glycoproteins and membrane layers (Chadha
et al., 2022a). MVs harbor a single lipid bilayer that is acquired from the endoplasmic reticulum of the infected cells. At the same time, EVs are characterized by the presence of an additional outer membrane (host-derived) that bears several entry-fusion complexes and even surface microtubules (Liang
et al., 2022). The virus is known to be resistant to both physical and chemical treatments, and shows high stability under ambient conditions for prolonged periods (EFSA, 2015). It has an exclusive survival potential in desiccated skin crusts (35 days), necrotic nodules (35 days), and air-dried hides (18 days). LSDV is unable to withstand incubation under high temperatures of 55˚C (2 h) and 65˚C (30 min). It remains persistent between pH 6.6 and 8.6 at 37˚C for five days but is highly susceptible to extreme alkaline or acidic conditions (EFSA, 2015). Lipidophilic detergents and sunlight can immediately predispose and inactivate the virus. LSDV is labile to chemical disinfectants such as chloroform, ether (20%), phenol (2% for 15 min), iodine compounds (1:33 dilution), formalin (1%), sodium hypochlorite (2–3%), and quaternary ammonium compounds (0.5%) (EFSA, 2015).
3. Epidemiological Trends and the Expanding Geographical Footprint of LSD: Unraveling the Viral Transition
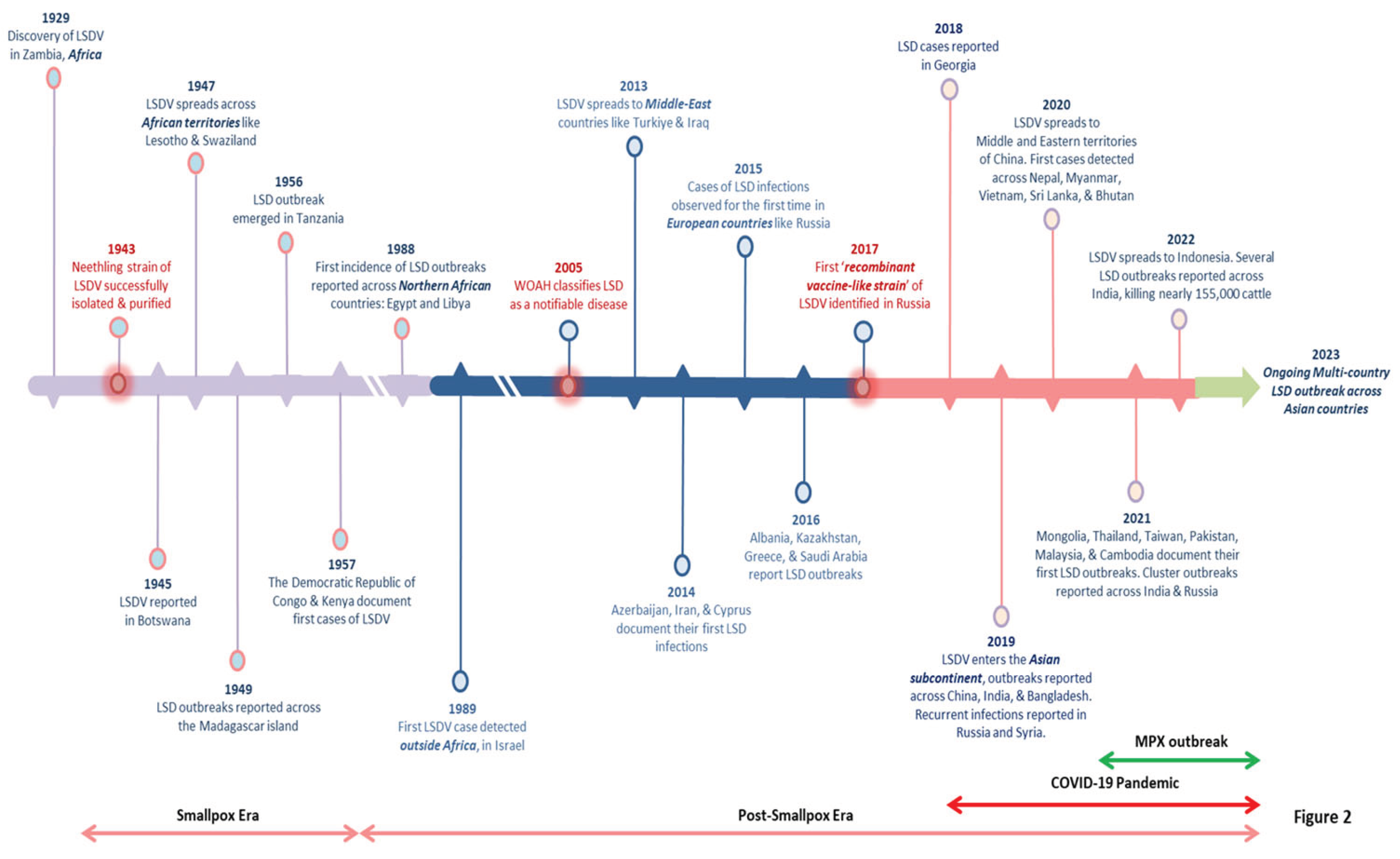
LSD first emerged in 1929 in Southern Africa's Zambia. The viral infection had spread across the southern African countries by the 1940s, decimating a large amount of domestic livestock. Previously, it was a notion that LSDV was endemic to only African countries, but very soon, the virus migrated to the Middle East (EFSA, 2015). The LSD steadily drifted towards the north in the following decades, and presently it is prevalent throughout African countries, including the sub-Saharan regions, and even Madagascar (Azeem
et al., 2022). The only untouched African nations include Libya, Algeria, Morocco, and Tunisia. In May 1988, Egypt reported its first LSD outbreak (Ali
et al., 1990), while Israel experienced the same in 1989 (Yeruham
et al., 1995). This was the first time LSDV crossed territorial borders, resulting in multiple cluster outbreaks outside the African subcontinent and north of the Sahara Desert. More outbreaks were recorded in Middle Eastern nations after 2000, and the disease is now regarded as endemic in these areas (Liang
et al., 2022). Near the end of 2013, the virus marked its entry into Turkey and Iraq, and it was subsequently reported in regions of Iran and Azerbaijan in 2014. Parallelly, the first confirmed case of LSDV appeared in Cyprus (Lojkic
et al., 2018). LSD was introduced in the Balkans and certain regions of the European continent in late 2014, primarily through Turkey, which functions as a connecting link between the Eurasian continents (
Figure 2). Also, the virus first surfaced in Russia in 2015, resulting in over 471 outbreaks over six years, followed by a gradual decline by 2020 (Byadovskaya
et al., 2022). In addition to Kazakhstan, Armenia, Russia, Georgia, and Saudi Arabia, LSDV was also reported in multiple clusters across South-Eastern European nations such as Albania, Bulgaria, Greece, Montenegro, North Macedonia, Kosovo, and Serbia, in 2016 (Lojkic
et al., 2018).
Besides, the disastrous effects of LSD outbreaks have recently been reported across multiple Asian countries, including India, Bangladesh, Pakistan, China, Myanmar, Thailand, Sri Lanka, Nepal, and Vietnam (Azeem
et al., 2022). In Southeast Asia, the first known LSD outbreak was identified in Bangladesh in July 2019 (Das
et al., 2021). In western China's Xinjiang province (Uyghur Autonomous Region), which shares a border with Kazakhstan, the infection emerged in August 2019 (Lu
et al., 2020). Interestingly, LSD was first documented in India in the same month. Successively, India experienced three LSD outbreaks in Odisha, an eastern coastal state (Sudhakar
et al., 2020). According to the statistics from surveillance studies, out of 2,539 suspected animals, 182 were identified to be clinically infected with a morbidity of 7.1%, but no deaths were reported (Sudhakar
et al., 2020). About a year after, in June 2020, Nepal declared an LSD outbreak in some adjacent livestock farms in the Morang district, which borders India (Koirala
et al., 2022), China reported a widespread LSD outbreak again in June 2020, implying the continued presence of LSDV across the country. Since then, the LSDV has crossed several other territorial borders and spread across Mongolia, the Lao People's Democratic Republic, Malaysia, and Cambodia between May and September 2021. Moreover, the disease has re-emerged in its most violent form ever recorded in the Indian subcontinent, resulting in the death of over 155,000 domestic and wild cattle (Kumar and Tripathi, 2022). The timeline illustrating the prime events and the major LSD outbreaks has been depicted in
Figure 2. Considering the trends and caseload reported for the LSD outbreaks in the past five years; it is clearly evident that the virus is undergoing an epidemiological shift due to cross-border animal trade (both legal and illegal), international transport of infected animals, and insect vectors. As a result, the LSDV has been expanding its geographical footprint in non-endemic countries, maintaining its infection cycle and resulting in massive cluster outbreaks. Hence, there is an urgent need to activate high-level vigilance and global surveillance programs to control this multi-country outbreak of LSD.
4. Transmission, Reservoirs, and Hosts of LSDV: A Panoramic Yet Distal View
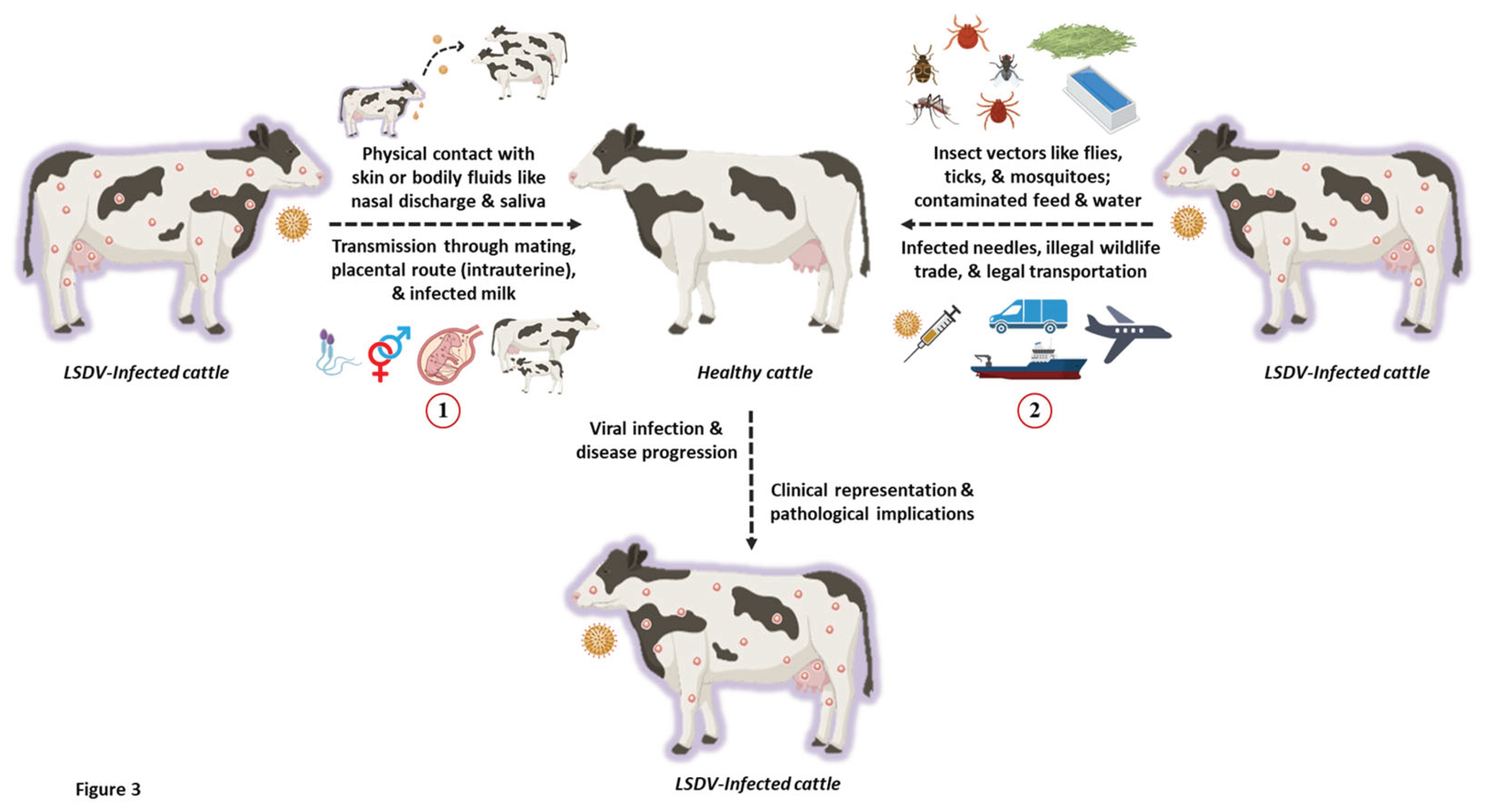
The transmission of poxviruses is multifactorial, involving direct contact through aerosols, bodily fluids like semen, and indirect dissemination through animal or insect vectors, reservoirs, and even fomites (Sprygin
et al., 2019). Like any other poxvirus, LSD is a host-specific disease that is transmitted mechanically through arthropod vectors, with its infective host(s) being bovine animals like cows and buffaloes (
Figure 3). Interestingly, LSD is a non-zoonotic disease with no traceable history or evidence of human infection to date.
Recent studies have demonstrated that both direct and indirect contact can spread LSDV. The virus can also be acquired vertically through the intrauterine route in infected cattle (Rouby and Aboulsoud, 2016). It is known to be transmitted from an infected dam to its calf through skin lesions on the udder or via contaminated milk (Tuppurainen et al., 2017). Experimental evidence.
LSD is primarily transmitted through insect vectors that act as natural reservoirs of the virus and infest healthy livestock. The mechanical transmission of LSD occurs via various species of mosquitoes, biting flies, and ticks (Liang et al., 2022). Biting flies like Stomoxy calictrans and Biomyia fasciata are known to be responsible for the vector-borne pathophysiology of LSD. Nonetheless, the potential role of non-biting flies in transmitting LSD has also been elucidated (Melcher et al., 2018). Ticks like Amblyomma hebraeum, Rhipicephalus appendiculatus, and Rhipicephalus decoloratus also serve as reservoirs of the LSDV (Melcher et al., 2018). Additionally, mosquitoes like Culex mirificens and Aedes natrionus are also known to transmit LSD. On the other hand, Anopheles stephensi Liston and Culex quinquefasciatus Say have been identified as potent carriers of LSDV. Still, their role in disease transmission has not been documented (Chihota et al., 2003). Amidst the LSD outbreaks in Russia in 2015, ixodid ticks were speculated to play a critical role in the transmitting LSD. This has been recently confirmed using molecular studies where ixodid ticks recovered from LSD-infected cattle tested positive for viral DNA (El-Ansary et al., 202). Apart from this, surveillance studies in Bulgaria have also indicated the presence of LSDV DNA in other ticks like Hyalomma marginatum and Rhipicephalus bursa (Sprygin et al., 2018).
The Asian water buffalos (Bubalus bubalis) and cattle (Bos Taurus and Bos indicus) are both severely affected by LSD (Azeem et al., 2022). Compared to cattle, buffalos have a significantly lower risk of morbidity associated with LSDV (Tuppurainen et al., 2017). This fact has been hypothesized and correlated based on the thick skin texture of buffaloes, which is difficult to be pricked by the frail mouthparts of blood-sucking vectors such as flies, ticks, and mosquitoes thereby lowering the possibility of viral transmission and susceptibility to LSD (Chihota et al., 2003; Neamat-Allah and Mahmoud, 2019). Since buffaloes tend to evade hot summer seasons by taking refuge in ponds, it has been postulated that this physiological behavior lowers the tendency to be attacked by insect vectors (Jainudeen, 2002). Hence, it becomes challenging for the insect vectors to establish direct contact with the animal skin, consequently lowering the risk of LSD infection. Regardless of age, cattle of both sexes are vulnerable to this virus. The immunological status and physiological well-being of the animal also plays a pivotal role in determining the disease severity (Tuppurainen et al., 2017). Bos indicus shows low vulnerability to clinical illness than Bos taurus (Sudhakar et al., 2020). Furthermore, younger animals show greater vulnerability and severity toward LSD infection than adult cattle (Elhaig et al., 2011). Wild animals are naturally immune to LSD infection, but in experimental settings, oryx (Oryx gazelle), springbok (Antidorcas marsupialis), Thomson's gazelle, giraffe (Giraffe camelopardalis), and impala (Aepyceros melampus), and have all been shown to develop clinical lesions and disease symptoms (Fagbo et al., 2014; Dao et al., 2022). Typically, it has been shown that natural wildlife plays a minor role in the spread and persistence of LSDV.
The exact mechanism behind the mechanical transmission of LSDV remains unclear. It is difficult to claim whether the transmittance is attained by contaminated mouthpart or if other intricate interactions are involved. In severe infections, high viral titers are present in the skin lesions, which serve as a potential source of contamination for arthropod vectors (Babiuk et al., 2008). For biting and blood-feeding insects like mosquitoes, a lower level of viremia has been detected that usually lasts for 12 days or less (Tuppurainen et al., 2005). Interestingly, the caseload of LSD attains a peak during the summer and rainy seasons, which usually coincides with the high prevalence of arthropod-based vectors, especially the blood-feeding insects (Melcher et al., 2018; Kahana-Sutin et al., 2017). This raises further speculations that such insects may be critical to transmitting LSDV. However, outbreaks beyond the vector prevalence period support the existence of an additional yet undiscovered mode of transmission of LSDV. Moreover, a few reports also propose that LSDV transmission is not confined to any specific season (Sprygin et al., 2019). Frequently, migration of domestic or wild cattle has also been correlated to the widespread transmission of LSDV (Sprygin et al., 2019). Hence, other naturally-existing reservoirs of LSDV must be identified, and their vectoring potentials be scrutinized, especially for the insects pertaining to livestock and farm animals. Despite numerous reports indicating vector-borne transmission, LSD outbreaks have been observed to occur, even in the complete absence of insect vectors. This suggests that LSDV may possibly employ other means for viral transmission, in addition to vector-assisted routes.
5. Pathogenesis and Clinical Representations of LSDV: From Signs to Symptoms
The clinical representation of LSDV infections shows a remarkable variation, including short- and long-term subclinical infections, and even death (Badhy et al., 2021). Once the virus has been successfully transmitted to its natural host, the incubation period varies from 7 to 28 days (Das et al., 2021). LSD is characterized by the presence of numerous skin lesions which are well-circumscribed and range between 2 to 7 cm in diameter, appear solid with flat-topped papules and nodules, and multiple coalescing centers (Liang et al., 2022). The virus persists in skin lesions, blood, scabs, oral, nasal, and ocular fluids, semen, and occasionally in animal skin without any noticeable symptoms (Khalafalla, 2022). Following LSDV infection, the virus replicates in the epidermal tissue, resulting in viremia and sudden onset of fever in the animal. LSDV localizes in the cutaneous tissue and then causes the nodules to develop (Namazi et al., 2021). The nodules involve both the dermis as well as epidermis, but sometimes extend to the hypodermis, and rarely to the adjacent striated muscle. LSDV exhibits a wide tissue tropism, but the preferred sites are the skin on the neck, head, limbs, perineum, udder, and genitalia (Beard, 2016; Sevik and Dogan, 2017). During the initial days of viral infection, the nodules appear grayish-white (internally) and may also exude serum. However, following disease progression (~ 14 days), the nodules may develop a cone-shaped central core or sequestrum of necrotic material called the "sit-fast" (Nielsen et al., 2022). As soon as the infected nodules on the mouth, nose, eyes, udder, genitalia, and rectum; begin to ulcerate, LSDV finds passage into all bodily fluids like saliva, nasal and ocular secretions, and even the genital discharge. Consequently, many cattle suffer from significant emaciation and weakness, resulting in the loss of animal productivity for several months, which may further inflict permanent damage to the hides (Khalafalla, 2022).
According to recent studies, most tissues and organs of the infected animals exhibit pathological alterations such as mastitis, orchitis, necrotic hepatitis, lymphadenitis, and disseminated vasculitis (Khalafalla, 2022). Tracheitis, cardiac damage, and other pathological alterations are also seen in a few cattle. These pathological abnormalities might induce varying degrees of injury to the animal, making LSDV infection more detrimental (Ali et al., 2021). A clinical study on LSDV surfaced previously, indicating the oxidation-anti-oxidation state imbalance in infected cattle, thereby invoking a significant rise in the levels of pro-inflammatory cytokines, extending negative consequences on animal health (Kamr et al., 2022). This was positively correlated with histopathological outcomes in infected animals, which showed signs of profuse necrosis, mononuclear cell infiltration, intracytoplasmic inclusion bodies, and severe vasculitis. The dysregulation of organ functions is known to be triggered by the metabolite buildup in the heart, liver, and kidney, causing hypophosphatemia, which exacerbates the symptoms of hemolytic anemia. Experimental findings from hematological and biochemical studies in LSD-infected animals have also revealed that infected animals suffer from pancytopenia, hyperproteinemia, hyperkalemia, hyperchloremia, and decreased creatinine content (Abutarbush et al., 2015; Liang et al., 2022). Hence, these indicators/markers may be used as an index for assessing the disease prognosis, severity, and timely management or control of LSD. It has been speculated that young cows, lactating mothers, and underweight livestock are more vulnerable to infection by LSDV, possibly due to poor or impaired immunity (Babiuk et al., 2008; Gaber et al., 2022). Interestingly, disease-recovered animals have been shown to harbor lifelong immunity against the virus (Namazi et al., 2021). Calves from the infected mothers exhibit resistance towards LSDV for nearly six months because of the acquired maternal antibodies (Tuppurainen et al., 2005). Nonetheless, animals that withstand the wrath of LSD infection show complete clearance of the viral load and do not act as carriers for LSDV (Tuppurainen et al., 2017).
6. Diagnosis, Preventive Measures, and Treatment of LSD
LSDV infection is diagnosed based on classical clinical symptoms, such as lymphadenopathy and typical nodular skin lesions, in conjunction with confirming the presence of the virus or viral antigen in immunodiagnostic tests. Conventional PCR (Zheng et al., 2007) and real-time PCR (Babiuk et al., 2008) are molecular techniques that are often employed to validate LSDV infections. Real-time PCR is employed to diagnose and clinically differentiate LSDV from other animal-associated poxviruses like GTPV and SPPV (Lamien et al., 2011). Furthermore, restriction fragment length polymorphism (RFLP) is another technique that is being exploited to distinguish vaccine strains from virulent LSDV (Menasherow et al., 2014). Besides RFLP, LSDV is also identified using electron microscopy, virus isolation, and virus neutralization tests (VNT) (Namazi et al., 2021). Virus neutralization is known to be the gold-standard for detecting antibodies raised against Capripoxviruses. Nonetheless, the disease can also be diagnosed by serological tests, including VNT, indirect fluorescent antibody test (IFAT), serum neutralization test (SNT), and indirect immunofluorescence test (Molla et al., 2017). However, ELISA is more sensitive and selective than IFTA or VNT (Aleksandr et al., 2020). Another approach for LSD diagnosis is the immuno-peroxidase monolayer assay (IPMA), a relatively cheap and convenient technique. It has greater sensitivity and specificity than VNT and commercially available ELISA kits (Bedekovic et al., 2018). Moreover, owing to higher costs and tedious operations, western blot, an extremely sensitive and specific technique, is seldom used to detect LSDV (Namazi et al., 2021).
In recent years, the spread and recurrent outbreaks of the Capripoxviruses point towards major issues like inconsistencies and inefficiencies in vaccination programs, poor economic conditions, and unawareness among farmers in endemic and non-endemic areas, legal as well as illegal trade of livestock, and global climatic changes. To date, the line of action taken to cure LSD is solely symptomatic which mainly targets on prevention against secondary microbial infections. This includes various combinations of anti-inflammatory, antimicrobials, supportive therapy, and anti-septics (Namazi et al., 2021). Currently, no effective antiviral drugs are available to treat LSD. Nonetheless, FDA-approved drugs and phytocompounds that are effective against other poxviruses may be repurposed against the LSDV (Gulati et al., 2023). The disease can only be controlled in endemic areas via mass vaccinations, imposing movement restrictions (quarantine), and removing suspected or infected animals (Sevik and Dogan, 2017). Culling of infected/suspected animals, transportation/movement restrictions, and mandatory and uniform immunization have all been suggested as control measures to minimize the possible transboundary spread of this disease (Beard, 2016; Tuppurainen et al., 2017). Moreover, due to the cardinal role of arthropod vectors in transmitting LSD, their eradication becomes more challenging. In addition, the delayed disposal of diseased animals or carcasses makes the situation even worse (Tuppurainen et al., 2017). However, to stop the spread of disease through vectors, certain control measures, such as the use of insecticides, pesticides, and vector traps, has been recommended in regions with a high vector population (Gupta et al., 2020). Besides, there are risk factors associated with such control activities. Further, creating awareness among veterinarians and farm/livestock workers regarding the disease will also enable rapid diagnosis of clinical cases, permitting timely management of the disease, thereby breaking the transmission chain and preventing cluster outbreaks (Beard, 2016).
The widespread administration of appropriate vaccines is quintessential for preventing and eradicating the virus. Currently, live-attenuated vaccines based on the LSDV, SPPV or GTPV strains make up most of the commercially available LSD vaccines. The presently-administered LSDV vaccines and efficacies have been summarized in
Table 1. Live-attenuated LSD vaccine are usually formulated by the conventional South African Neethling strain or the Kenyan sheep and goat pox strains, KSGP O-180 and O-240, respectively (Tuppurainen
et al., 2021). For preparing attenuated vaccines, the Neethling strain (virulent) has been subjected to serial passaging (61 times) in lamb kidney cells (LK), followed by 20 passages in the chorioallantoic membrane of embryonated chicken eggs, and subsequently back in LK cells (3 times) (Kitching, 2003). Another virulent strain, i.e., the Madagascan LSDV strain, requires 101 passages in rabbit kidney cells, followed by five passages in fetal calf kidney cells for its potential use as a vaccine (Kitching, 2003). After immunization with homologous booster LSD vaccines, animals may experience adverse effects such as allergic reactions at vaccination site or typical skin nodules accompanied by reduction in lactation (Tuppurainen
et al., 2021). This reaction is often called the "Neethling response/disease."
In 2021, the homologous live-attenuated LSD vaccines, including Herbivac LS, Lumpy Skin Disease Vaccine, Kenyavac (South Africa), and Lumpyvax (South Africa), and Vaccin LSD Neethling O vivant (Morocco) were clinically tested by researchers (Haegeman et al., 2021). Interestingly, none of the aforementioned vaccines adversely affected general health or animal behavior in any of the experimental groups, including feed intake, albeit these were known to induce fever in some animals (Haegeman et al., 2021). Nevertheless, in animals immunized with Herbivac LS vaccine, swollen lymph nodes were detected, while the other three South African vaccines showed clinical manifestations of Neethling disease upon vaccination. Small nodules, not as large as those reported in sick animals, appeared in the Moroccan Neethling vaccine group (Haegeman et al., 2021). Since LSDV also shares more than 97% of its nucleic acid sequences with that of GTPV and SPPV, immunization with live-attenuated goat or sheep pox vaccines also confers cross-immunity in susceptible animals against the LSDV. This has been typically employed in clinical settings to circumvent LSD.
In the recent past, various immunization studies have reported that the wild-type ‘Neethling’ strain can be chemically inactivated using ethylenimine and coupled with various adjuvants like the Montanide adjuvant (Hamdi et al., 2020) and a low molecular weight copolymer (Polygen, MVP Adjuvants®, named as Adjuvant A) (Wolff et al., 2021), to provide adequate protection against LSDV. Hamdi et al. tested the efficacies of both live-attenuated and inactivated vaccines prepared using LSDV Neethling strain. Both vaccines successfully elicited a protective immune response in cattle against the virulent LSDV Israeli field isolate (Hamdi et al., 2020). Interestingly, in this particular scenario, the inactivated vaccines induced a higher antibody response (87%) in comparison with the live-attenuated jabs (50%), at 28 days post-vaccination (Hamdi et al., 2020). Another bivalent inactivated vaccine conjugated with oil adjuvants against the LSDV and bluetongue virus was reported recently, which could stimulate the production of neutralizing antibodies at high titers (Es-sadeqy et al., 2021). Moreover, recombinant LSD vaccines, namely, LSDV-WB005KO and LSDV-WB008KO, have also been developed using a homologous recombination technique by deleting the LSDV open reading frames 005 and 008 (Liang et al., 2022). Further, clinical investigations have discovered that combining these two vaccines can significantly enhance the titers of neutralizing antibodies in immunized cattle, which can eventually fend off any infection or invasion by LSDV (Kara et al., 2018).
Table 1.
Commercially available live-attenuated LSDV vaccines and information about their formulations and protective efficacies.
Table 1.
Commercially available live-attenuated LSDV vaccines and information about their formulations and protective efficacies.
7. Current Scenario & Economic Repercussions of LSD Outbreaks
Over the past few years, the world has witnessed an unprecedented wave of LSD outbreaks across diverse geographical boundaries, pioneering from Africa and spreading beyond the Middle East to southeast Europe and the Asian subcontinent (Tuppurainen and Oura, 2012). Several factors have influenced the transboundary spread of LSD to non-endemic countries. These constitute both legal/illegal transportation and trade of livestock, cross-border passage of insect vectors/reservoirs, deceleration of vaccination drives, and reduced global surveillance (Liang
et al., 2022). In recent times, animal disease databases like EMPRES-i (FAO) and WAHIS (WOAH) have allowed real-time monitoring of the global disease situation, which helps in collating data for continuous risk assessments and associated trade recommendations for animals and related products (Azeem
et al., 2022; Liang
et al., 2022). The LSDV has been spreading like wildfire across the Asian subcontinent, inflicting high mortality and incurring huge economic losses in some countries. Statistics from the past two years suggest a total of 3,562 outbreaks worldwide, with most cases reported in Asian countries like India, China, Nepal, Vietnam, Thailand, and Sri Lanka (Kumar and Tripathi, 2022). Countries that share territorial borders with regions having active LSDV infections are at a significantly higher risk of incursion by the virus. With the first outbreaks of this disease in non-endemic countries like China, India, Pakistan, Afghanistan, and Bangladesh, LSDV has been expanding its geographical range at a global level (Liang
et al., 2022). In 2022 alone, India witnessed around 3 million LSD infections of cattle, with a mortality rate of nearly 6%, resulting in 155,000 deaths (Kumar and Tripathi, 2022).
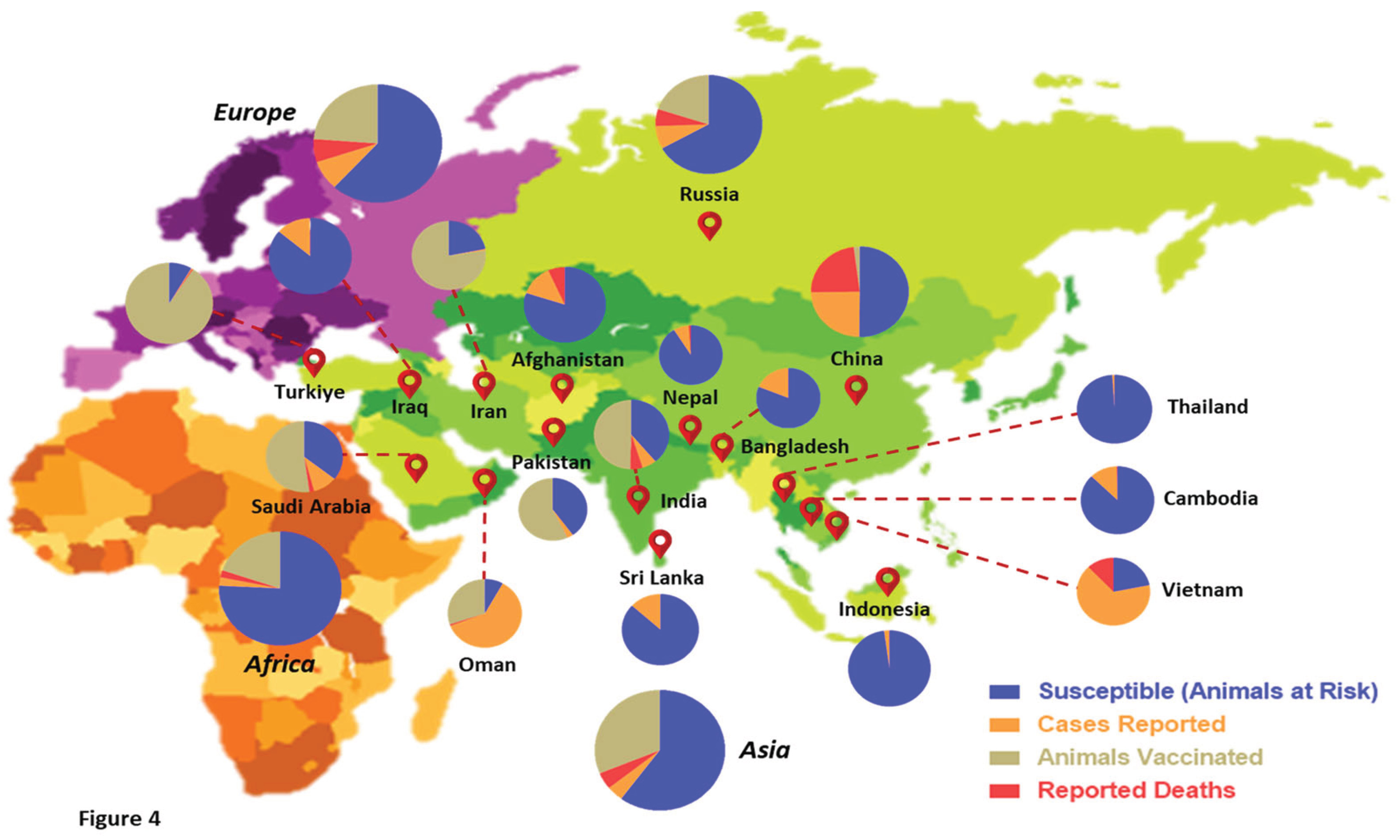
Figure 4 represents a comprehensive geographical overview of LSD outbreaks reported in the past five years at the global level. The statistics have been quantified based on the categorization made by WOAH with respect to LSDV infections (Cases reported, deaths, susceptible, and vaccinated animals). Considering these grim statistics in the wake of the current multi-country LSD outbreak, this viral disease has raised serious concerns for the livestock industry. Although a few authorities actively undertake global surveillance of LSD, their consistent efforts towards disease tracking have not been successful enough to curb the transboundary spread of this virus. Hence, it is the need of the hour that animal healthcare agencies, state governments, academia, and relevant stakeholders collaborate to establish synchrony for ensuring active surveillance programs for the timely identification of LSD outbreaks/clusters.
To curb the spread of LSD, the Food and Agricultural Organization of the United Nations (FAO) has drafted necessary guidelines and protocols, laid down templates for contingency plans, and awareness measures (FAO, 2017). To provide the best protection, FAO recommends annual vaccination of livestock and dairy animals in LSD-affected nations and coordinated vaccination drives across countries. Newly-born calves from uninfected mothers must be immunized at any early age, while calves from naturally-infected or immunized dams should be vaccinated between three to six months post-partum. Regionally-harmonized vaccinations have also been proposed before massive herd movements, for instance, before the commencement of seasonal grazing (FAO, 2017). According to the FAO guidelines, the principal foundation for LSD surveillance programs should be passive disease reporting and, secondarily, risk-based surveillance based on detecting clinical signs in both wild and domestic farm animals. Along with vector surveillance, emphasis must be placed on monitoring susceptible hosts, wildlife animals, and even small ruminants. Serological surveillance can also be used for retrospective analyses in affected areas or to predict possible LSD outbreaks (FAO, 2017). However, insights into the recent genomic studies highlight the need for large-scale genome surveillance and close monitoring to track the LSDV for building better algorithms, disease prediction models, logistics, and diagnostics in the coming future. For example, genome sequences of six viral isolates retrieved from infected animals during the recent LSDV outbreak in India have indicated the presence of several genomic mutations, strongly suggesting that the presently-circulating LSDV strains have evolved from a distinct lineage, giving rise to genetic variants of this animal virus (Bhatt et al., 2022). Hence, immediate attention must be given to the global surveillance of LSD, which may add new dimensions to understanding the viral disease better and controlling future outbreaks.
Since LSDV primarily targets livestock animals like cattle and buffaloes, any outbreak in countries that heavily depend on the productivity of dairy industries takes a huge financial hit. With more than 650 million head of cattle and buffaloes, Asia is a major contributor towards the global livestock industry, accounting for a mammoth share of 39% (Pineda et al., 2021). Most of these animals are concentrated in South and Southeast Asia, with India being on the top with a whopping 300 million head, followed by China and Pakistan with approximately 90 and 85 million, respectively (Xavier-Roche et al., 2020). India is also a lead exporter in the beef market, with nearly 527 tons of carcass weight equivalent exported in 2018 alone (Xavier-Roche et al., 2020). With the death of nearly 155,000 cows in India during the 2022 LSD outbreak, it has been estimated that the country faced a direct economic loss of nearly 3 billion Indian Rupees (Kumar and Tripathi, 2022). Therefore, the transboundary spread of LSD is bound to substantially impact the economies of agro- and dairy-based Asian countries. The recurrent outbreaks of LSDV directly affect the dairy, meat, and tannery industries because of decreased meat and milk production, damaged cattle skins, fertility problems, abortions, and, ultimately, the death of severely affected animals (Kiplagat et al., 2020). Restrictions on intra- and inter-country trade and movement of cattle also incur indirect losses. The high costs of disease diagnosis, management, treatment, and vaccination also add to the economic burden (Casal et al., 2018). Considering the strategic positions held by China and India in the global meat and dairy markets, any significant impact on their livestock industries will certainly be felt across the global markets. Despite the gruesome figures coming out of India, the United States Department of Agriculture (USDA) suggests that the disease outbreak in India had a marginal impact on the gross milk production and net revenues (The Hindu, 2022). This points towards Western nations' ignorance and double standards in undermining infectious diseases like LSD that are not prevalent or endemic within their territorial boundaries. The present LSD outbreak must be viewed very critically by the global economies as a wake-up call to adopt integrated approaches toward the surveillance and management of LSD. In addition, the unprecedented spread of LSDV has a direct implication towards escalating antimicrobial and anthropogenic resistance in the environment due to the extensive use of broad-spectrum antibiotics in livestock for treating secondary bacterial infections and large-scale application of insecticides to kill insect vectors (Tuppurainen et al., 2021).
8. Causes of LSDV Resurgence: from Ground Reality to Speculations, and Beyond
Several reasons can be speculated for the unprecedented outbreaks of LSDV in recent years. The scenario draws an analogy with many other re-emerging viruses like the MPXV, SARS-CoV-2, Nipah, and influenza virus (Chadha
et al., 2022a). Of lately, the recent outbreaks of LSDV have majorly affected the developing countries of the Asian subcontinent and the Middle East. In most South Asian territories, the disease erupted unexpectedly and began to spread rapidly during monsoons. High rainfall creates ambient environments for the propagation and multiplication of insect vectors like mosquitoes, flies, and ticks, which directly increases the risk of disease transmission (Campbell-Lendrum
et al., 2015). Parallels can be drawn with chikungunya and dengue, which are vector-borne viral diseases and are also known to peak in Asian countries during rainy seasons when mosquito populations are at their maximum (Tuladhar
et al., 2019). Notably, the sudden rise in LSDV infections coincided with weather conditions that resonate with high amounts of precipitation. The increased rainfall and humidity make it difficult to control insect vectors and treat the infected animals as open skin wounds (lesions) take a longer time to heal. This increases the susceptibility of animals to secondary bacterial infections. Interestingly, climate change is also conjectured to play a pivotal role in expanding the geographical niche of this disease (
Figure 5). Reports have shed light on this matter, stating that the LSDV may be transported internationally across territorial borders due to altered wind direction and velocity (Rouby and Aboulsoud, 2016). Therefore, the climate is believed to be critical in shaping LSD outbreaks.
Since LSDV is a DNA virus, its genome was believed to be stable for many years. Field isolates of LSDV recovered over decades in Africa exhibited minimal genetic alterations from the parental strain, which was first identified in Zambia in 1929 (Tuppurainen
et al., 2011). Moreover, LSDV strains retrieved from the successive outbreaks that occurred in the Middle East and Europe, post-2012 and -2015, respectively, did not show any signs of divergence or mutations in their DNA genomes (Tuppurainen
et al., 2011; Alkhamis
et al., 2016; Agianniotaki
et al., 2017). As a result, the genomic stability of the virus was exploited to develop live-attenuated vaccines against LSDV, where the virus could be easily differentiated from the contemporary field isolates (Menasherow
et al., 2014; Gelaye
et al., 2015). But surprisingly, LSDV strains recovered from infected animals in Russia between 2017 and 2019 exhibited vaccine-like characteristics, prompting an immediate shift towards this dynamics (Kononov
et al., 2019; Uddin
et al., 2020). Some of these variants contained a 12-nucleotide insertion in the GPCR gene, similar to the vaccine strains, while others displayed a 27-nucleotide deletion in the ORF LSDV 126, like the LSDV Neethling vaccine strain. Recombination between the field virus strains and the Neethling vaccine strain was believed to be the primary cause for the emergence of these LSDV variants (Uddin
et al., 2020). On similar lines, the virus strains recovered from the LSD outbreaks in China also demonstrated GPCR profiles similar to that of LSDV vaccines with the 27-nucleotide deletion (Melcher
et al., 2018; Uddin
et al., 2020). Moreover, recent studies have demonstrated that these recombinant LSDV strains can induce more severe disease than their parental field strains (Kononova
et al., 2020). Other than contributing towards increased virulence, these genomic mutations may also play a critical role in altering the virus’ mode of transmission (
Figure 5). These new variants can possibly lead to a direct, cattle-to-cattle transfer of viral infection through semen or other body fluids like saliva and nasal discharge. Similar concerns have been raised in the case of MPXV, which was previously known to be transmitted only through direct contact and aerosols. But recent studies hint towards a possible role of sexual transmission since most of the monkeypox infections were reported in homosexual men (Chadha
et al., 2022a). Hence, the recently identified mutations in the LSDV genome may be attributed to the ongoing multi-country outbreak of LSD.
In most developing South-Asian countries, domestic livestock are allowed to roam outdoors freely and consume wild vegetation. From a broader perspective, this communal grazing system can also be deemed responsible for the escalating LSDV infections. The impact of open grazing is two-fold for disease epidemiology. The nutritional composition of wild vegetation in the meadows is not defined as compared to regular farm fodder, which upon consumption, may lead to the deficiency of essential nutrients in the livestock (Zeballos and Chelius, 2021). This scarcity of nutrients can impair the immune system of farm animals, making them more susceptible to diseases (Kegley
et al., 2016), such as LSD. Secondly, infected animals grazing in open fields are more likely to spread infection
via insect vectors (
Figure 5). Such animals can transmit the disease to arthropod vectors, which can infest healthy livestock, making them more vulnerable to viral infection. This can ultimately result in an unprecedented outbreak at livestock farms, making disease containment even more challenging.
LSDV is not a novel virus; the disease is almost a century old. Despite the availability of LSD vaccines for several decades, the virus is currently spreading like wildfire across countries. It must be noted that vaccines formulated from the erstwhile LSDV, SPPV or GTPV strains are still being used (Morgenstern and Klement, 2020). Hence, the lack of vaccine upgradation is speculated to be another possible reason behind the resurgence of LSD. These vaccines have not been upgraded according to the currently-circulating LSDV strains and, therefore, are now failing to protect animals worldwide (
Figure 5). This implies serious doubts over vaccine efficacy. Similar issues have been brought up with the COVID-19 vaccines, as the current vaccine formulations are based on the SARS-CoV-2 strain that first emerged in the Wuhan province of China more than 3 years ago (Nohynek and Wilder-Smith, 2022). Since then, more than a hundred variants of SARS-CoV-2 with distinct PANGO lineages have emerged, including the alpha, beta, gamma, delta, and presently-spreading omicron variants (Young
et al., 2022). These variants are known to evade the vaccine-induced neutralizing antibodies and even the host immune system (Chadha
et al., 2022b). The limited protection against the new sub-variants is believed to be a key factor in recurrent cluster outbreaks causing multiple unprecedented infection waves across international borders (Chadha
et al., 2022b). Hence, there is a pressing need to modernize and re-formulate LSD vaccines with the presently existing or circulating virus strains. In this direction, Indian scientists have successfully isolated and characterized a recently circulating LSDV strain, termed as LSDV/India/2019, which is closely related to the Kenyan LSDV strains (Kumar
et al., 2021). Further attempts have been made to formulate a novel homologous live-attenuated vaccine against LSDV, Lumpi-ProVac
Ind, which has been shown to confer complete protection against LSD by eliciting humoral and cell-mediated immune .responses (Kumar
et al., 2022; Kumar and Tripathi, 2022). Also, field trails revealed that the seroconversion rate with Lumpi-ProVac
Ind was nearly 85% (30 days post-vaccination) without inducing any local or systemic reactions in vaccinated animals (Kumar
et al., 2022). Interestingly, sera samples obtained from Lumpi-ProVac
Ind-immunized animals was also able to effectively neutralize the recent LSDV strain (LSDV/India/2022), thereby proving its prophylactic efficacy against the disease (Kumar and Tripathi, 2022). Also, most existing research and field trials on LSD have been conducted with mildly virulent strains of LSDV (Liang
et al., 2022). Therefore, the efficacy of existing vaccines will actually unfold only when largescale field trials are conducted at different geographical locations across the world. In addition, the recombination events between live-attenuated (vaccine) and field strains of LSD have recently been shown to result in the emergence of new variants (Uddin
et al., 2020). Hence, the presently used and commercially available live-attenuated LSD vaccines incur serious risks of generating new LSDV variants. Such vaccines also put livestock animals at risk of vaccine-derived LSD (
Figure 5), a scenario that has been previously reported with the live-attenuated oral polio vaccine (OPV). The OPV is infamously associated with the incidence of vaccine-derived polio, wherein the OPV strains undergo partial reversion to become virulent again (Lai
et al., 2022). Consequently, the developed nations have ceased administering OPV and replaced it with the inactivated polio vaccine (IPV) (Lai
et al., 2022). Similar instances may be possible with LSDV, where partial reversion to a virulent type may be responsible for inflicting vaccine-derived LSD. Nonetheless, such possibilities warrant strong scientific upholding and further scrutiny to determine the fate of existing LSDV vaccines.
WOAH has categorized LSDV as a notifiable transboundary disease (Anwar
et al., 2022). The movement of the arthropod vectors or other reservoirs over long distances due to high wind currents or natural migration may also be attributed to virus transmission across borders. Parallel to the meat and dairy industries, illegal wildlife trade and unlawful selling of livestock across territories is another potential reason behind the transboundary spread of LSDV (Rush
et al., 2021). When introduced into the unaffected areas through illegal activities, the infected animals from endemic regions can also transmit the virus to healthy livestock, eventually causing a widespread outbreak of the disease (
Figure 5). Importantly, the ongoing COVID-19 pandemic also played a critical role in shaping today’s scenario concerning the LSD outbreak. The deadly SARS-CoV-2 occupied the center stage and drew the attention of researchers/clinicians globally, sidelining other important human diseases, including cancer(s), leave alone animal diseases (Boniface and Tapia-Rico, 2022). The unprecedented COVID-19 pandemic compelled the global economies to enforce nationwide lockdowns and restrict human movement, which exacerbated the existing challenges for veterinary personnel and scientific laboratories working on disease diagnosis and prediction of possible outbreaks. This ultimately impeded disease monitoring, surveillance, and the subsequent enactment of containment strategies (
Figure 5). In summary, the present-day multi-country outbreak of LSD has multidimensional aspects that have been illustrated in
Figure 5. Considering the speculations raised in this review, it can be believed that the current scenario was already in the making until the LSDV infections exploded beyond comprehension. Hence, necessary control measures are needed to end the wrath of LSDV. Ensuring coordinated attempts from the international regulatory bodies, governmental authorities, and veterinary scientists is critical to managing disease treatment, prevention, and relevant control measures against LSDV. Devising alternate intervention strategies, creating awareness among livestock farm owners, promoting regular vaccination campaigns for susceptible animals, and implementing strict laws to prevent wildlife trafficking across borders are a few necessary steps that can be taken to curb recurrent LSD outbreaks. Apart from this, regular screening of farm animals and maintaining high-level surveillance programs lay a strong foundation for preventing cluster outbreaks of LSD.
9. Conclusion
Livestock animals are a critical pillar of the dairy industries that contribute greatly to the world economy. The global livestock sector has been severely affected by recurrent LSD outbreaks, resulting in the large-scale death of cows and buffaloes. Apart from being fatal, the virus reduces overall productivity in livestock animals which incurs tremendous revenue losses to the agro-dependent nations. LSDV was previously thought to be endemic in Africa, but the recent trends and unprecedented resurgence indicate the virus’ expanding geographical foothold in non-endemic countries. This becomes critically important since LSD is an economically important transboundary disease that lacks proper global surveillance and data acquisition, making the existing statistics deficient, fragmented, and unreliable. The present situation has worsened with the identification of recombinant virus strains and several mutations in the LSDV genome. With the ongoing COVID-19 pandemic and multi-country monkeypox outbreak, the gravity of the present situation has increased significantly, demanding immediate attention towards stringent global surveillance and healthcare systems. There is a dire need to upgrade the vaccine formulations with the presently-circulating LSDV strains and devise alternative intervention strategies to combat LSD outbreaks. Extensive research from academia, strong inter-organization engagement, and collaborations between the relevant stakeholders are paramount to controlling and managing the recurrent outbreaks of LSD. It is high time that all nations join hands to work collaboratively on a common platform to ensure that such viral outbreaks are not transformed into widespread epidemics or pandemics.
Funding
The authors did not receive support from any organization for the submitted work.
Acknowledgments
SC would like to thank the Indian Council of Medical Research (ICMR) for their financial support. Financial assistance from ICMR, New Delhi for providing fellowship (SRF) to JC and LK is also appreciated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
COVID-19: Coronavirus disease 2019, EV: Enveloped virion, FAO: Food and agricultural organization, GTPV: Goat poxvirus, IMPA: Indirect fluorescent antibody test, IPV: Inactivated polio vaccine, LSD: Lumpy skin disease, LSDV: Lumpy skin disease virus, MPXV: Monkeypox virus, MV: Mature virion, OPV: Oral polio vaccine, RFLP: Restriction fragment length polymorphism, SARS-CoV-2: Severe acute respiratory syndrome virus 2, SNT: Serum neutralization test, SPPV: Sheep poxvirus, VNT: Virus neutralization test, WHOA: World organization for animal health.
References
- Abutarbush, S.M.; Ababneh, M.M.; Al Zoubi, I.G.; Al Sheyab, O.M.; Al Zoubi, M.G.; Alekish, M.O.; Al Gharabat, R.J. Lumpy Skin Disease in Jordan: Disease Emergence, Clinical Signs, Complications and Preliminary-associated Economic Losses. Transbound. Emerg. Dis. 2013, 62, 549–554. [CrossRef]
- Agianniotaki, E.I.; Mathijs, E.; Vandenbussche, F.; Tasioudi, K.E.; Haegeman, A.; Iliadou, P.; Chaintoutis, S.C.; Dovas, C.I.; Van Borm, S.; Chondrokouki, E.D.; et al. Complete Genome Sequence of the Lumpy Skin Disease Virus Isolated from the First Reported Case in Greece in 2015. Genome Announc. 2017, 5, e00550-17. [CrossRef]
- Aleksandr, K.; Olga, B.; David, W.B.; Pavel, P.; Yana, P.; Svetlana, K.; Alexander, N.; Vladimir, R.; Dmitriy, L.; Alexander, S. Non-vector-borne transmission of lumpy skin disease virus. Sci. Rep. 2020, 10, 1–12. [CrossRef]
- Ali AA, Esmat M, Attia H, et al. (1990) Clinical and pathological studies on lumpy skin disease in Egypt. Vet Rec. 127(22), 549-50.
- Ali AA, Neamat-Allah ANF, Sheire HA, et al. (2021) Prevalence, intensity, and impacts of noncutaneous lesions of lumpy skin disease among some infected cattle flocks in Nile Delta governorates, Egypt. Comparative Clinical Pathology. 30(4), 693-700. [CrossRef]
- Alkhamis MA, VanderWaal K. (2016) Spatial and Temporal Epidemiology of Lumpy Skin Disease in the Middle East, 2012–2015. Frontiers in Veterinary Science. 3, 19. [CrossRef]
- Annandale, C.H.; Holm, D.E.; Ebersohn, K.; Venter, E.H. Seminal Transmission of Lumpy Skin Disease Virus in Heifers. Transbound. Emerg. Dis. 2014, 61, 443–448. [CrossRef]
- Anwar A, Na-Lampang K, Preyavichyapugdee N, et al. (2022) Lumpy Skin Disease Outbreaks in Africa, Europe, and Asia (2005–2022): Multiple Change Point Analysis and Time Series Forecast. Viruses. 14(10), 2203. [CrossRef]
- Ayelet, G.; Haftu, R.; Jemberie, S.; Belay, A.; Gelaye, E.; Sibhat, B.; Skjerve, E.; Asmare, K. Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of the virus. Rev. Sci. et Tech. de l'OIE 2014, 33, 877–887. [CrossRef]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Manning, L.; Neufeld, J.; Embury-Hyatt, C.; Copps, J.; Boyle, D.B. Quantification of Lumpy Skin Disease Virus Following Experimental Infection in Cattle. Transbound. Emerg. Dis. 2008, 55, 299–307. [CrossRef]
- Badhy, S.C.; Chowdhury, M.G.A.; Settypalli, T.B.K.; Cattoli, G.; Lamien, C.E.; Fakir, M.A.U.; Akter, S.; Osmani, M.G.; Talukdar, F.; Begum, N.; et al. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet. Res. 2021, 17, 61. [CrossRef]
- Beard, P.M. Lumpy skin disease: a direct threat to Europe. Veter- Rec. 2016, 178, 557–558. [CrossRef]
- Bedeković T, Šimić I, Krešić N, et al. (2018) Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transboundary and Emerging Diseases. 65(2), 491-496. [CrossRef]
- Bhanuprakash, V.; Hosamani, M.; Singh, R. Prospects of control and eradication of capripox from the Indian subcontinent: A perspective. Antivir. Res. 2011, 91, 225–232. [CrossRef]
- Bhatt, L.; Bhoyar, R.C.; Jolly, B.; Israni, R.; Vignesh, H.; Scaria, V.; Sivasubbu, S. The genome sequence of lumpy skin disease virus from an outbreak in India suggests a distinct lineage of the virus. Arch. Virol. 2023, 168, 1–5. [CrossRef]
- Boniface, D.; Tapia-Rico, G. Oncology During the COVID-19 Pandemic: a Lockdown Perspective. Curr. Oncol. Rep. 2022, 24, 1219–1235. [CrossRef]
- Byadovskaya, O.; Prutnikov, P.; Shalina, K.; Babiuk, S.; Perevozchikova, N.; Korennoy, F.; Chvala, I.; Kononov, A.; Sprygin, A. The changing epidemiology of lumpy skin disease in Russia since the first introduction from 2015 to 2020. Transbound. Emerg. Dis. 2022, 69, E2551–E2562. [CrossRef]
- Campbell-Lendrum D, Manga L, Bagayoko M, et al. (2015) Climate change and vector-borne diseases: what are the implications for public health research and policy? Philosophical Transactions of the Royal Society B: Biological Sciences. 370(1665), 20130552. [CrossRef]
- Casal J, Allepuz A, Miteva A, et al. (2018) Economic cost of lumpy skin disease outbreaks in three Balkan countries: Albania, Bulgaria and the Former Yugoslav Republic of Macedonia (2016-2017). Transboundary and Emerging Diseases. 65(6), 1680-1688. [CrossRef]
- Chadha J, Khullar L, Gulati P, et al. (2022a) Insights into the monkeypox virus: Making of another pandemic within the pandemic? Environmental Microbiology. 24(10), 4547-4560. [CrossRef]
- Chadha J, Khullar L Mittal N. (2022b) Facing the wrath of enigmatic mutations: a review on the emergence of severe acute respiratory syndrome coronavirus 2 variants amid coronavirus disease-19 pandemic. Environmental Microbiology. 24(6), 2615-2629. [CrossRef]
- Chihota, C.M.; Rennie, L.F.; Kitching, R.P.; Mellor, P.S. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med Veter- Èntomol. 2003, 17, 294–300. [CrossRef]
- Dao, T.D.; Tran, L.H.; Nguyen, H.D.; Hoang, T.T.; Nguyen, G.H.; Tran, K.V.D.; Nguyen, H.X.; Van Dong, H.; Bui, A.N.; Bui, V.N. Characterization of Lumpy skin disease virus isolated from a giraffe in Vietnam. Transbound. Emerg. Dis. 2022, 69, E3268–E3272. [CrossRef]
- Das, M.; Chowdhury; Akter, S.; Mondal, A.; Uddin; Rahman An updated review on lumpy skin disease: a perspective of Southeast Asian countries. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 322. [CrossRef]
- Diallo A, Viljoen GJ. (2007) Genus Capripoxvirus. In: Mercer, A.A., Schmidt, A., Weber, O. (eds) Poxviruses. Birkhäuser Advances in Infectious Diseases. Birkhäuser Basel. 167-181.
- EFSA (2015) Scientific Opinion on lumpy skin disease: EFSA Panel on Animal Health and Welfare (AHAW) EFSA Journal. 13(1), 3986.
- El-Ansary RE, El-Dabae WH, Bream AS, et al. (2022) Isolation and molecular characterization of lumpy skin disease virus from hard ticks, Rhipicephalus (Boophilus) annulatus in Egypt. BMC Veterinary Research. 18(1), 1-10. [CrossRef]
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy skin disease in cattle: Frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort J. Veter- Res. 2017, 84, 6 pages–e6. [CrossRef]
- Es-Sadeqy, Y.; Bamouh, Z.; Ennahli, A.; Safini, N.; El Mejdoub, S.; Tadlaoui, K.O.; Gavrilov, B.; El Harrak, M. Development of an inactivated combined vaccine for protection of cattle against lumpy skin disease and bluetongue viruses. Veter- Microbiol. 2021, 256, 109046. [CrossRef]
- Fagbo S, Coetzer JAW, Venter EH. (2014) Seroprevalence of Rift Valley fever and lumpy skin disease in African buffalo Syncerus caffer in the Kruger National Park and Hluhluwe iMfolozi Park, South Africa. Journal of the South African Veterinary Association. 85(1), e1-e7.
- FAO (2017) LUMPY SKIN DISEASE A field manual for veterinarians. In Food and Agriculture Organization of the United Nations (FAO). URL https://www.fao.org/3/i7330e/i7330e.pdf [Accessed: 31st Jan, 2023].
- Gaber, A.; Rouby, S.; Elsaied, A.; El-Sherif, A. Assessment of heterologous lumpy skin disease vaccine-induced immunity in pregnant cattle vaccinated at different times of gestation period and their influence on maternally derived antibodies. Veter- Immunol. Immunopathol. 2022, 244, 110380. [CrossRef]
- Gelaye E, Belay A, Ayelet G, et al. (2015) Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antiviral Research. 119, 28-35. [CrossRef]
- Gulati, P.; Chadha, J.; Harjai, K.; Singh, S. Targeting envelope proteins of poxviruses to repurpose phytochemicals against monkeypox: An in silico investigation. Front. Microbiol. 2023, 13, 1073419. [CrossRef]
- Gupta, T.; Patial, V.; Bali, D.; Angaria, S.; Sharma, M.; Chahota, R. A review: Lumpy skin disease and its emergence in India. Veter- Res. Commun. 2020, 44, 111–118. [CrossRef]
- Haegeman, A.; De Leeuw, I.; Mostin, L.; Van Campe, W.; Aerts, L.; Venter, E.; Tuppurainen, E.; Saegerman, C.; De Clercq, K. Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines. Vaccines 2021, 9, 473. [CrossRef]
- Hamdi, J.; Boumart, Z.; Daouam, S.; El Arkam, A.; Bamouh, Z.; Jazouli, M.; Tadlaoui, K.O.; Fihri, O.F.; Gavrilov, B.; El Harrak, M. Development and Evaluation of an Inactivated Lumpy Skin Disease Vaccine for Cattle. Veter- Microbiol. 2020, 245, 108689. [CrossRef]
- Jainudeen MR. (2002) Buffalo husbandry. Asia. Encyclopedia of Dairy Sciences. 186-193. [CrossRef]
- Kahana-Sutin, E.; Klement, E.; Lensky, I.; Gottlieb, Y. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Med Veter- Èntomol. 2016, 31, 150–160. [CrossRef]
- Kamr, A.; Hassan, H.; Toribio, R.; Anis, A.; Nayel, M.; Arbaga, A. Oxidative stress, biochemical, and histopathological changes associated with acute lumpy skin disease in cattle. Veter- World 2022, 15, 1916–1923. [CrossRef]
- Kara, P.D.; Mather, A.S.; Pretorius, A.; Chetty, T.; Babiuk, S.; Wallace, D.B. Characterisation of putative immunomodulatory gene knockouts of lumpy skin disease virus in cattle towards an improved vaccine. Vaccine 2018, 36, 4708–4715. [CrossRef]
- Kegley, E.B.; Ball, J.J.; Beck, P. 121 Impact of mineral and vitamin status on beef cattle immune function and health. J. Anim. Sci. 2016, 94, 59–59. [CrossRef]
- Khalafalla A. (2022) Lumpy Skin Disease: An Economically Significant Emerging Disease. In: Cattle Diseases - Molecular and Biochemical Approach. 1-14.
- Khan, Y.R.; Ali, A.; Hussain, K.; Ijaz, M.; Rabbani, A.H.; Khan, R.L.; Abbas, S.N.; Aziz, M.U.; Ghaffar, A.; Sajid, H.A. A review: Surveillance of lumpy skin disease (LSD) a growing problem in Asia. Microb. Pathog. 2021, 158, 105050. [CrossRef]
- Kiplagat, S.K.; Kitala, P.M.; Onono, J.O.; Beard, P.M.; Lyons, N.A. Risk Factors for Outbreaks of Lumpy Skin Disease and the Economic Impact in Cattle Farms of Nakuru County, Kenya. Front. Veter- Sci. 2020, 7, 259. [CrossRef]
- Kitching RP. (2003) Vaccines for lumpy skin disease, sheep pox and goat pox. Dev Biol (Basel). 114, 161-167.
- Koirala, P.; Meki, I.K.; Maharjan, M.; Settypalli, B.K.; Manandhar, S.; Yadav, S.K.; Cattoli, G.; Lamien, C.E. Molecular Characterization of the 2020 Outbreak of Lumpy Skin Disease in Nepal. Microorganisms 2022, 10, 539. [CrossRef]
- Kononov, A.; Prutnikov, P.; Shumilova, I.; Kononova, S.; Nesterov, A.; Byadovskaya, O.; Pestova, Y.; Diev, V.; Sprygin, A. Determination of lumpy skin disease virus in bovine meat and offal products following experimental infection. Transbound. Emerg. Dis. 2019, 66, 1332–1340. [CrossRef]
- Kononova S, Kononov A, Shumilova I, et al. (2020) A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate. Transboundary and Emerging Diseases. 68(3), 1377-1383. [CrossRef]
- Kumar, N.; Chander, Y.; Kumar, R.; Khandelwal, N.; Riyesh, T.; Chaudhary, K.; Shanmugasundaram, K.; Kumar, S.; Kumar, A.; Gupta, M.K.; et al. Isolation and characterization of lumpy skin disease virus from cattle in India. PLOS ONE 2021, 16, e0241022. [CrossRef]
- Kumar, N.; Tripathi, B.N. A serious skin virus epidemic sweeping through the Indian subcontinent is a threat to the livelihood of farmers. Virulence 2022, 13, 1943–1944. [CrossRef]
- Kumar, N.; Barua, S.; Kumar, R.; Khandelwal, N.; Kumar, A.; Verma, A.; Singh, L.; Godara, B.; Chander, Y.; Kumar, G.; et al. Evaluation of the safety, immunogenicity and efficacy of a new live-attenuated lumpy skin disease vaccine in India. Virulence 2023, 14, 2190647. [CrossRef]
- Lai, Y.A.; Chen, X.; Kunasekaran, M.; Rahman, B.; MacIntyre, C.R. Global epidemiology of vaccine-derived poliovirus 2016–2021: A descriptive analysis and retrospective case-control study. EClinicalMedicine 2022, 50, 101508. [CrossRef]
- Lamien CE, Lelenta M, Goger W, et al. (2011) Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. Journal of Virological Methods. 171(1), 134-140. [CrossRef]
- Liang, Z.; Yao, K.; Wang, S.; Yin, J.; Ma, X.; Yin, X.; Wang, X.; Sun, Y. Understanding the research advances on lumpy skin disease: A comprehensive literature review of experimental evidence. Front. Microbiol. 2022, 13, 1065894. [CrossRef]
- Lojkić, I.; Šimić, I.; Krešić, N.; Bedeković, T. Complete Genome Sequence of a Lumpy Skin Disease Virus Strain Isolated from the Skin of a Vaccinated Animal. Genome Announc. 2018, 6. [CrossRef]
- Lu G, Xie J, Luo J, et al. (2020) Lumpy skin disease outbreaks in China, since 3 August 2019. Transboundary and Emerging Diseases. 68(2), 216-219. [CrossRef]
- Sprygin, A.; Babin, Y.; Pestova, Y.; Kononova, S.; Wallace, D.B.; Van Schalkwyk, A.; Byadovskaya, O.; Diev, V.; Lozovoy, D. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLOS ONE 2018, 13, e0207480. [CrossRef]
- Menasherow, S.; Rubinstein-Giuni, M.; Kovtunenko, A.; Eyngor, Y.; Fridgut, O.; Rotenberg, D.; Khinich, Y.; Stram, Y. Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). J. Virol. Methods 2014, 199, 95–101. [CrossRef]
- Molla, W.; de Jong, M.C.M.; Frankena, K. Temporal and spatial distribution of lumpy skin disease outbreaks in Ethiopia in the period 2000 to 2015. BMC Veter- Res. 2017, 13, 310. [CrossRef]
- Morgenstern, M.; Klement, E. The Effect of Vaccination with Live Attenuated Neethling Lumpy Skin Disease Vaccine on Milk Production and Mortality—An Analysis of 77 Dairy Farms in Israel. Vaccines 2020, 8, 324. [CrossRef]
- Mourya, D.; Yadav, P.; Ullas, P.; Bhardwaj, S.; Sahay, R.; Chadha, M.; Shete, A.; Jadhav, S.; Gupta, N.; Gangakhedkar, R.; et al. Emerging/re-emerging viral diseases & new viruses on the Indian horizon. Indian J. Med Res. 2019, 149, 447–467. [CrossRef]
- Namazi F, Khodakaram-Tafti A. (2021) Lumpy skin disease, an emerging transboundary viral disease: A review. Veterinary Medicine and Science. 7(3), 888-896. [CrossRef]
- Neamat-Allah ANF, Mahmoud EA (2019) Assessing the possible causes of hemolytic anemia associated with lumpy skin disease naturally infected buffaloes. Comparative Clinical Pathology. 28(3), 747-753. [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; et al. Assessment of the control measures for category A diseases of Animal Health Law: Lumpy Skin Disease. EFSA J. 2022, 20, e07121. [CrossRef]
- Nohynek H, Wilder-Smith A. (2022) Does the World Still Need New Covid-19 Vaccines? New England Journal of Medicine. 386(22), 2140-2142. [CrossRef]
- Pineda, P.S.; Flores, E.B.; Herrera, J.R.V.; Low, W.Y. Opportunities and Challenges for Improving the Productivity of Swamp Buffaloes in Southeastern Asia. Front. Genet. 2021, 12, 629861. [CrossRef]
- Rouby, S.; Aboulsoud, E. Evidence of intrauterine transmission of lumpy skin disease virus. Veter- J. 2016, 209, 193–195. [CrossRef]
- Rush ER, Dale E, Aguirre AA. (2021) Illegal Wildlife Trade and Emerging Infectious Diseases: Pervasive Impacts to Species, Ecosystems and Human Health. Animals. 11(6), 1821. [CrossRef]
- Selim, A.; Manaa, E.; Khater, H. Molecular characterization and phylogenetic analysis of lumpy skin disease in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101699. [CrossRef]
- Şevik, M.; Doğan, M. Epidemiological and Molecular Studies on Lumpy Skin Disease Outbreaks in Turkey during 2014-2015. Transbound. Emerg. Dis. 2017, 64, 1268–1279. [CrossRef]
- Sprygin A, Pestova Y, Prutnikov P, et al. (2018) Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transboundary and Emerging Diseases. 65(5), 1137-1144. [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.; Tuppurainen, E.; Kononov, A. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [CrossRef]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Jhade, S.K.; Hemadri, D.; Sood, R.; Bal, G.C.; Nayak, M.K.; Pradhan, S.K.; Singh, V.P. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transbound. Emerg. Dis. 2020, 67, 2408–2422. [CrossRef]
- The Hindu (Businessline). (2022) Slight impact. Lumpy skin disease had marginal impact on India’s milk output this year: USDA. URL https://www.thehindubusinessline.com/economy/agri-business/lumpy-skin-diseaseoutbreak-marginally-impacted-indias-milk-output-this-year-usda/article66065825.ece [Accessed: 31st Jan, 2023].
- Tuladhar, R.; Singh, A.; Banjara, M.R.; Gautam, I.; Dhimal, M.; Varma, A.; Choudhary, D.K. Effect of meteorological factors on the seasonal prevalence of dengue vectors in upland hilly and lowland Terai regions of Nepal. Parasites Vectors 2019, 12, 1–15. [CrossRef]
- Tulman ER, Afonso CL, Lu Z, et al. (2001) Genome of Lumpy Skin Disease Virus. Journal of Virology. 75(15), 7122-7130.Azeem S, Sharma B, Shabir S, et al. (2022) Lumpy skin disease is expanding its geographic range: A challenge for Asian livestock management and food security. The Veterinary Journal. 279, 105785. [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Sur, J.-H.; Sandybaev, N.T.; Kerembekova, U.Z.; Zaitsev, V.L.; Kutish, G.F.; Rock, D.L. The Genomes of Sheeppox and Goatpox Viruses. J. Virol. 2002, 76, 6054–6061. [CrossRef]
- Tuppurainen, E.; Dietze, K.; Wolff, J.; Bergmann, H.; Beltran-Alcrudo, D.; Fahrion, A.; Lamien, C.E.; Busch, F.; Sauter-Louis, C.; Conraths, F.J.; et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines 2021, 9, 1136. [CrossRef]
- Tuppurainen ESM, Oura CAL. (2012) Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases. 59(1), 40-48. [CrossRef]
- Tuppurainen, E.S.M.; Stoltsz, W.H.; Troskie, M.; Wallace, D.B.; Oura, C.A.L.; Mellor, P.S.; Coetzer, J.A.W.; Venter, E. A Potential Role for Ixodid (Hard) Tick Vectors in the Transmission of Lumpy Skin Disease Virus in Cattle. Transbound. Emerg. Dis. 2011, 58, 93–104. [CrossRef]
- Tuppurainen, E.S.; Venter, E.H.; Coetzer, J.A. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J. Veter. Res. 2005, 72, 153–164. [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2015, 64, 729–745. [CrossRef]
- Uddin JM, Sprygin A, Pestova Y, et al. (2020) Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS One. 15(5), e0232584. [CrossRef]
- Wolff, J.; Tuppurainen, E.; Adedeji, A.; Meseko, C.; Asala, O.; Adole, J.; Atai, R.; Dogonyaro, B.; Globig, A.; Hoffmann, D.; et al. Characterization of a Nigerian Lumpy Skin Disease Virus Isolate after Experimental Infection of Cattle. Pathogens 2021, 11, 16. [CrossRef]
- Xavier-Roche AR, TagoPacheco D, Kamata A, et al. (2020) Introduction and spread of lumpy skin disease in South, East and Southeast Asia - Qualitative risk assessment and management. FAO animal production and health. 1-50.
- Yeruham, I.; Nir, O.; Braverman, Y.; Davidson, M.; Grinstein, H.; Haymovitch, M.; Zamir, O. Spread of lumpy skin disease in Israeli dairy herds. Veter- Rec. 1995, 137, 91–93. [CrossRef]
- Young M, Crook H, Scott J, et al. (2021) Covid-19: virology, variants, and vaccines. BMJ Medicine. 1(1), e000040. [CrossRef]
- Zeballos, E.; Chelius, C. The effects of grazing on daily caloric intake and dietary quality. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 1–14. [CrossRef]
- Zheng, M.; Liu, Q.; Jin, N.; Guo, J.; Huang, X.; Li, H.; Zhu, W.; Xiong, Y. A duplex PCR assay for simultaneous detection and differentiation of Capripoxvirus and Orf virus. Mol. Cell. Probes 2007, 21, 276–281. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).