Submitted:
31 January 2023
Posted:
03 February 2023
You are already at the latest version
Abstract
Keywords:
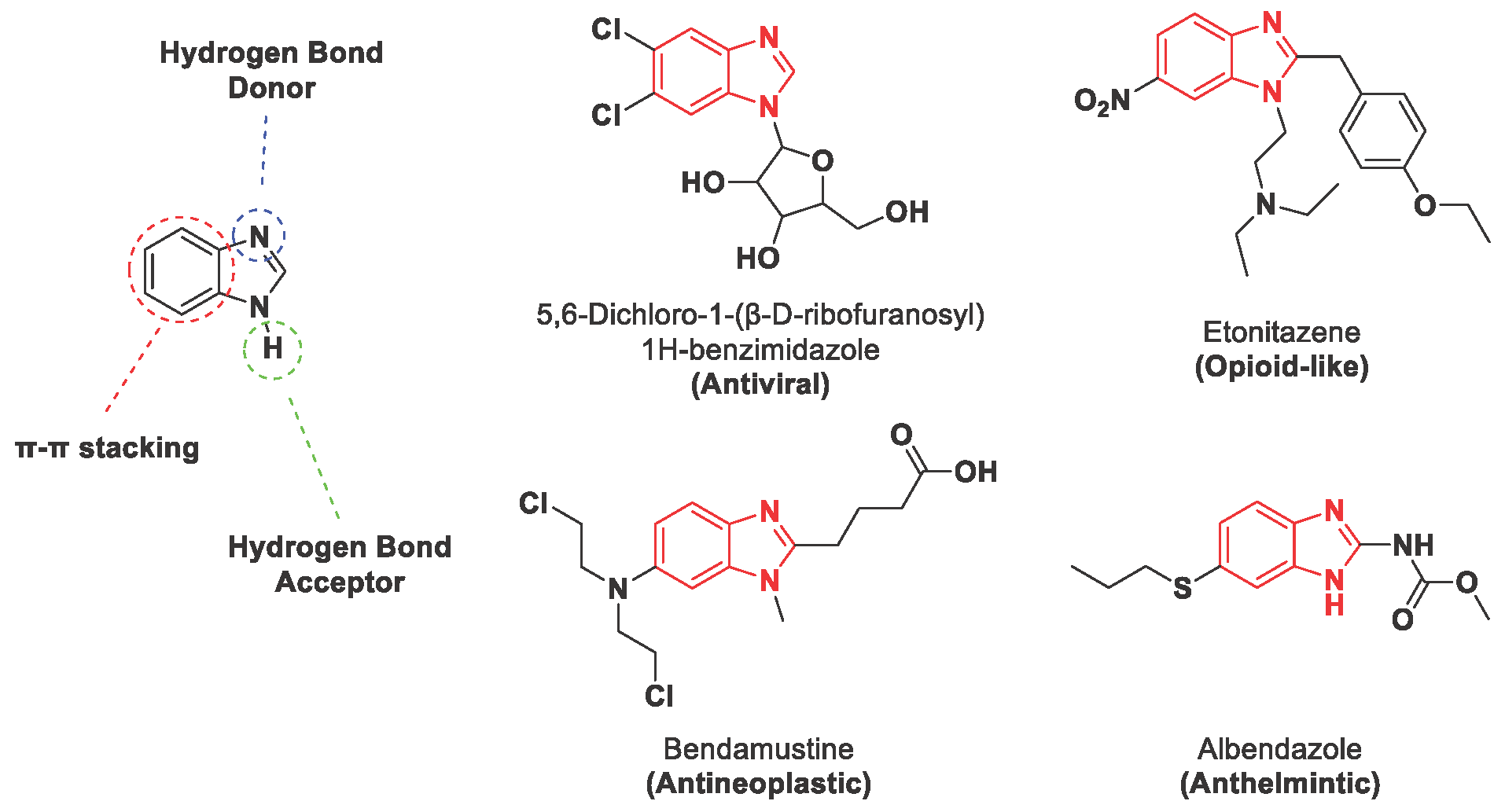
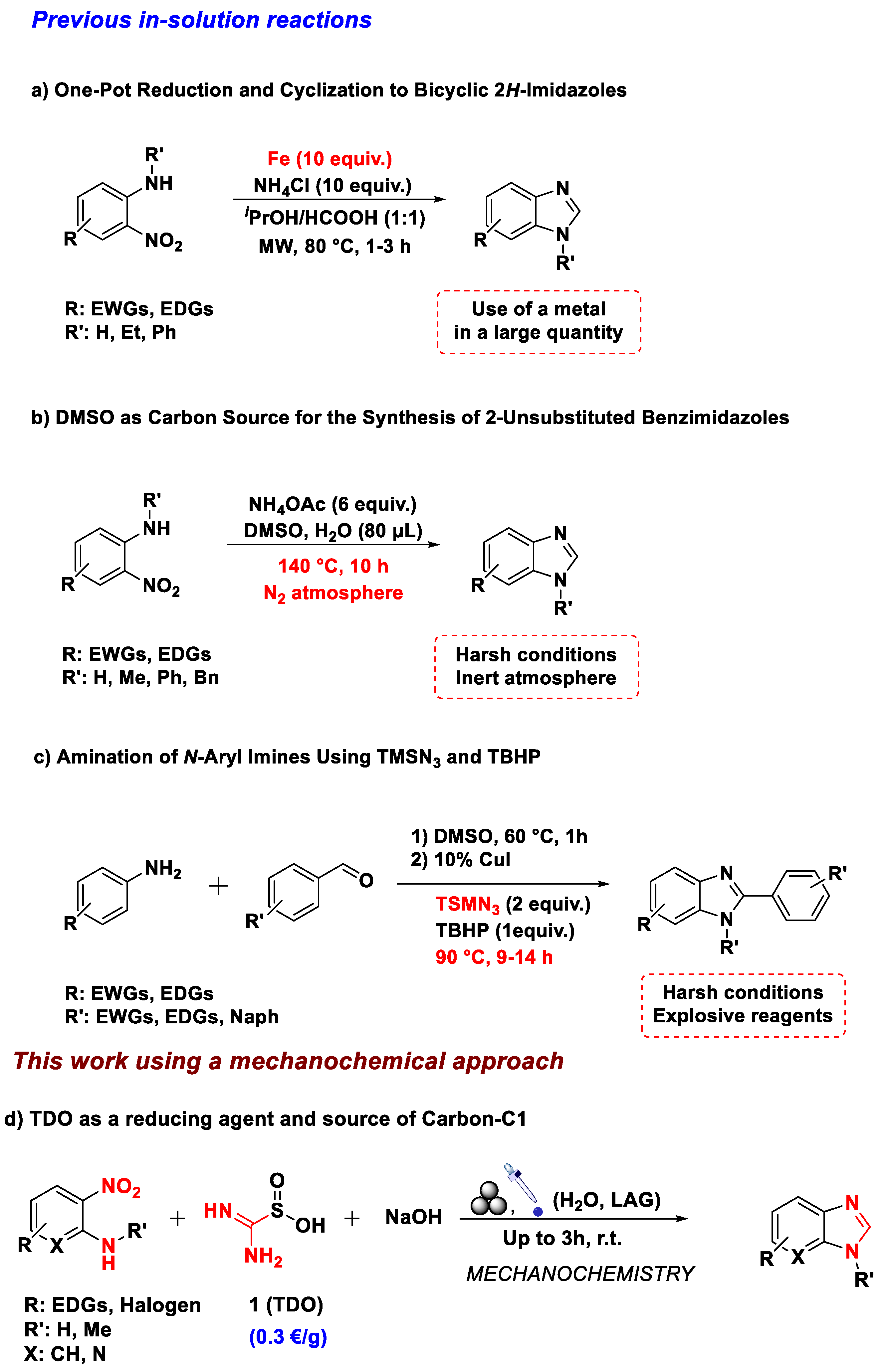
1. Introduction
2. Results and Discussion
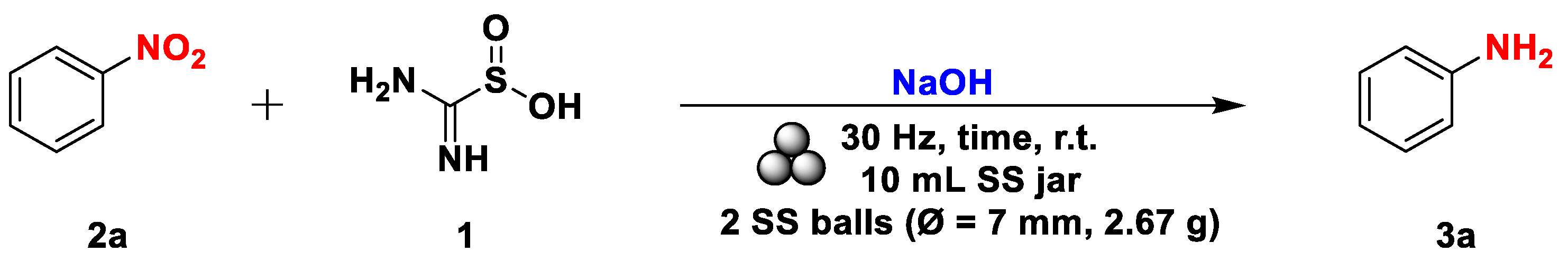
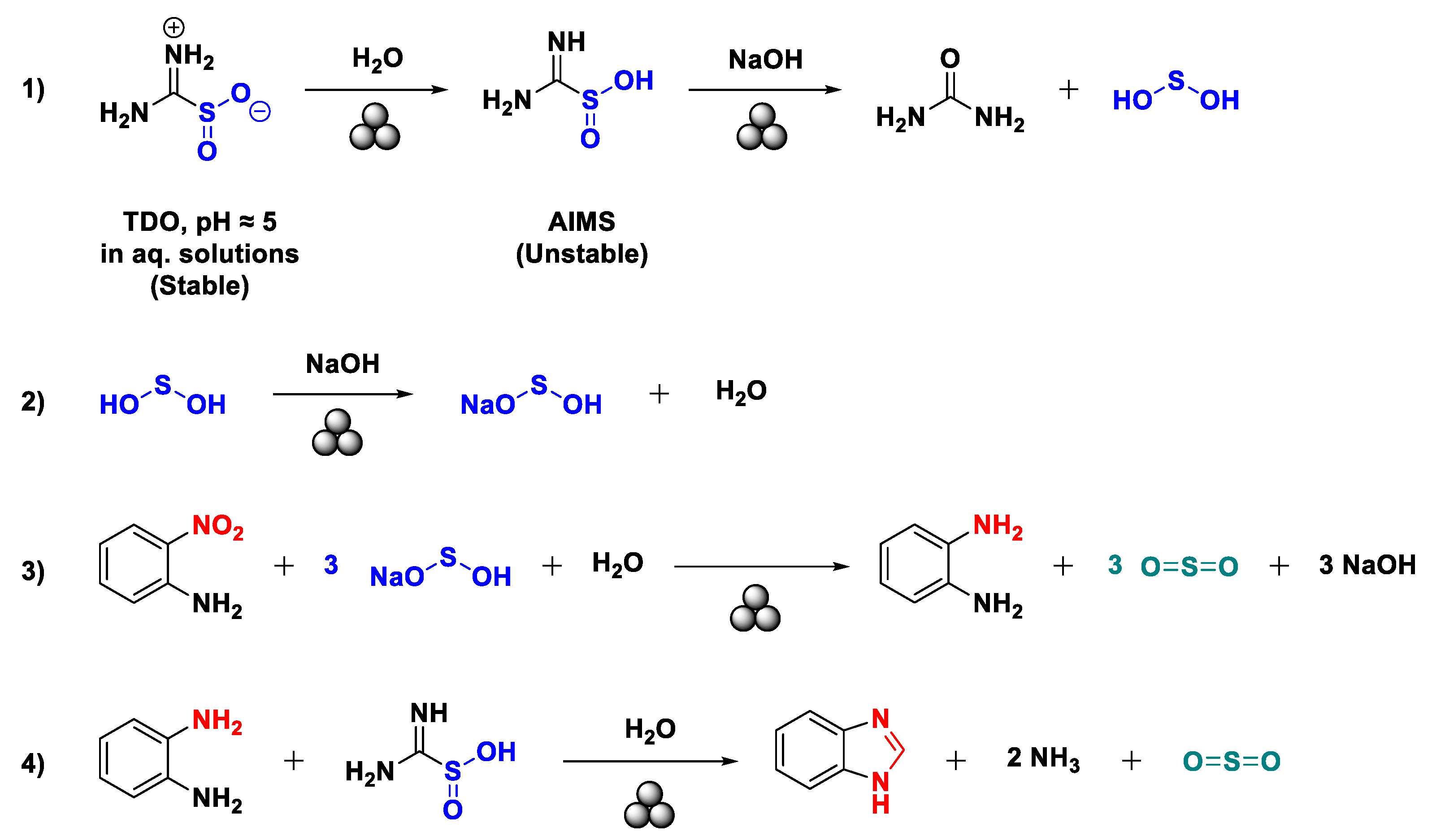
2.1. TDO as a reducing agent
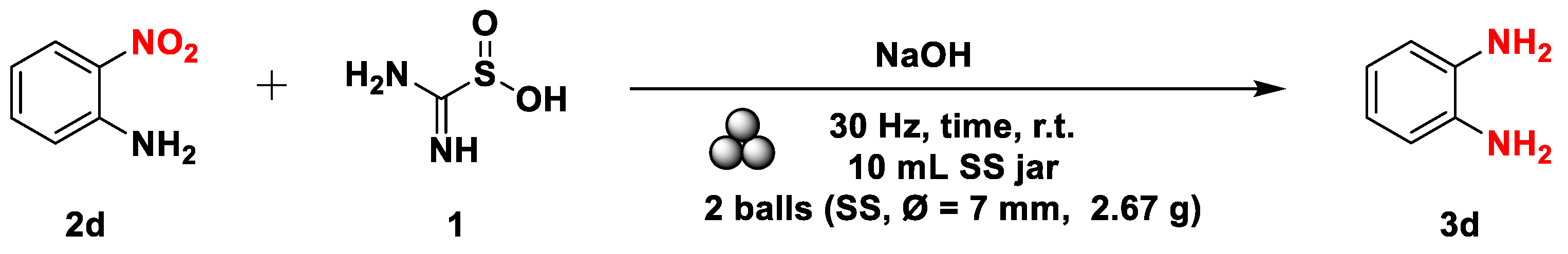
| Entry | TDO eq. | Base eq. | Reaction Time (h) | Additivesb | Yieldsa |
|---|---|---|---|---|---|
| 1 | 3 | 6 | 1.5 | / | 37% |
| 2c | 3 | 6 | 2 | / | 45% |
| 3d | 3 | 6 | 2 | / | 54% |
| 4e | 3 | 6 | 2 | / | Complex mixture |
| 5f | 3 | 6 | 2 | MeOH | 0% |
| 6 | 3 | 6 | 2 | H2O | 98% |
| 7g | 3 | 3 | 2 | H2O | 1% |
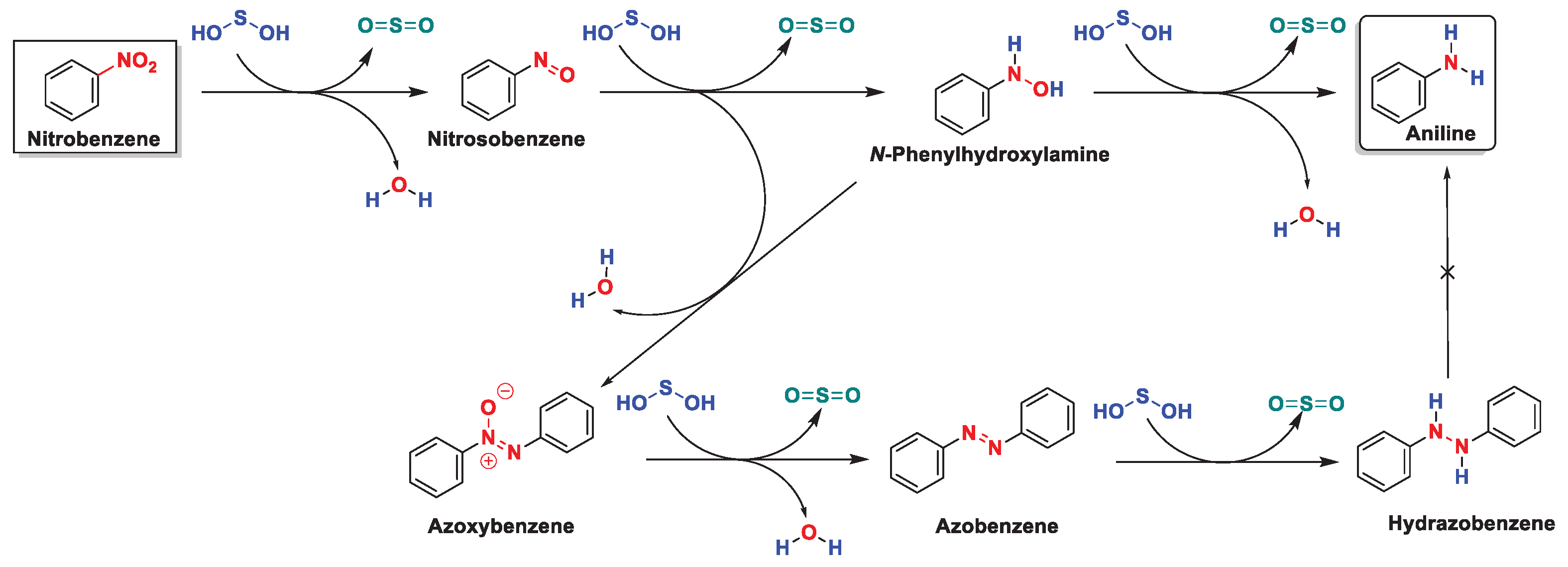
2.2. TDO as an electrophile
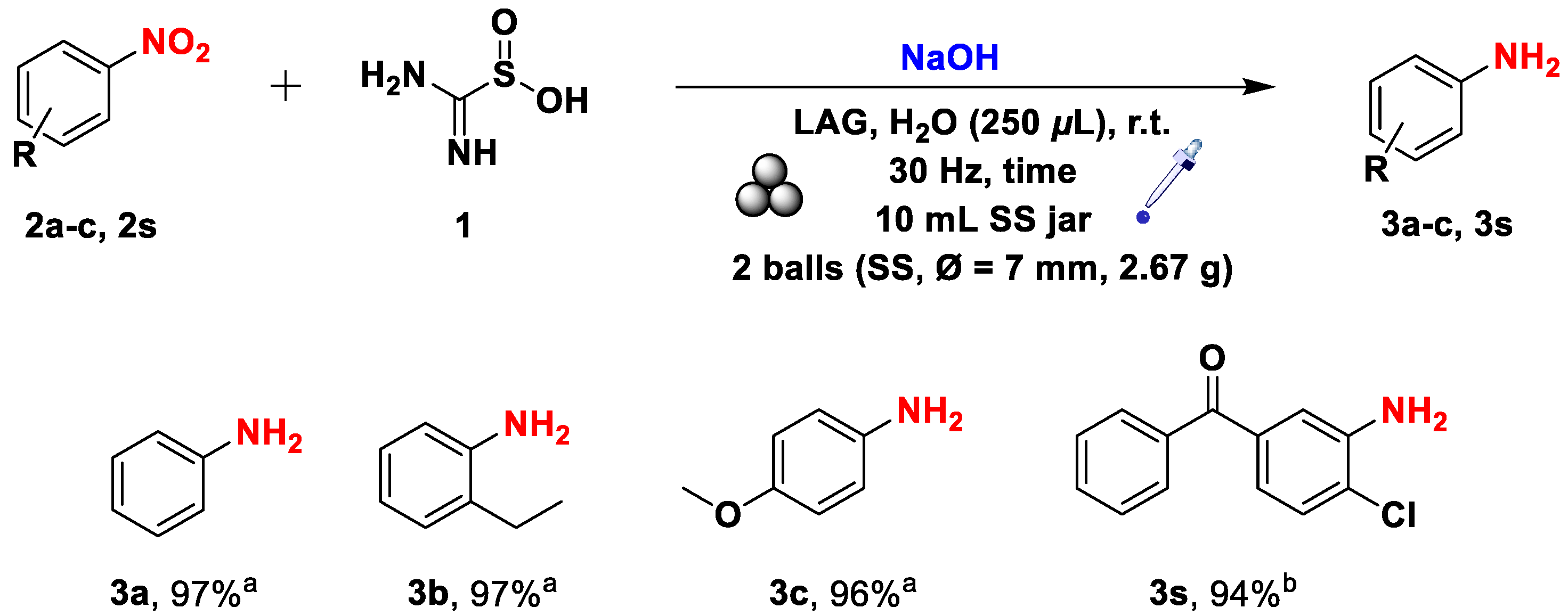
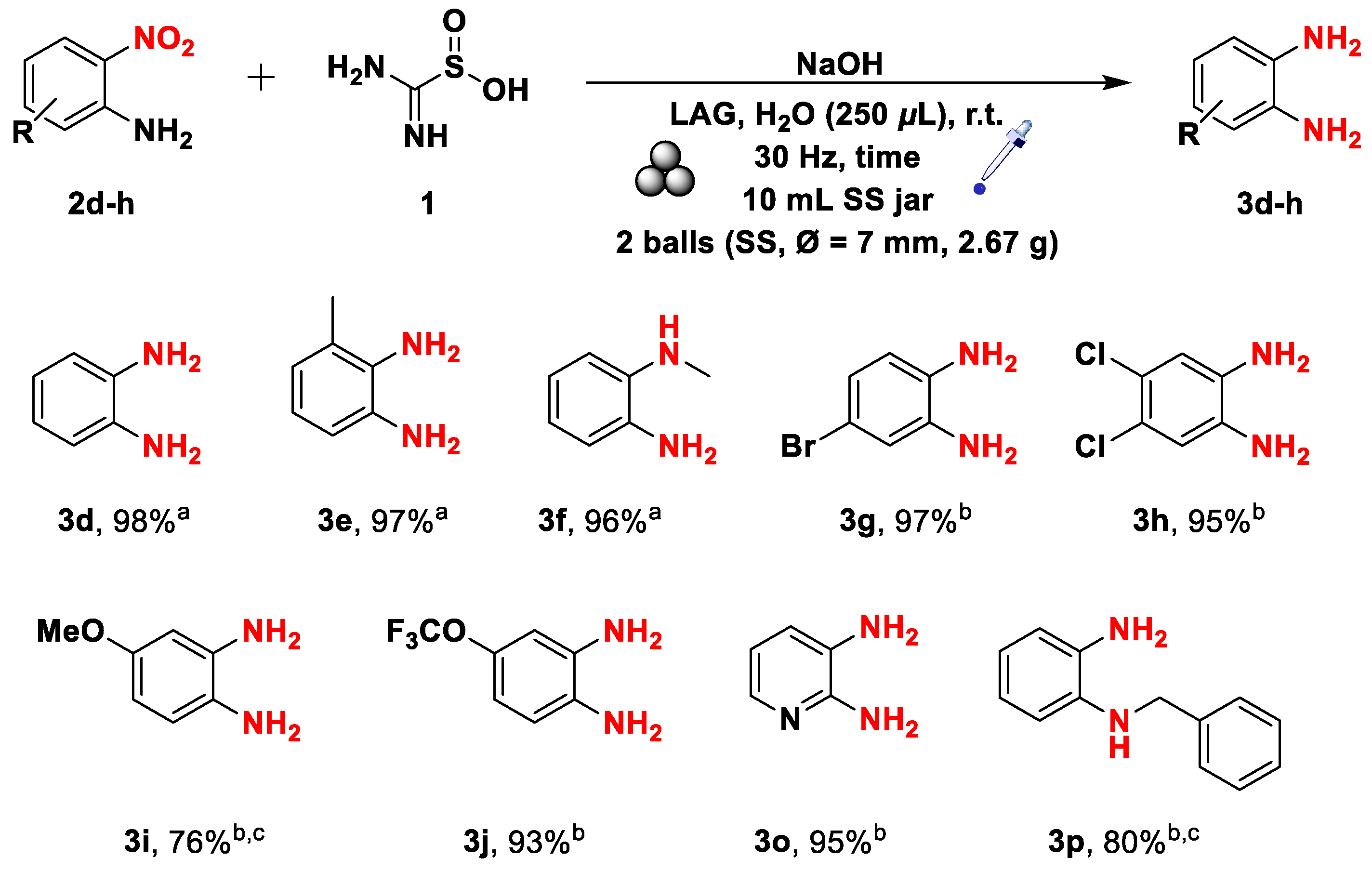
2.2.1. Procedure A
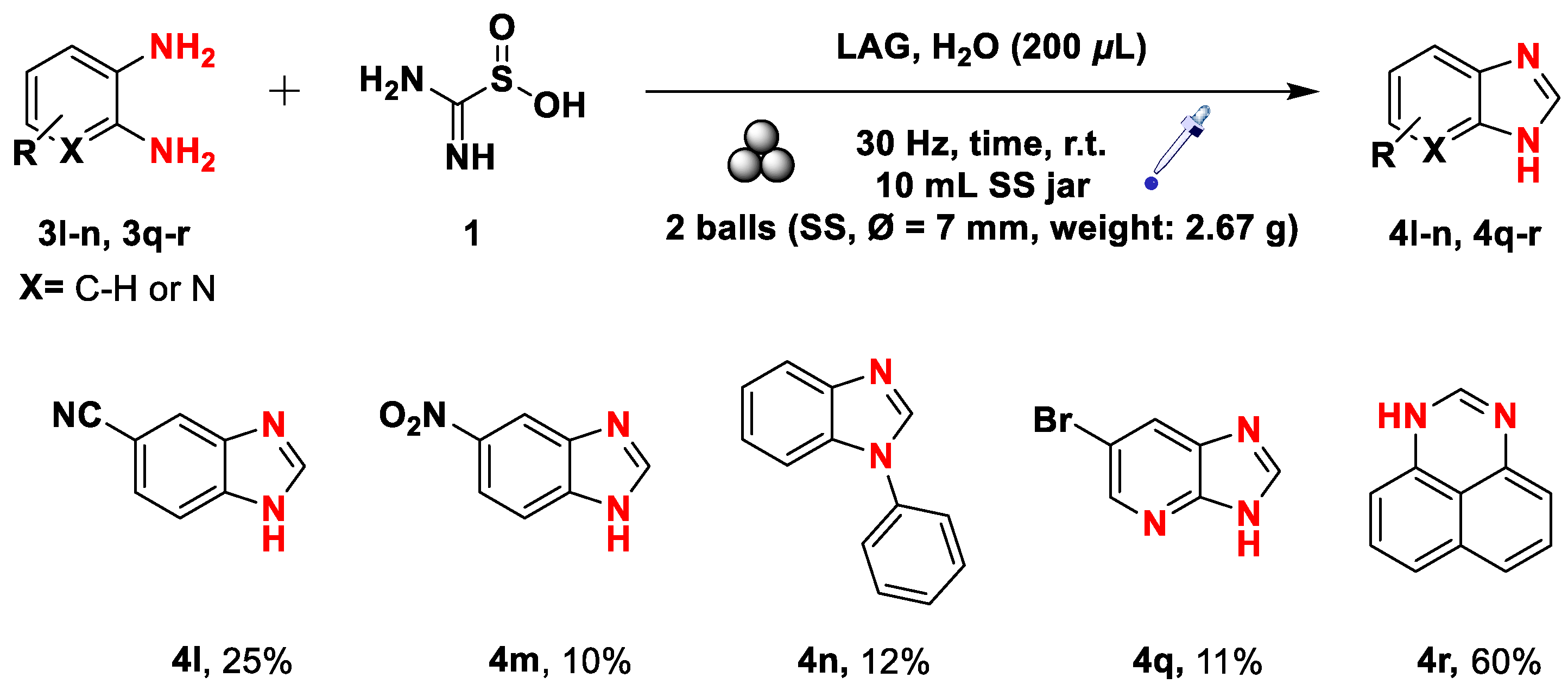
2.2.2. Procedure B
3. Materials and Methods
3.1. Materials
3.2. General procedure A for anilines and o-phenylenediamines 3a-j, 3o-p, 3s synthesis from 2- nitroanilines 2a-j, 2o-p, 2s
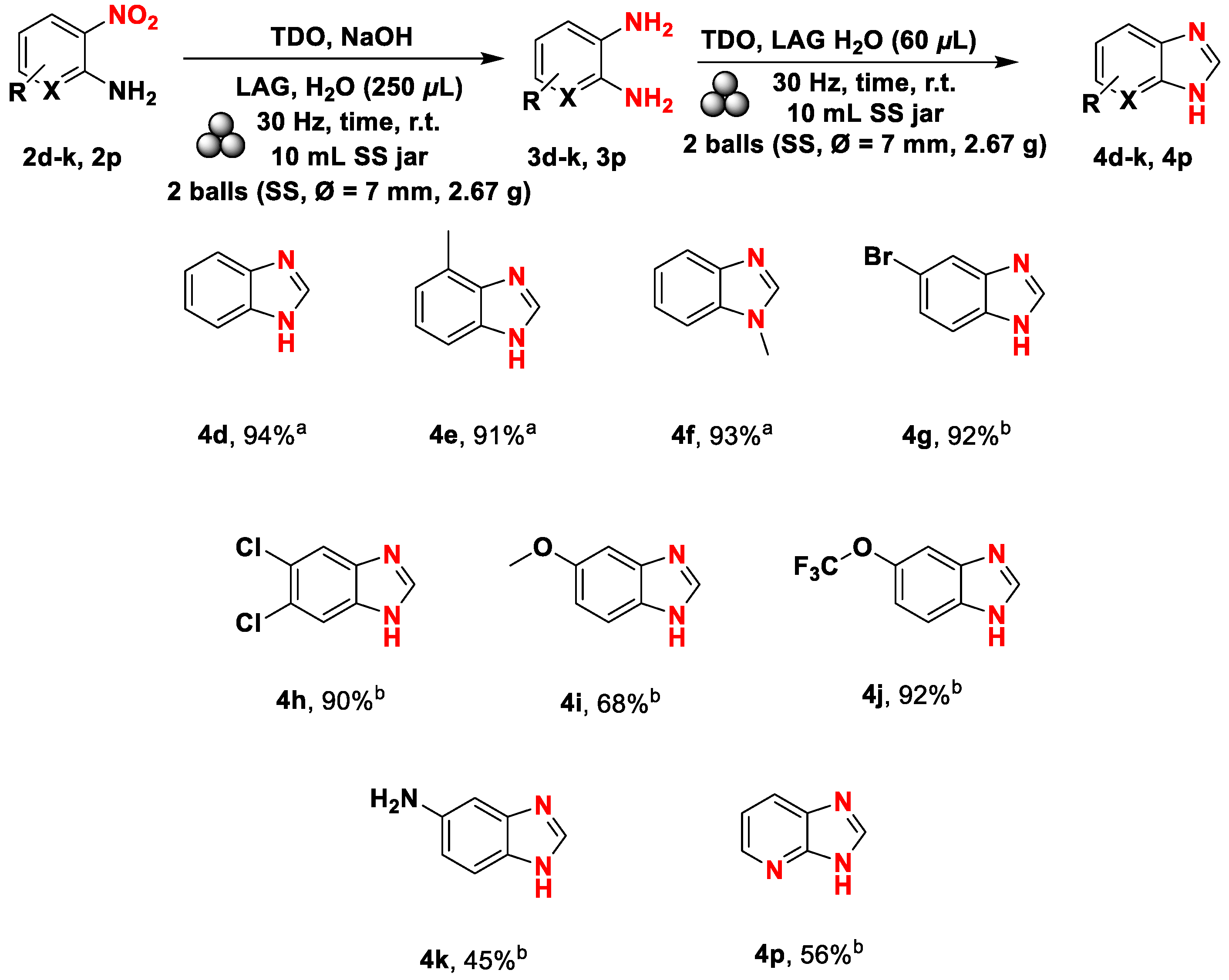
3.3. General procedure B for heterocycles 4l-n, 4q-r synthesis from o-phenylenediamines 3l-n, 3q-r
3.4. General procedure C for heterocycles 4d-k, 4p synthesis from 2-nitroanilines 2d-k, 2p
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elattar, K.M.; Mert, B.D.; Monier, M.; El-Mekabaty, A. Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido[1,6-a]pyrimidine and pyrimido[1,6-c]pyrimidine scaffolds. RSC Adv. 2020, 10, 15461–15492. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.M.; Gaffer, H.E.; Elattar, K.M. Insights into the medicinal chemistry of heterocycles integrated with a pyrazolo[1,5-a]pyrimidine scaffold. RSC Med Chem 2022, 13, 1150–1196. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Naim, M.J.; Alam, M.J.; Nawaz, F.; Ahmed, S.; Alam, O. Benzimidazole Scaffold as Anticancer Agent: Synthetic Approaches and Structure-Activity Relationship. Arch Pharm (Weinheim) 2017, 350. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Kakkar, S.; Narasimhan, B. Benzimidazole scaffolds as promising antiproliferative agents: a review. BMC Chem 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Luxami, V.; Paul, K. Benzimidazole-biologically attractive scaffold for protein kinase inhibitors. RSC Adv. 2014, 4, 12422–12440. [Google Scholar] [CrossRef]
- Mudi, P.K.; Mahanty, A.K.; Kotakonda, M.; Prasad, S.; Bhattacharyya, S.; Biswas, B. A benzimidazole scaffold as a promising inhibitor against SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Gutiérrez, A.; Curiel-Quesada, E.; Correa-Basurto, J.; Martínez-Muñoz, A.; Reyes-Arellano, A. N-Heterocycles Scaffolds as Quorum Sensing Inhibitors. Design, Synthesis, Biological and Docking Studies. Int. J. Mol. Sci. 2020, 21, 9512. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.K.; Ibrahim, N.A.; El-Kaed, S.A.; El-Helw, E.A.E. New potential fungicides pyrazole-based heterocycles derived from 2-cyano-3-(1,3-diphenyl-1H-pyrazol-4-yl) acryloyl isothiocyanate. J. Sulfur Chem. 2021, 42, 529–546. [Google Scholar] [CrossRef]
- Merel, S.; Benzing, S.; Gleiser, C.; Di Napoli-Davis, G.; Zwiener, C. Occurrence and overlooked sources of the biocide carbendazim in wastewater and surface water. Environ. Pollut. 2018, 239, 512–521. [Google Scholar] [CrossRef]
- Han, P.; Rios-Miguel, A.B.; Tang, X.; Yu, Y.; Zhou, L.-J.; Hou, L.; Liu, M.; Sun, D.; Jetten, M.S.M.; Welte, C.U.; et al. Benzimidazole fungicide biotransformation by comammox Nitrospira bacteria: Transformation pathways and associated proteomic responses. J. Hazard. Mater. 2023, 445, 130558. [Google Scholar] [CrossRef]
- Chu, B.; Liu, F.; Li, L.; Ding, C.; Chen, K.; Sun, Q.; Shen, Z.; Tan, Y.; Tan, C.; Jiang, Y. A benzimidazole derivative exhibiting antitumor activity blocks EGFR and HER2 activity and upregulates DR5 in breast cancer cells. Cell. Death Dis. 2015, 6, e1686. [Google Scholar] [CrossRef]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw 2020, 20, e29. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: an emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef]
- Ersan, S.; Nacak, S.; Noyanalpan, N.; Yeşilada, E. Studies on analgesic and anti-inflammatory activities of 1-dialkylaminomethyl-2-(p-substituted phenyl)-5-substituted benzimidazole derivatives. Arzneimittelforschung 1997, 47, 834–836. [Google Scholar]
- Ujváry, I.; Christie, R.; Evans-Brown, M.; Gallegos, A.; Jorge, R.; de Morais, J.; Sedefov, R. DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles. ACS Chem. Neurosci. 2021, 12, 1072–1092. [Google Scholar] [CrossRef] [PubMed]
- Satija, G.; Sharma, B.; Madan, A.; Iqubal, A.; Shaquiquzzaman, M.; Akhter, M.; Parvez, S.; Khan, M.A.; Alam, M.M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heterocycl. Chem. 2022, 59, 22–66. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Nazreen, S.; Elbehairi, S.E.I.; Asad, M.; Shati, A.A.; Alfaifi, M.Y.; Alhadhrami, A.; Elhenawy, A.A.; Alorabi, A.Q.; Asiri, A.M.; et al. Design, synthesis, anticancer activity and molecular docking studies of new benzimidazole derivatives bearing 1,3,4-oxadiazole moieties as potential thymidylate synthase inhibitors. New J. Chem. 2022, 46, 14967–14978. [Google Scholar] [CrossRef]
- Hosamani, K.M.; Hiremath, V.B.; Keri, R.S.; Harisha, R.S.; Halligudi, S.B. Synthesis of novel 2-alkyl substituted oleobenzimidazole derivatives using ethylene glycol as solvent. Can. J. Chem. 2008, 86, 1030–1033. [Google Scholar] [CrossRef]
- Hanan, E.J.; Chan, B.K.; Estrada, A.A.; Shore, D.G.; Lyssikatos, J.P. Mild and General One-Pot Reduction and Cyclization of Aromatic and Heteroaromatic 2-Nitroamines to Bicyclic 2H-Imidazoles. Synlett 2010, 2010, 2759–2764. [Google Scholar] [CrossRef]
- Nale, D.B.; Bhanage, B.M. N-Substituted Formamides as C1-Sources for the Synthesis of Benzimidazole and Benzothiazole Derivatives by Using Zinc Catalysts. Synlett 2015, 26, 2835–2842. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Kuang, D.; Deng, G.; Yang, Y.; Yu, J.; Liang, Y. K2S as Sulfur Source and DMSO as Carbon Source for the Synthesis of 2-Unsubstituted Benzothiazoles. Org. Lett. 2020, 22, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, D.; Sadhu, P.; Punniyamurthy, T. Copper(I)-Catalyzed Regioselective Amination of N-Aryl Imines Using TMSN3 and TBHP: A Route to Substituted Benzimidazoles. J. Org. Chem. 2015, 80, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, S.; Kumar, M.; Bhalla, V. “Metal-Free” Nanoassemblies of AIEE-ICT-Active Pyrazine Derivative: Efficient Photoredox System for the Synthesis of Benzimidazoles. J. Org. Chem. 2020, 85, 13906–13919. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Mondal, A.; Srimani, D. Selective Synthesis of 2-Substituted and 1,2-Disubstituted Benzimidazoles Directly from Aromatic Diamines and Alcohols Catalyzed by Molecularly Defined Nonphosphine Manganese(I) Complex. J. Org. Chem. 2018, 83, 9553–9560. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Jones, B.P.; Wei, L. Preparation of Substituted Benzimidazoles and Imidazopyridines Using 2,2,2-Trichloroethyl Imidates. Synthesis 2012, 44, 3049–3054. [Google Scholar] [CrossRef]
- Fischer, K.; Marquardt, K.; Schlüter, K.; Gebert, K.; Borschel, E.-M.; Heimann, S.; Kromm, E.; Giesen, V.; Schneider, R.; Lee Wayland Jr., R. Textile Auxiliaries. In Ullmann’s Encycl. Ind. Chem.
- Obtemper, S.I.; Zlobin, V.K. Application of Formamidinesulfinic acid for Separate Spectrophotometric Determination of para Nitrophenol, ortho Nitrophenol and meta Nitrophenol in their Mutual Presence. 1974, 29, 609-611.
- Obtemper, S.I.; Zlobin, V.K. Use of Thiourea Dioxide in Organic-Analysis-Determination of Nitric-Acid Esters, Nitroso and Azo-Compounds. 1974, 247-249.
- Koniecki, W.B.; Linch, A.L. Determination of Aromatic Nitro Compounds. Anal. Chem. 1958, 30, 1134–1137. [Google Scholar] [CrossRef]
- de Barry Barnett, E. VII.—The action of hydrogen dioxide on thiocarbamides. J. Chem. Soc., Trans. 1910, 97, 63–65. [Google Scholar] [CrossRef]
- De Filippo, D.; Ponticelli, G.; Trogu, E.F.; Lai, A. Spectrochemical study of aminoiminomethanesulphinic acid and related NN′-substituted derivatives. J. Chem. Soc., Perkin Trans. II 1972, 1500–1502. [Google Scholar] [CrossRef]
- Havel, J.J.; Kluttz, R.Q. A Synthesis of Formamidinesulfinic Acids and Formamidines. Synth. Comm. 1974, 4, 389–393. [Google Scholar] [CrossRef]
- Dictionary. In Gardner’s Commercially Important Chemicals; 2005; pp. 2-682.
- Pu, S.; Liang, Q.; Luo, X.; Luo, J. Convenient Two-step One-pot Synthesis of Benzimidazoles Using 2-nitroanilines and Thiourea Dioxide. J. Chem. Res. 2014, 38, 118–120. [Google Scholar] [CrossRef]
- Hamad, M.O.; Kiptoo, P.K.; Stinchcomb, A.L.; Crooks, P.A. Synthesis and hydrolytic behavior of two novel tripartate codrugs of naltrexone and 6β-naltrexol with hydroxybupropion as potential alcohol abuse and smoking cessation agents. Bioorg. Med. Chem. 2006, 14, 7051–7061. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Gupta, S.; Sureshbabu, P.; Sabiah, S.; Kandasamy, J. A metal free reduction of aryl-N-nitrosamines to the corresponding hydrazines using a sustainable reductant thiourea dioxide. Green Chem. 2016, 18, 6215–6221. [Google Scholar] [CrossRef]
- Chatterjie, N.; Umans, J.G.; Inturrisi, C.E. Reduction of 6-ketones of the morphine series with formamidinesulfinic acid. Stereoselectivity opposite to that of hydride reductions. J. Org. Chem. 1976, 41, 3624–3625. [Google Scholar] [CrossRef] [PubMed]
- Svarovsky, S.A.; Simoyi, R.H.; Makarov, S.V. Reactive oxygen species in aerobic decomposition of thiourea dioxides . J. Chem. Soc., Dalton Trans. 2000, 511–514. [Google Scholar] [CrossRef]
- He, F.-S.; Yang, M.; Ye, S.; Wu, J. Sulfonylation from sodium dithionite or thiourea dioxide. Chin. Chem. Lett. 2021, 32, 461–464. [Google Scholar] [CrossRef]
- Verma, S.; Singh, R.; Tripathi, D.; Gupta, P.; Bahuguna, G.M.; Jain, S.L. Thiourea dioxide with TBHP: a fruitful and greener recipe for the catalytic oxidation of alcohols. RSC Advances 2013, 3, 4184–4188. [Google Scholar] [CrossRef]
- Zhou, L.H.; Jin, Y.J.; Ma, L.F.; Huang, W.H.; Wu, Y. Highly Efficient and Catalyst-Free Synthesis of Benzimidazoles in Aqueous Media. Russ. J. Org. Chem. 2021, 57, 825–830. [Google Scholar] [CrossRef]
- Kahl, T.; Schröder, K.-W.; Lawrence, F.R.; Marshall, W.J.; Höke, H.; Jäckh, R. Aniline. In Ullmann’s Encycl. Ind. Chem.
- Available online: https://archive.vn/20020219104231/http://www.the-innovation-group.com/ChemProfiles/Aniline.
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bu, J.; Wang, J.; Sun, C.; Zhao, D.; Sheng, G.; Xie, X.; Sun, M.; Yu, L. Highly Efficient Hydrogenation of Nitrobenzene to Aniline over Pt/CeO2 Catalysts: The Shape Effect of the Support and Key Role of Additional Ce3+ Sites. ACS Catal. 2020, 10, 10350–10363. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Prashad, M.; Repič, O.; Blacklock, T.J. A Practical and Chemoselective Reduction of Nitroarenes to Anilines Using Activated Iron. Adv. Synth. Catal. 2005, 347, 217–219. [Google Scholar] [CrossRef]
- Anjali, K.; Ahmed, M.; Christopher, J.; Sakthivel, A. Rhodium-calix[4]pyrrole and rhodium-tetraphenyl porphyrin: preparation, surface grafting and their catalytic application in nitro-benzene reduction. Dalton Trans. 2018, 47, 12353–12361. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R.; Prashanth, V.; Rao, G.M.; Shivakumara, C. Structure and Catalytic Activity of Cr-Doped BaTiO3 Nanocatalysts Synthesized by Conventional Oxalate and Microwave Assisted Hydrothermal Methods. Inorg. Chem. 2016, 55, 4795–4805. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Purkait, M.K. Green synthesized iron nanoparticle-embedded pH-responsive PVDF-co-HFP membranes: Optimization study for NPs preparation and nitrobenzene reduction. Sep. Sci. Technol. 2017, 52, 2338–2355. [Google Scholar] [CrossRef]
- Leng, F.; Gerber, I.C.; Lecante, P.; Moldovan, S.; Girleanu, M.; Axet, M.R.; Serp, P. Controlled and Chemoselective Hydrogenation of Nitrobenzene over Ru@C60 Catalysts. ACS Catal. 2016, 6, 6018–6024. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, S.; Zhao, Z.; Hao, F.; Cai, Z.; Liu, P.; Zhang, H.; Luo, H. Highly uniform Ni particles with phosphorus and adjacent defects catalyze 1,5-dinitronaphthalene hydrogenation with excellent catalytic performance. Front. Chem. Sci. Eng. 2021, 15, 998–1007. [Google Scholar] [CrossRef]
- Gong, W.; Lin, Y.; Chen, C.; Al-Mamun, M.; Lu, H.-S.; Wang, G.; Zhang, H.; Zhao, H. Nitrogen-Doped Carbon Nanotube Confined Co–Nx Sites for Selective Hydrogenation of Biomass-Derived Compounds. Adv. Mater. 2019, 31, 1808341. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; chen, Y.; Zhao, Z.; Deng, H.; Zhou, D.; Wei, C.; Nie, R.; Xia, Q. Highly selective one-step hydrogenation of nitrobenzene to cyclohexylamine over the supported 10% Ni/carbon catalysts doped with 3‰ Rh. RSC Advances 2016, 6, 15354–15361. [Google Scholar] [CrossRef]
- Diao, S.; Qian, W.; Luo, G.; Wei, F.; Wang, Y. Gaseous catalytic hydrogenation of nitrobenzene to aniline in a two-stage fluidized bed reactor. Appl. Catal. A: Gen. 2005, 286, 30–35. [Google Scholar] [CrossRef]
- Krishnan, S.; Patel, P.N.; Balasubramanian, K.K.; Chadha, A. Yeast supported gold nanoparticles: an efficient catalyst for the synthesis of commercially important aryl amines. New J. Chem. 2021, 45, 1915–1923. [Google Scholar] [CrossRef]
- Daems, N.; Wouters, J.; Van Goethem, C.; Baert, K.; Poleunis, C.; Delcorte, A.; Hubin, A.; Vankelecom, I.F.J.; Pescarmona, P.P. Selective reduction of nitrobenzene to aniline over electrocatalysts based on nitrogen-doped carbons containing non-noble metals. Appl. Catal. B: Environ. 2018, 226, 509–522. [Google Scholar] [CrossRef]
- Niknam, T.; Bornapour, M.; Gheisari, A.; Bahmani-Firouzi, B. Impact of heat, power and hydrogen generation on optimal placement and operation of fuel cell power plants. Int. J. Hydrog. Energy 2013, 38, 1111–1127. [Google Scholar] [CrossRef]
- Sheng, X.; Wouters, B.; Breugelmans, T.; Hubin, A.; Vankelecom, I.F.J.; Pescarmona, P.P. Cu/CuxO and Pt nanoparticles supported on multi-walled carbon nanotubes as electrocatalysts for the reduction of nitrobenzene. Appl. Catal. B: Environ. 2014, 147, 330–339. [Google Scholar] [CrossRef]
- Sheng, X.; Wouters, B.; Breugelmans, T.; Hubin, A.; Vankelecom, I.F.J.; Pescarmona, P.P. Pure and Alloyed Copper-Based Nanoparticles Supported on Activated Carbon: Synthesis and Electrocatalytic Application in the Reduction of Nitrobenzene. ChemElectroChem 2014, 1, 1198–1210. [Google Scholar] [CrossRef]
- Zhang, T.; Xie, Z.; Jiang, L.; Zhao, W.; Cao, S.; Wang, B.; Si, R.; Zhang, R.; Liu, Y.; Zhao, Z. Selective transfer hydrogenation coupling of nitroaromatics to azoxy/azo compounds by electron-enriched single Ni-N4 sites on mesoporous N-doped carbon. Chem. Eng. J. 2022, 443, 136416. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yang, B.; Ma, L.; Wang, N.; Wei, X. Facile Synthesis of a Novel Heterogeneous Rh/COF Catalyst and Its Application in Tandem Selective Transfer Hydrogenation and Monomethylation of Nitro Compounds with Methanol. Ind. Eng. Chem. Res. 2022, 61, 1066–1077. [Google Scholar] [CrossRef]
- Moran, M.J.; Martina, K.; Baricco, F.; Tagliapietra, S.; Manzoli, M.; Cravotto, G. Tuneable Copper Catalysed Transfer Hydrogenation of Nitrobenzenes to Aniline or Azo Derivatives. Adv. Synth. Catal. 2020, 362, 2689–2700. [Google Scholar] [CrossRef]
- Xu, D.; Liu, R.; Li, J.; Zhao, H.; Ma, J.; Dong, Z. Atomically dispersed Co-N4 sites anchored on N-doped carbon for aqueous phase transfer hydrogenation between nitroarenes and saturated N-heterocycles. Appl. Catal. B: Environ. 2021, 299, 120681. [Google Scholar] [CrossRef]
- Dai, X.; Cui, X.; Yuan, H.; Deng, Y.; Shi, F. Cooperative transformation of nitroarenes and biomass-based alcohols catalyzed by CuNiAlOx. RSC Adv. 2015, 5, 7970–7975. [Google Scholar] [CrossRef]
- Liu, H.; Khuan Chuah, G.; Jaenicke, S. Alumina-entrapped Ag catalyzed nitro compounds coupled with alcohols using borrowing hydrogen methodology. Phys. Chem. Chem. Phys. 2015, 17, 15012–15018. [Google Scholar] [CrossRef]
- Wei, R.P.; Shi, F. Controllable synthesis of azoxybenzenes and anilines with alcohol as the reducing agent promoted by KOH. Synth. Commun. 2019, 49, 688–696. [Google Scholar] [CrossRef]
- Bigelow, H.E.; Robinson, D.B. Org. Synth. 1942, 22, 28. [CrossRef]
- Srilakshmi, C.; Vijay Kumar, H.; Praveena, K.; Shivakumara, C.; Muralidhar Nayak, M. A highly efficient iron doped BaTiO3 nanocatalyst for the catalytic reduction of nitrobenzene to azoxybenzene. RSC Adv. 2014, 4, 18881–18884. [Google Scholar] [CrossRef]
- Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A.M.; Yang, W.; Chen, Y.I. Mechanochemistry: A force in disguise and conditional effects towards chemical reactions. Chem. Comm. 2021, 57, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef] [PubMed]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical synthesis of small organic molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Mal, P. Mechanochemistry of supramolecules. Beilstein J. Org. Chem. 2019, 15, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Shearouse, W.C.; Korte, C.M.; Mack, J. A two-step ball milling method synthesizes and purifies α,β-unsaturated esters. Green Chem. 2011, 13, 598–601. [Google Scholar] [CrossRef]
- Do, J.-L.; Mottillo, C.; Tan, D.; Štrukil, V.; Friščić, T. Mechanochemical Ruthenium-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2015, 137, 2476–2479. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.N.; Bolm, C. Mechanochemical Rhodium(III)-Catalyzed C–H Bond Amidation of Arenes with Dioxazolones under Solventless Conditions in a Ball Mill. ACS Catal. 2017, 7, 4592–4596. [Google Scholar] [CrossRef]
- Nakagawa, K.M.; Kawamura, S.; Minami, K. Reduction of Organic Compounds with Thiourea Dioxide. II Reduction of Aromatic Nitro Compounds and Synthesis of Hydrazo Compounds. Yakugaku Zasshi 1977, 97, 1253–1256. [Google Scholar] [CrossRef]
- Huang, S.-L.; Chen, T.-Y. Reduction of Organic Compounds with Thiourea Dioxide II. The Reduction of Organic Nitrogen Compounds. J. Chin. Chem. Soc. 1975, 22, 91–94. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Central Science 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: from curiosity to established protocols. Chemical Society Reviews 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer? Chem. sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Howard, J.L.; Brand, M.C.; Browne, D.L. Switching chemoselectivity: using mechanochemistry to alter reaction kinetics. Ang. Chem. 2018, 130, 16336–16340. [Google Scholar] [CrossRef]
- Lewis, D.; Mama, J.; Hawkes, J. An Investigation into the Structure and Chemical Properties of Formamidine Sulfinic Acid. Appl. Spectrosc. 2014, 68, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-C.; Lu, Y.-T.; Mai, Y.-W.; Zhang, C.; Xia, J.; Yao, P.-F.; Wang, H.-G.; Huang, S.-L.; Huang, Z.-S. Design, synthesis and biological evaluation of novel perimidine o-quinone derivatives as non-intercalative topoisomerase II catalytic inhibitors. Bioorg. Chem. 2019, 91, 103131. [Google Scholar] [CrossRef] [PubMed]
- Dymińska, L. Imidazopyridines as a source of biological activity and their pharmacological potentials—Infrared and Raman spectroscopic evidence of their content in pharmaceuticals and plant materials. Bioorg. Med. Chem. 2015, 23, 6087–6099. [Google Scholar] [CrossRef]
- Scribner, A.; Dennis, R.; Hong, J.; Lee, S.; McIntyre, D.; Perrey, D.; Feng, D.; Fisher, M.; Wyvratt, M.; Leavitt, P.; et al. Synthesis and biological activity of imidazopyridine anticoccidial agents: part I. Eur. J. Med. Chem. 2007, 42, 1334–1357. [Google Scholar] [CrossRef]
- Blazquez-Barbadillo, C.; González, J.F.; Porcheddu, A.; Virieux, D.; Menéndez, J.C.; Colacino, E. Benign synthesis of therapeutic agents: domino synthesis of unsymmetrical 1,4-diaryl-1,4-dihydropyridines in the ball-mill. Green Chem. Lett. Rev. 2022, 15, 881–892. [Google Scholar] [CrossRef]
- Makarov, S.V.; Sal’nikov, D.S.; Pogorelova, A.S. Acid-base properties and stability of sulfoxylic acid in aqueous solutions. Russ. J. Inorg. Chem. 2010, 55, 301–304. [Google Scholar] [CrossRef]
- Büeseken, J. Étude sur les Oxydes de Thiourée, I. Sur le dioxyde de thiourée. Recl. Trav. Chim. Pays-Bas 1936, 55, 1040–1043. [Google Scholar] [CrossRef]
- Sullivan, R.A.L.; Hargreaves, A. The crystal and molecular structure of thiourea dioxide. Acta Crystallogr. 1962, 15, 675–682. [Google Scholar] [CrossRef]
- Kis, Z.; Makarov, S.V.; Silaghi-Dumitrescu, R. Computational investigations on the electronic structure and reactivity of thiourea dioxide: sulfoxylate formation, tautomerism and dioxygen liberation. J. Sulfur Chem. 2010, 31, 27–39. [Google Scholar] [CrossRef]
- Grady, B.J.; Dittmer, D.C. Reaction of perfluoroaryl halides with reduced species of sulfur dioxide (HSO2−, SO22−, S2O42−). J. Fluor. Chem. 1990, 50, 151–172. [Google Scholar] [CrossRef]
- Krug, P. Thiourea Dioxide (Formamidinesulphinic Acid) A New Reducing Agent for Textile Printing. J. Soc. Dye. 1953, 69, 606–611. [Google Scholar] [CrossRef]
- Makarov, S.V.; Horváth, A.K.; Silaghi-Dumitrescu, R.; Gao, Q. Recent Developments in the Chemistry of Thiourea Oxides. Chem. Eur. J. 2014, 20, 14164–14176. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, H.F.; Mattern, J.A.; Fernelius, W.C. Sulfites and Pyrosulfites of the Alkali Metals. In Inorg. Synth.; Inorganic Syntheses; 1946; pp. 162-167.
- Miller, A.E.; Bischoff, J.J.; Pae, K. Chemistry of aminoiminomethanesulfinic and-sulfonic acids related to the toxicity of thioureas. Chem. Res. Toxicol. 1988, 1, 169–174. [Google Scholar] [CrossRef]
- Surasani, S.R.; Maity, S. Deciphering Intermediates and Additives Effect on the Reduction of Nitrobenzene by SmI2. ChemistrySelect 2017, 2, 598–603. [Google Scholar] [CrossRef]
- Böeseken, J. Etude sur les Oxydes de Thiouree. IV. Recl. Trav. Chim. Pays-Bas 1948, 67, 603–621. [Google Scholar] [CrossRef]
- Knopp, C. Zur verwendung von aminoiminomethanesulfinsaure ais antioxidans. Sci. Pharm 1983, 51, 283–290. [Google Scholar]
- Brown, D. A new synthesis of formamidine. J. Appl. Chem. 1952, 2, 202–203. [Google Scholar] [CrossRef]
- Dunitz, J.D. The structure of sodium dithionite and the nature of the dithionite ion. Acta Crystallogr. 1956, 9, 579–586. [Google Scholar] [CrossRef]
- Hartwig, U.; Pritzkow, H.; Rall, K.; Sundermeyer, W. Bis (trifluoromethyl) sulfene (CF3)2C SO2, Isolated as Adduct. Angew. Chem., Int. Ed. Engl. 1989, 28, 221–223. [Google Scholar] [CrossRef]
- Weber, H.P.; Craven, B.M. Structure and charge density of the 1: 1 complex of thiourea with parabanic acid at 298 K. Acta Crystallogr. B: Struct. Sci. 1987, 43, 202–209. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, N.L.; Pai, C.T. Charge density study of thiourea S, S-dioxide. Inorg. Chem. 1990, 29, 3256–3259. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. Benzimidazole: Journey from Single Targeting to Multitargeting Molecule. In Key Heterocycle Cores for Designing Multitargeting Molecules; Elsevier: 2018; pp. 31-52.
- Wang, S.; Gao, Q.; Wang, J. Thermodynamic analysis of decomposition of thiourea and thiourea oxides. J. Phys. Chem. B 2005, 109, 17281–17289. [Google Scholar] [CrossRef]
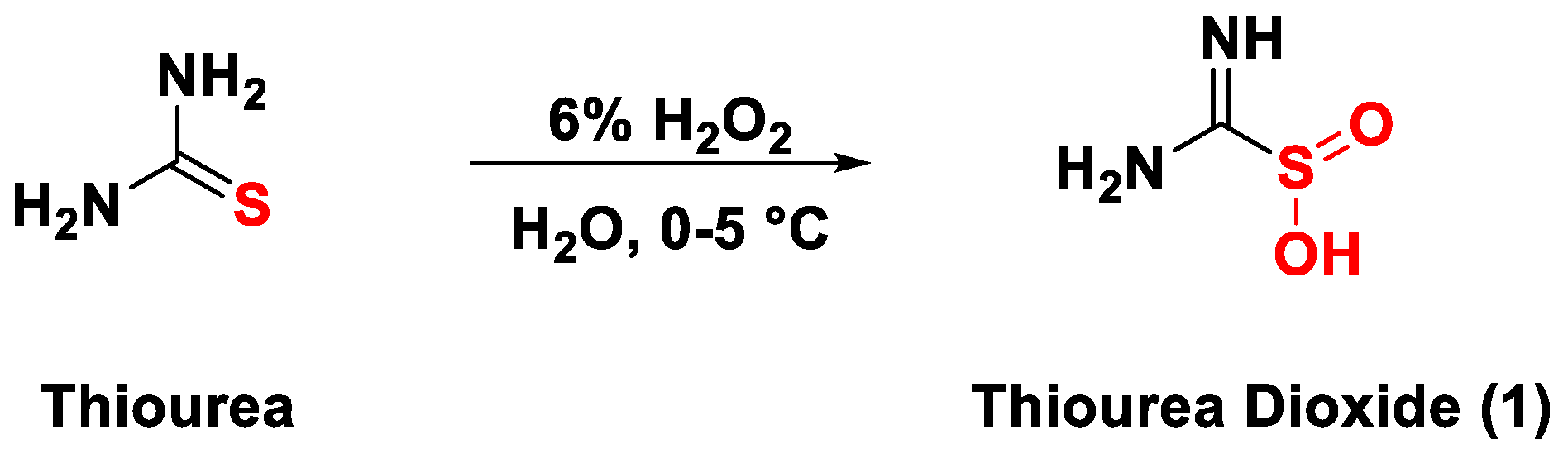


| Entry | TDO eq. | Base eq. | Reaction Time (h) | Additivesb | Yieldsa |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | / | 0% |
| 2 | 3 | 6 | 1 | / | 2% |
| 3 | 6 | 6 | 1 | / | 21% |
| 4 | 6 | 6 | 2 | / | 5% |
| 5 | 10 | 10 | 2 | / | 29% |
| 6c | 6 | 6 | 2 | / | 0% |
| 7 | 3 | 6 | 1.5 | Decane | 2% |
| 8 | 3 | 6 | 1.5 | Toluene | 4% |
| 9 | 3 | 6 | 1.5 | iPrOH | 3% |
| 10 | 3 | 6 | 1.5 | Acetone | 5% |
| 11d | 3 | 6 | 1.5 | MeOH | Complex Mixture |
| 12 | 3 | 6 | 1.5 | H2O | 89% |
| 13e | 3 | 6 | 1.5 | H2O | 0% |
| 14 | 3 | 6 | 2 | H2O | 97% |
| 15f | 3 | 6 | 1.5 | H2O | 20% |
| 16g | 3 | 6 | 1.5 | H2O | 1% |
| 17h | 3 | 3 | 2 | H2O | <5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





