1. Introduction
The study on solvate formation in crystalline compounds is becoming increasingly a significant topic, given the academic and industrial interest in elucidating the properties and variations in morphology of different crystal forms [
1,
2]. Many times the crystallization solvent can explain the observed changes in physical properties of a crystalline material, and this investigation can assist in the ongoing efforts to improve the design of crystallization processes from solution [
1,
2,
3,
4,
5].
In the coordination chemistry of Re(IV), the polymophism and the effect of the solvent on the magnetic properties of Re(IV) complexes have been relatively little studied [
6,
7]. The mononuclear complexes based on halides of Re(IV) ion have been investigated during decades and are characterized by large values of magnetic anisotropy and significant intermolecular magnetic exchange [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Indeed, these compounds can display relatively strong dipolar exchange through Re–X···X–Re type contacts (X = halogen), which can result in a magnetic order as, for instance, metamagnetism or spin canting [
23,
24,
25].
The neutral mononuclear [ReCl
4(bpym)] complex has been previously studied, showing remarkable properties [
26]. It displays potent in vitro anti-proliferative activity against a series of cancer cells [
27]. Regarding its magnetic properties, it exhibits magnetic ordering below 7.0 K through the spin-canting phenomenon, with values of the coercive field (Hc) and remanent magnetization (Mr) of 1750 G and 0.05 μB, respectively [
28]. Besides, it has been employed as starting material for the preparation of rhenium-based compounds as, for instance, the first heterodinuclear 2,2′-bipyrimidine-bridged complex magnetostructurally studied, of the formula [ReCl
4(μ-bpym)NiBr
2(H
2O)
2] [
29], and the chiral, photoluminescent, and spin-canted compound of formula {CuReCl
4(μ-Cl)(μ-pyz)[ReCl
4(μ-bpym)]}·MeNO
2 [
30]. More recently, two one-dimensional coordination polymers of general formula {[ReCl
4(μ-bpym)CuX
2]·solvent}
n have been characterized structurally and magnetically [
31].
To develop our investigation on the effect of the solvent and the intermolecular interactions on the magnetic properties of Re(IV) compounds, we herein report the synthesis, crystal structure and magnetic properties of two solvated Re(IV) complexes based on the 2,2′-bipyrimidine ligand, with formula [ReCl4(bpym)]·MeCN (1) and [ReCl4(bpym)]·CH3COOH·H2O (2), which are compared with the unsolvated complex.
2. Results and Discussion
2.1. Description of the crystal structures
The crystal structures of
1 and
2 were studied through the single-crystal X-ray diffraction technique. Both compounds crystallize in the monoclinic crystal system with space groups P2
1/n and P2
1/c, respectively (
Table 1). The not solvated [ReCl
4(bpym)] complex crystallizes in the orthorhombic space group P2
12
12
1 [
26]. The crystal structure of
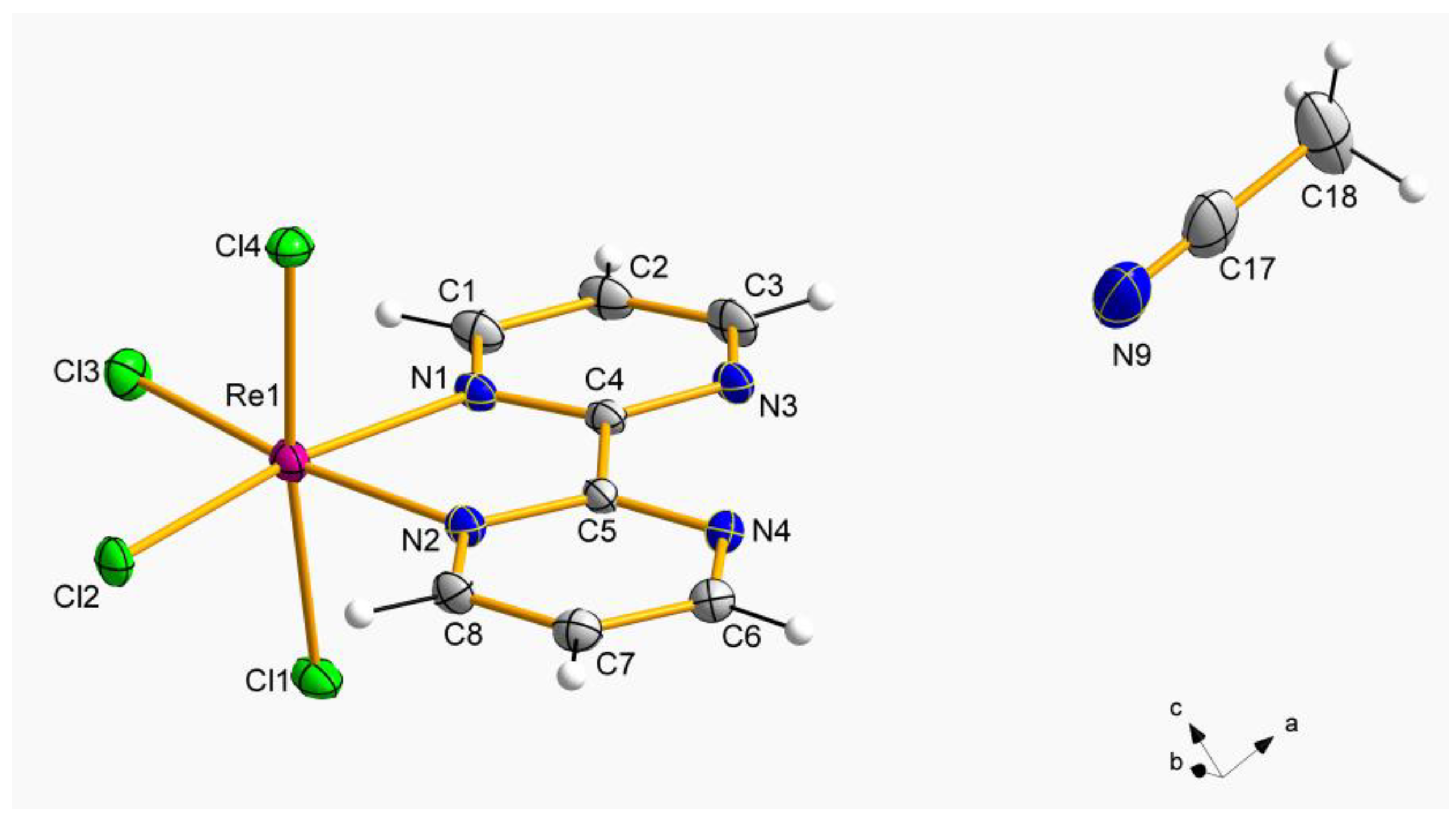
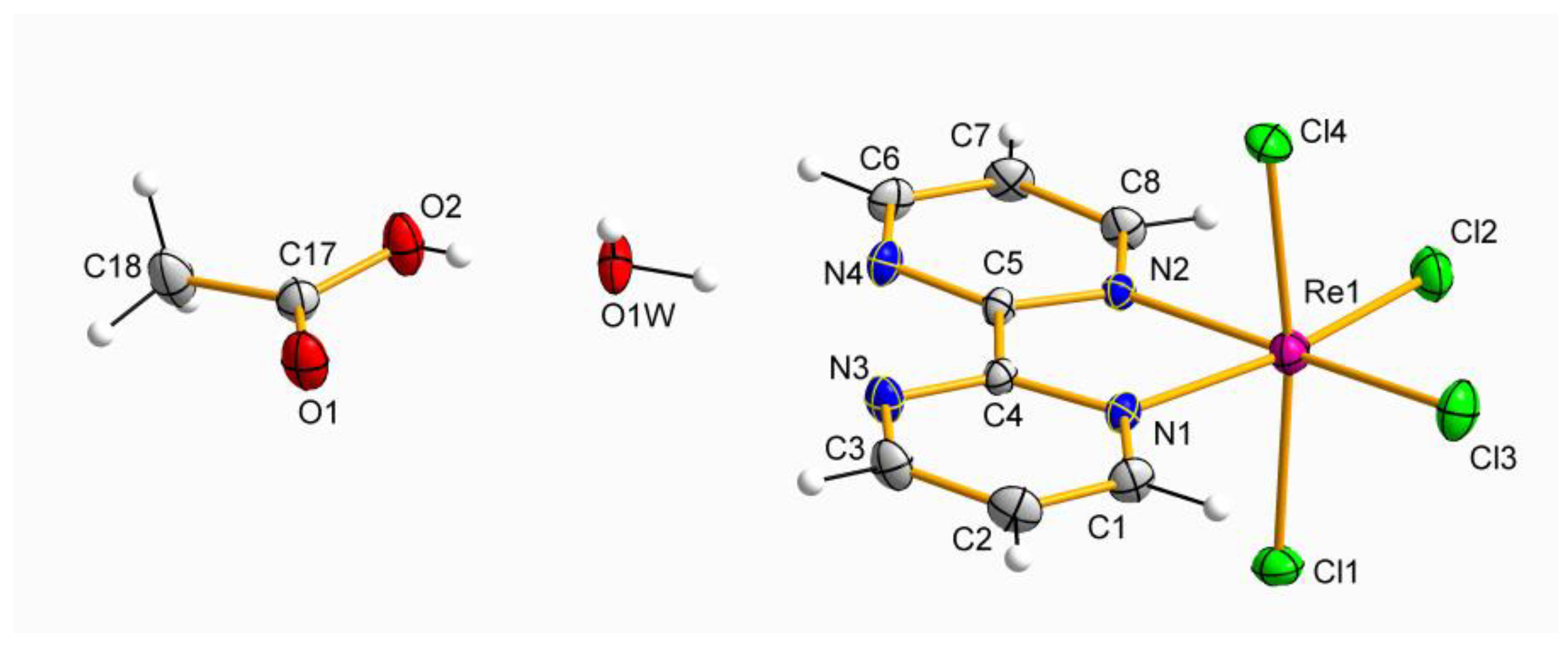
1 is made up of neutral [ReCl
4(bpym)] complexes and MeCN molecules, whereas the crystal structure of
2 is based on [ReCl
4(bpym)], CH
3COOH and H
2O molecules, as showed in their respective asymmetric units (
Figure 1 and
Figure 2).
In the mononuclear [ReCl
4(bpym)] complexes of
1 and
2, each Re(IV) ion is bonded to four chloride ions and two nitrogen atoms of a 2,2′-bipyrimidine molecule forming a distorted octahedral geometry around the metal ion. In both compounds, the average values of the Re–N bond lengths [2.129(1) Å for
1 and 2.137(1) Å for
2] are shorter than those of the Re–Cl bond lengths [2.317(1) Å for
1 and 2.308(1) Å for
2], displaying values which are in agreement with those previously published for similar Re(IV) complexes [
26,
32]. Besides, the Re-Cl bond lengths in axial positions are longer than those located in equatorial positions in both
1 and
2 (
Figure 1 and
Figure 2). The chelating 2,2′-bipyrimidine molecule is pretty much planar in both compounds, and exhibit average C−C, N−N, and C−N bond length values in agreement with those found in similar bpym-based complexes containing 4d and 5d metal ions [
33,
34].
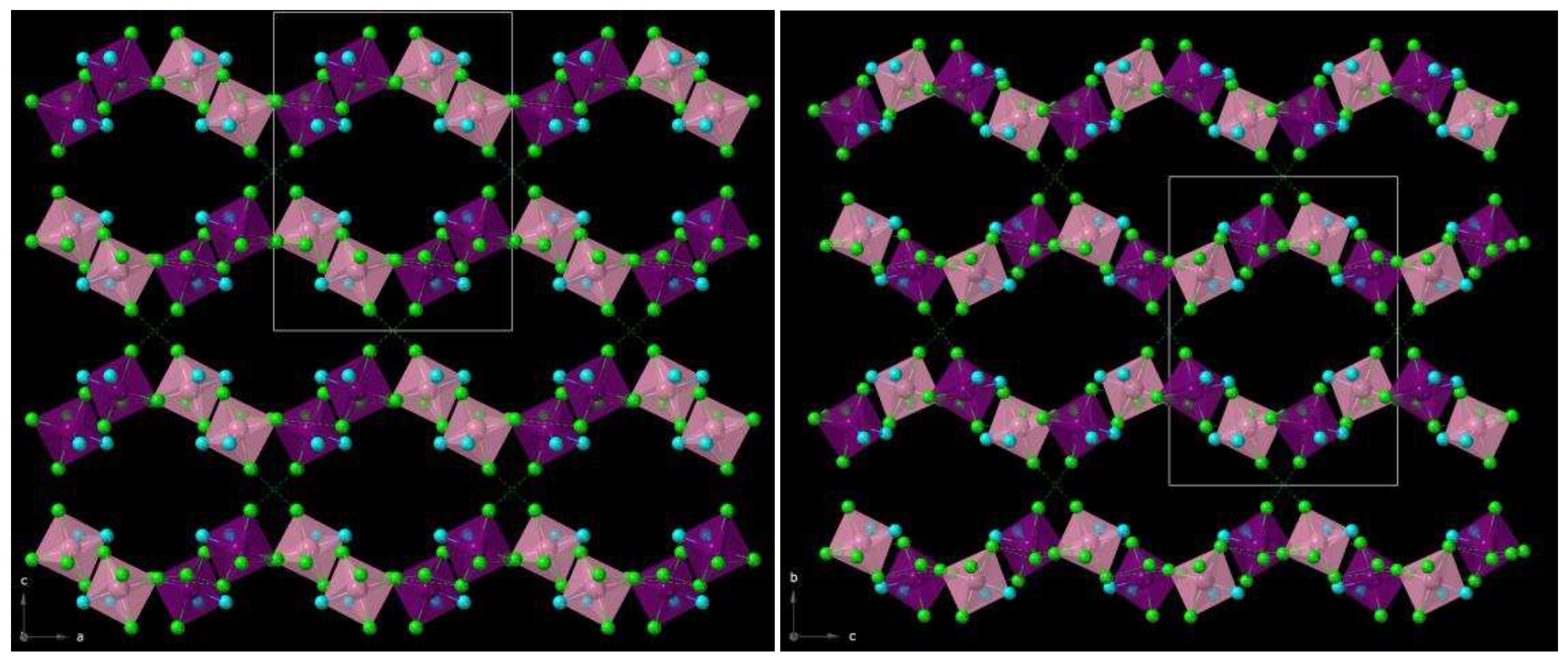
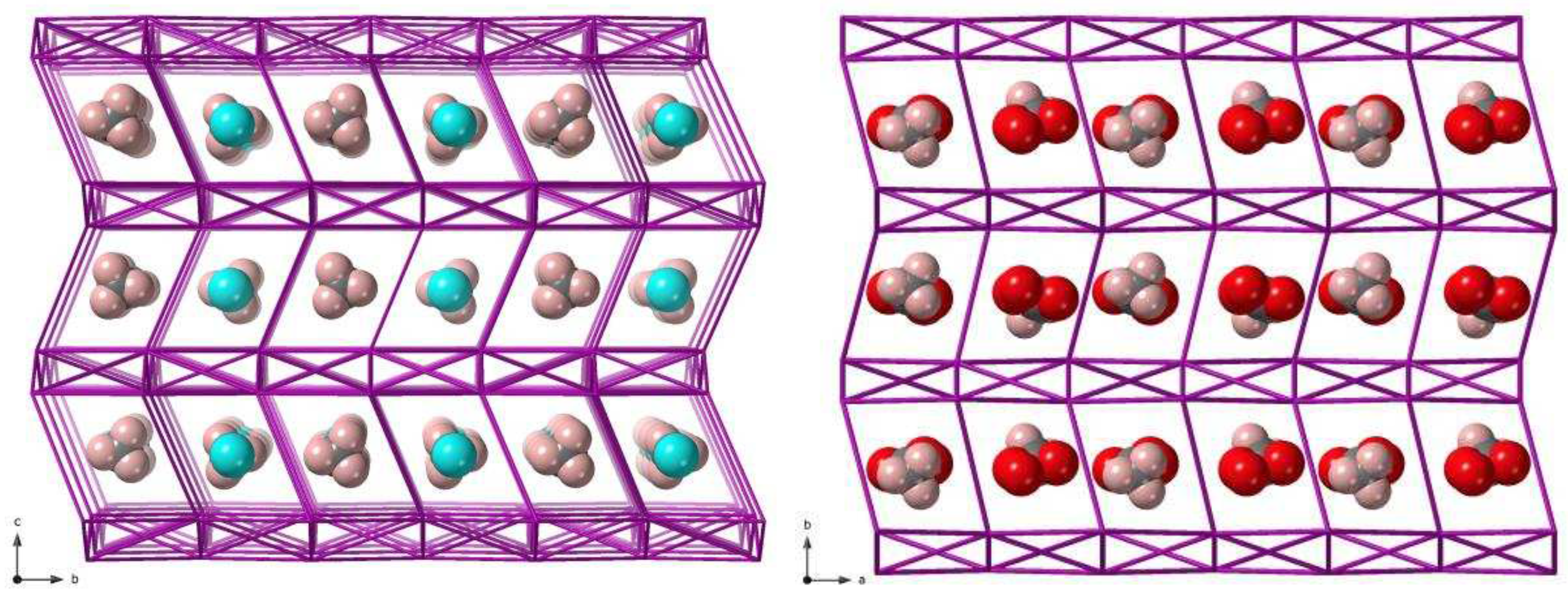
In the crystal packing of
1, intermolecular short Cl···Cl contacts of 3.559(1) Å [Cl(3)···Cl(7a) distance with (a) = x−1, y, z] and 3.560(1) Å [Cl(2)···Cl(6b) distance with (b) = x−1, y−1, z] direct alternate chains formed by complexes of Re(1) and Re(2) ions, which grow along the crystallographic c axis (
Figure 3). These chains are interlinked with each other through C-H···N interactions involving neighboring bpym ligands [C(15c)···N(4) distance being ca. 3.50 Å; (c) = x, y−1, z], and also with somewhat longer Cl···Cl contacts of ca. 3.667(1) Å, resulting in a corrugated layered structure that grows in the ac plane (
Figure 3). Further C-H···N interactions [C(6d)···N(9) distance being ca. 3.62 Å; (d) = −x+1, −y+2, −z+1] occur between [ReCl
4(bpym)] complexes and MeCN solvent molecules. These intermolecular interactions, along with π···Cl contacts of ca. 3.283(1) Å and several weak C-H···Cl interactions, draw together a 3D crystal structure in compound
1.
In the crystal packing of
2, several O-H···O and O-H···N hydrogen bonds connect the solvent molecules CH
3COOH and H
2O to the [ReCl
4(bpym)] complexes [O1w···N(7a) distance of ca. 2.866 Å; (a) = -x+1, y-1/2, -z+3/2] (
Table 2). As in
1, chains formed through alternate complexes of Re(1) and Re(2) ions are generated by short Cl···Cl contacts (
Figure 3). In the case of
2, this type of halogen···halogen bonds are shorter than those found in
1 [the shortest one being Cl(3)···Cl(7b) with distance of ca. 3.309 Å and (b) = x+1, -y+3/2, z+1/2] (
Figure 3). Further Cl···Cl contacts of approximately 3.708 Å, together with C-H···N interactions between bipyrimidine rings of adjacent [ReCl
4(bpym)] complexes, link the chains forming 2D sheets [C(6c)···N(8) distance of ca. 3.507 Å; (c) = x, -y+3/2, z+1/2] (
Figure 4).
In
2, there are π···Cl contacts, involving bipyrimidine rings and chloride anions, which are of approximately 3.283(1) Å, and also weak C-H···Cl interactions, which implicate the methyl group of the CH
3COOH molecules and chloride anions of the [ReCl
4(bpym)] complexes [C(18)···Cl(5d) distance of ca. 3.617 Å; (d) = x+1, -y+3/2, z-1/2]. These last intermolecular interactions also contribute to stabilizing the crystal structure in compound
2 (
Figure 4).
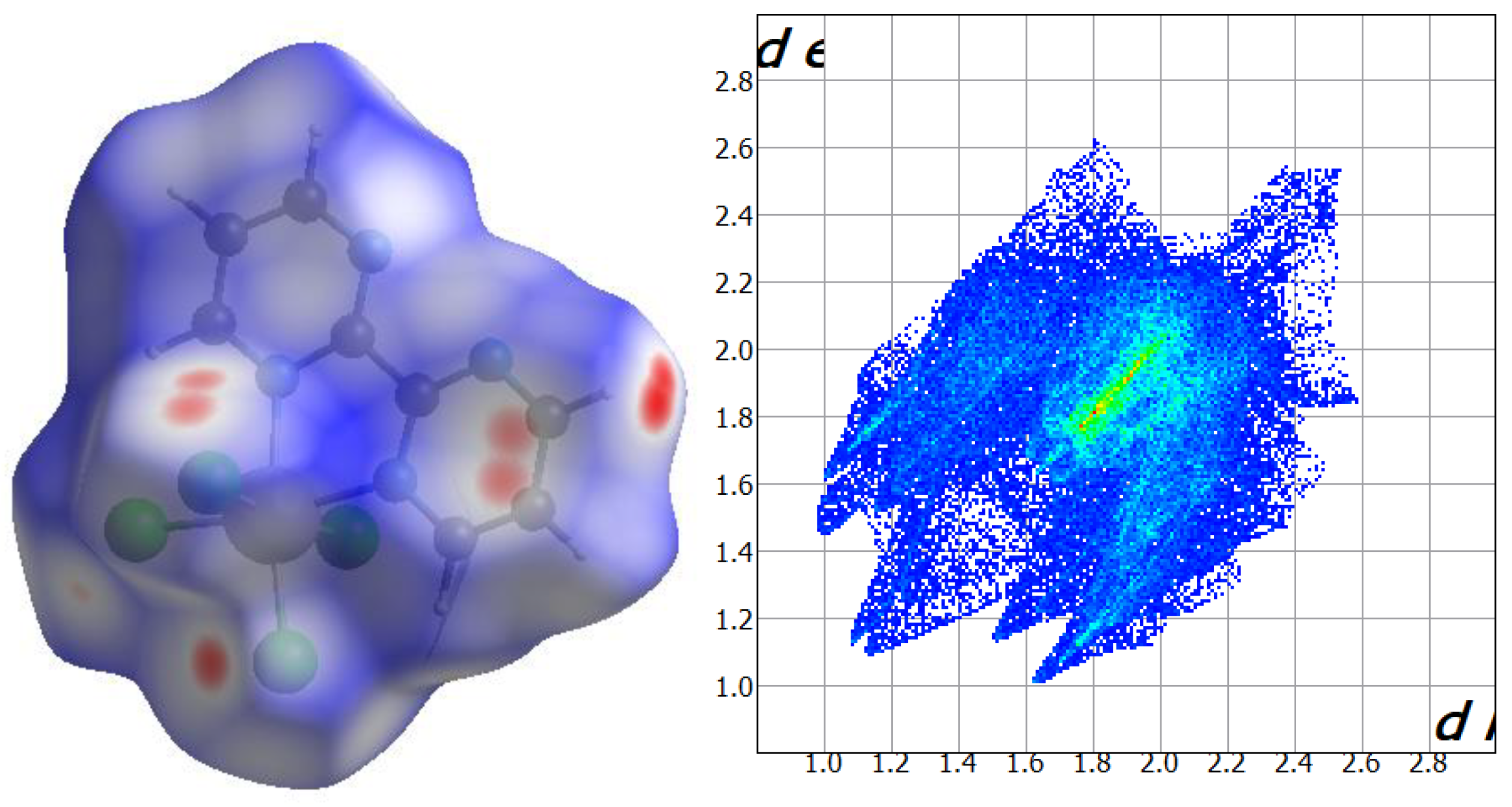
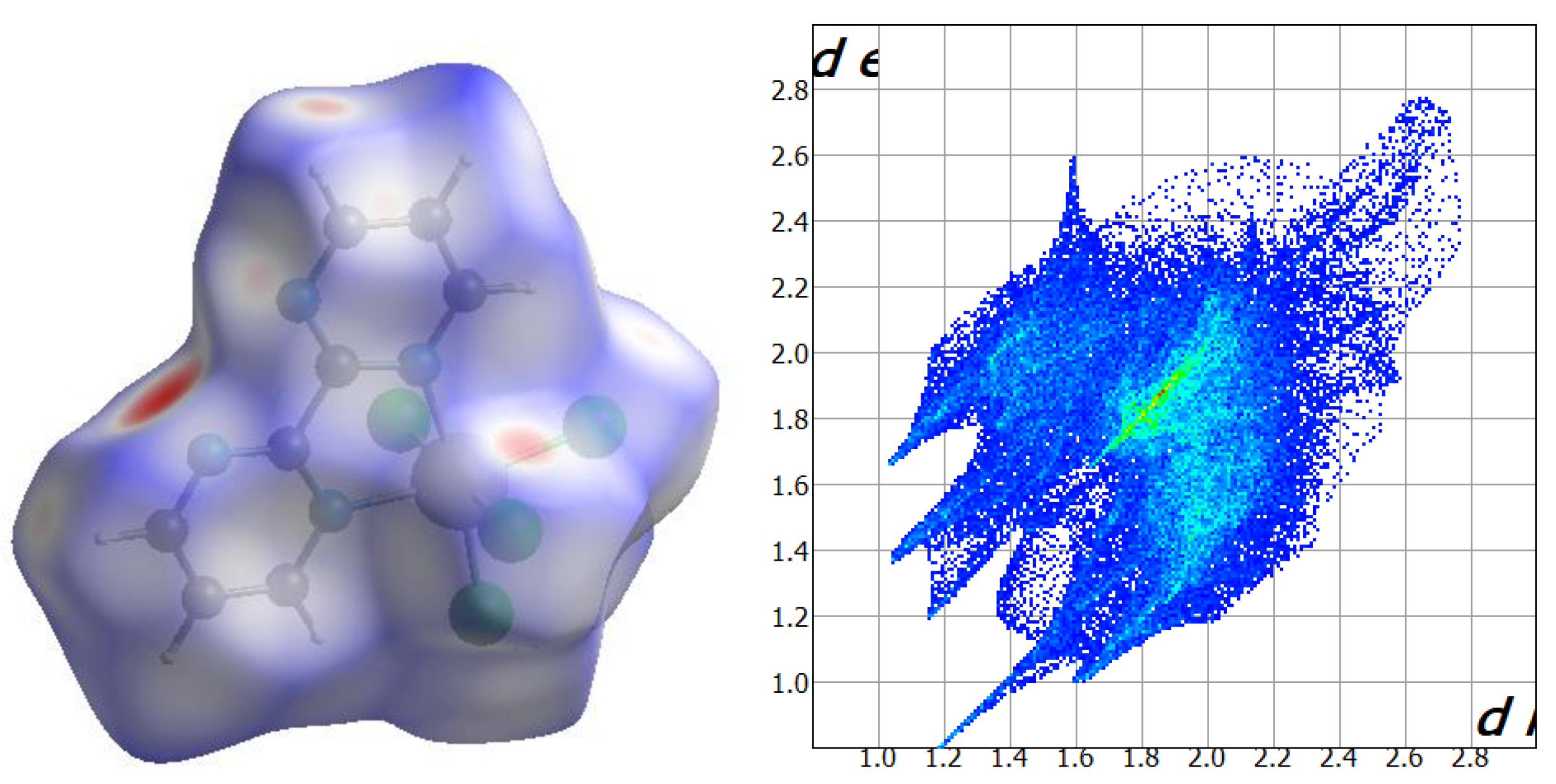
2.2. Hirshfeld Surface Analysis
Hirshfeld surfaces of the neutral [ReCl
4(bpym)] complex in compounds
1 and
2 were calculated and their close intermolecular interactions were analyzed through the CrystalExplorer program [
35,
36,
37]. CrystalExplorer calculates a series of surfaces which allow obtaining a both qualitative and quantitative visualization of the main intermolecular interactions, in this case, taking place in
1 and
2, the shorter contacts being shown with red color [
38,
39]. This is performed by mapping the distances from the surface to the nearest atom outside (d
e) and inside (d
i) of each surface and, at the same time, considering a normalized contact distance (d
norm) that takes into account some limitations generated by the atomic radii [
35,
36,
37]. The Hirshfeld surfaces for compounds
1 and
2 are given in
Figure 5 and
Figure 6, respectively. According to their fingerprint plots, both
1 and
2 show a very similar percentage regarding the intermolecular Cl···Cl contacts between adjacent [ReCl
4(bpym)] complexes [ca. 6.4% for
1 and ca. 6.3% for
2], which is consistent with the akin framework based on halogen bonds that both compounds exhibit (
Figure 7). The Cl···H contacts connecting mainly chloride anions and C-H groups of neighboring [ReCl
4(bpym)] complexes in
1 and
2, and also the methyl groups of CH
3COOH molecules in the case of
2, are the main interactions observed on the Hirshfeld surfaces for both compounds, which cover ca. 34.9% for
1 and ca. 39.2% for
2 on their respective fingerprint plots (
Figure 5 and
Figure 6). Finally, further N···H contacts involving solvent molecules, mainly MeCN (in
1) and H
2O (in
2), and N atoms and C-H groups of adjacent bipyrimidine rings are approximately 11.6% and 9.7% of the complete fingerprint plot of
1 and
2, respectively (
Figure 5 and
Figure 6).
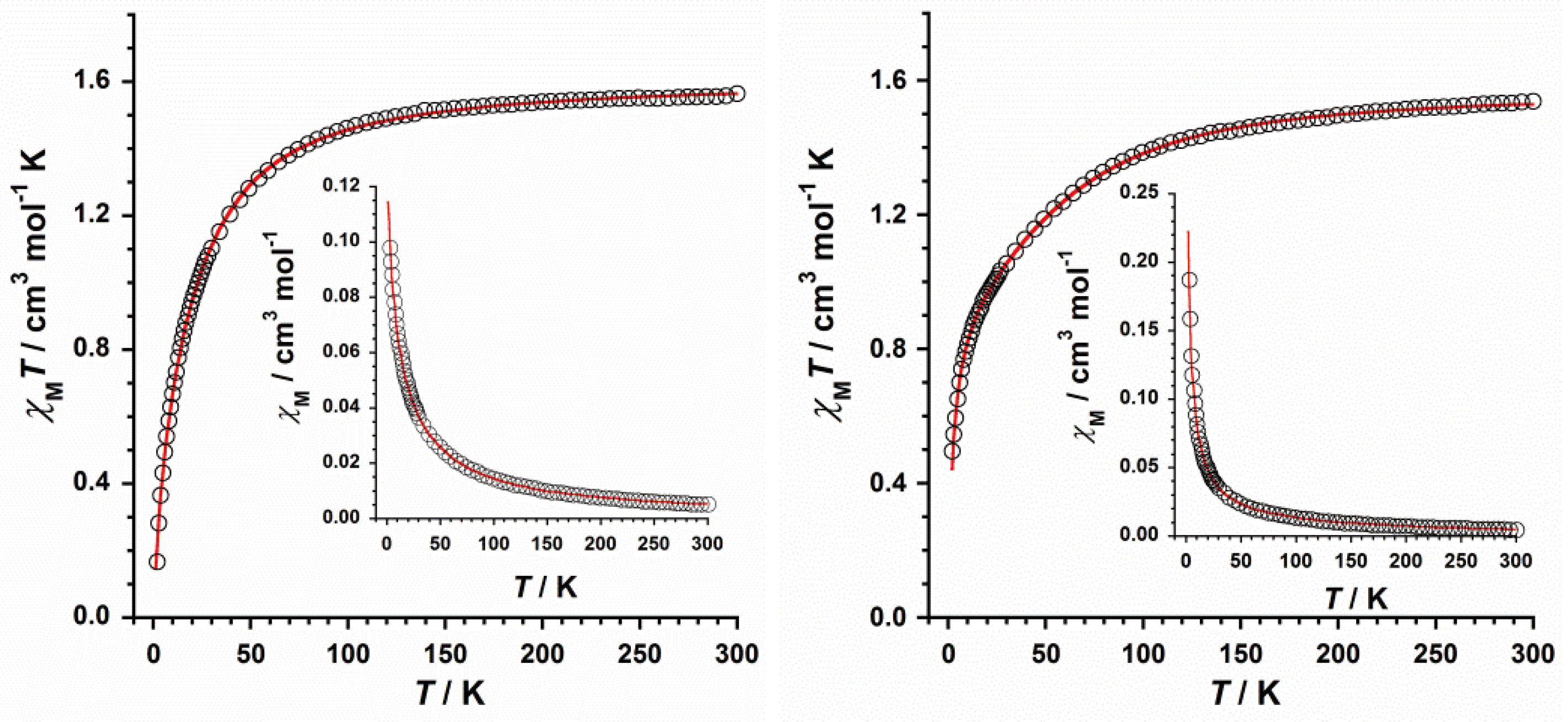
2.3. Magnetic Properties
Dc magnetic susceptibility measurements were performed on microcrystalline samples of
1 and
2 in the 2-300 K temperature range and under an external magnetic field of 0.5 T. The resulting χ
MT versus T plots (χ
M being the molar magnetic susceptibility per mononuclear Re(IV) complex) for compounds
1 and
2 are shown in
Figure 8. At 300 K, the χ
MT values are
ca. 1.57 (
1) and
ca. 1.54 cm
3mol
-1K (
2), which are very close to those earlier published for magnetically isolated complexes based on Re(IV) ion (5d
3 ion with S = 3/2) [
7]. Upon cooling, the curve that the χ
MT values draw for
1 follow the Curie law with decreasing temperature to approximately 100 K, before they decrease reaching minimum values of approximately 0.17 cm
3mol
-1K at 2.0 K (
Figure 8). In the case of
2, the χ
MT values decrease gradually with decreasing temperature, and more abruptly at approximately 50 K, reaching a minimum value of 0.49 cm
3mol
-1K at 2.0 K (
Figure 8). The decrease of the χ
MT value observed for both
1 and
2 would be assignable to antiferromagnetic interactions as well as zero-field splitting (ZFS) effects [
40,
41]. No maximum of the magnetic susceptibility is observed for either compound, as shown in their respective χ
M versus T plots (insets in
Figure 8).
As indicated in the description of the crystal structure of
1 and
2, both compounds exhibit short Cl···Cl contacts between the paramagnetic [ReCl
4(bpym)] complexes in their crystal lattices (
Figure 3). Hence, these relatively important through-space interactions between Re(IV) ions precludes the occurrence of SIM behavior [
40,
41]. Besides, the presence of solvent molecules changes drastically the magnetic properties, given that the unsolvated [ReCl
4(bpym)] complex exhibits magnetic ordering through spin-canting, as previously reported [
7,
28], a magnetic behaviour which is not observed neither in
1 nor in
2.
To analyze the magnetic behavior of
1 and
2, we have employed the Hamiltonian of equation (1) and its derived theoretical expression for the magnetic susceptibility, equation (2), by including a θ term to account for the observed intermolecular interactions, where
Ŝz is the easy-axis spin operator,
H is the applied field,
β is the Bohr magneton, the
g parameter is the Landé factor and
D is the zero-field splitting (ZFS) for the Re(IV) ion [
7]. Thus, the first term in equation (1) corresponds to the ZFS and the second term to the Zeeman effects. Besides, we have assumed that
gǀǀ =
g⊥ =
g for complexes
1 and
2. The best least-squares fit gave the parameters
D = 31.9 cm
-1,
g = 1.86, θ = -5.3 K and
R = 2.94·10
-5 for
1 and
D = 35.8 cm
-1,
g = 1.83, θ = - 8.8 K and
R = 4.52·10
-5 for
2 {
R being the agreement factor defined as Σ
i[(χ
MT)
iobs − (χ
MT)
icalcd]
2/[(χ
MT)
iobs]
2}. As shown in
Figure 8, the calculated curves (solid red lines) reproduce quite well the experimental magnetic data in the whole temperature range. The
D and
g values calculated for
1 and
2 are in agreement with those earlier reported for mononuclear Re(IV) complexes [
7,
8]. The sign and magnitude of the θ values corroborate the presence of relatively strong antiferromagnetic exchanges between the Re(IV) ions connected through intermolecular Re-Cl···Cl-Re pathways (
Figure 3), with the shorter interactions in
2 [ca. 3.6 Å (in
1)
versus ca. 3.3 Å (in
2)] resulting in stronger antiferromagnetic exchange.
3. Experimental Section
3.1. Materials
All manipulations were performed under aerobic conditions, using all the solvents and chemicals as received. The Re(IV) precursors, [ReCl
4(bpym)] [
26] and (NBu
4)
2[ReCl
4(ox)] [
42], were prepared following literature procedures.
3.2. Preparation of the complexes
3.2.1. Synthesis of [ReCl4(bpym)]·MeCN (1)
1 was prepared by dissolving [ReCl4(bpym)] (0.05 mmol, 24.3 mg) in 2 mL of a N,N-dimethylformamide-acetonitrile (1:1, v/v) mixture, followed by a slow diffusion in isopropanol at room temperature. Brown crystals of 1 were thus obtained in a few days and were suitable for single-crystal X-ray diffraction studies. Yield: ca. 75%. Anal. Calcd. for C10H9N5Cl4Re (1): C, 9.5; H, 3.2; N, 5.5. Found: C, 9.9; H, 3.0; N, 5.3. SEM-EDAX: a molar ratio of 1:4 for Re/Cl was found for 1. IR (KBr pellet) peaks are observed at 3100 (m), 3067 (s), 2245 (m), 1578 (vs), 1541 (m), 1453 (w), 1407 (vs), 1269 (w), 1213 (m), 1113 (m), 1021 (s), 987 (w), 818 (m), 747 (s), 693 (m), 665 (s), 490 (w), 440 (w) cm-1.
3.2.2. Synthesis of [ReCl4(bpym)]·CH3COOH·H2O (2)
2 was prepared through ligand substitution of the previously reported (NBu4)2[ReCl4(ox)] complex. (NBu4)2[ReCl4(ox)] (0.12 mmol, 110 mg) and 2,2′-bipyrimidine (0.25 mmol, 39.5 mg) were mixed in glacial acetic acid (4.0 mL). The mixture was heated to 100 °C and stirred for 4h. The resulting solution was filtered while hot and left to evaporate at room temperature. Dark orange crystals of 2 were grown in less than 1 week, and were suitable for X-ray diffraction data collection. Yield: ca. 60%. Anal. Calcd for C10H12N4O3Cl4Re (2): C, 21.3; H, 2.1; N, 9.9. Found: C, 21.4; H, 2.0; N, 9.6. SEM-EDAX: a molar ratio of 1:4 for Re/Cl was found for 2. IR (KBr pellet) peaks are observed at 3424 (br), 3091 (m), 3066 (m), 1711 (m), 1579 (vs), 1547 (m), 1452 (w), 1436 (w), 1406 (vs), 1303 (w), 1258 (m), 1211 (w), 1109 (m), 1024 (s), 989 (w), 877 (w), 819 (m), 748 (s), 695 (m), 669 (m), 617 (w), 515 (w) cm-1.
3.3. X-ray data collection and structure refinement
X-ray diffraction data collection on single crystals of dimensions 0.28 x 0.21 x 0.21 (
1) and 0.17 x 0.16 x 0.06 mm
3 (
2) were collected on a Bruker D8 Venture diffractometer with PHOTON II detector and by using monochromatised Mo-K
α radiation (λ = 0.71073 Å). Crystal parameters and refinement results for
1 and
2 are summarized in
Table 1. The structures were solved by standard direct methods and subsequently completed by Fourier recycling using the SHELXTL [
43] software packages and refined by the full-matrix least-squares refinements based on F
2 with all observed reflections. The final graphical manipulations were performed with the DIAMOND [
44] and CRYSTALMAKER [
45] programs. CCDC 2233243 and 2233244 for
1 and
2, respectively.
3.4. Physical measurements
Elemental analyses (C, H, N) were performed in an Elemental Analyzer CE Instruments CHNS1100 and the molar ratio between heavier elements was found by means of a Philips XL-30 scanning electron microscope (SEM-EDAX), equipped with system of X-ray microanalysis, in the Central Service for the Support to Experimental Research (SCSIE) at the University of Valencia. Infrared spectra (IR) of
1 and
2 were recorded with a PerkinElmer Spectrum 65 FT-IR spectrometer in the 4000-400 cm
-1 region. Variable-temperature, solid-state dc magnetic susceptibility data were collected on a Quantum Design MPMS-XL SQUID magnetometer equipped with a 5 T dc magnet. Experimental magnetic data were corrected for the diamagnetic contributions of both the sample holder and the eicosene. The diamagnetic contribution of the involved atoms was corrected by using Pascal’s constants [
46].
4. Conclusions
In summary, the synthesis, crystal structure and magnetic properties of two novel solvated Re(IV) complexes based on the 2,2′-bipyrimidine ligand, with formula [ReCl4(bpym)]·MeCN (1) and [ReCl4(bpym)]·CH3COOH·H2O (2), have been reported. In the crystal structures of 1 and 2, there are present intermolecular halogen⋯halogen and π⋯halogen type interactions and only in compound 2 there exist O-H···O and O-H···N hydrogen bonds. Having into account the presence of Re-Cl···Cl-Re contacts, both compounds exhibit a corrugated crystal framework, which host the solvent molecules. The investigation of the magnetic properties of 1 and 2 through dc magnetic susceptibility measurements reveals a similar magnetic behavior, since both compounds display antiferromagnetic exchange couplings between Re(IV) ions. The magnetic properties can be drastically modified by the presence of solvent molecules, given that the unsolvated [ReCl4(bpym)] complex exhibits a very different magnetic behaviour, that is, magnetic ordering through spin-canting, as previously reported. In this way, by changing suitable solvents, it is possible to tune the magnetic properties in this type of Re(IV) complexes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.M.-L.; funding acquisition, J.M.-L.; methodology, A.S.-P., M.O.-A., N.M. and J.M.-L.; investigation, A.S.-P., M.O.-A., N.M. and J.M.-L.; formal analysis, A.S.-P., M.O.-A., N.M. and J.M.-L.; writing-original draft preparation, J.M.-L.; writing-review and editing, J.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Spanish Ministry of Science and Innovation [Grant numbers PID2019-109735GB-I00 and CEX2019-000919-M (Excellence Unit “María de Maeztu”)].
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
A.S.-P. and M.O.-A. thank the Spanish “FPU fellowships” and “FPI fellowships” programs, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Price, C.P.; Glick, G.D.; Matzger, A.J. Dissecting the Behavior of a Promiscuous Solvate Former. Angew. Chem., Int. Ed. 2006, 45, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Takieddin, K.; Khimyak, Y.Z.; Fábián, L. Prediction of Hydrate and Solvate Formation Using Statistical Models. Cryst. Growth Des. 2016, 16, 70–81. [Google Scholar] [CrossRef]
- Braga, D.; Casali, L.; Grepioni, F. The Relevance of Crystal Forms in the Pharmaceutical Field: Sword of Damocles or Innovation Tools? Int. J. Mol. Sci. 2022, 23, 9013. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Armentano, D.; Mastropietro, T.F.; Julve, M.; Faus, J.; De Munno, G. Self-Assembled One- and Two-Dimensional Networks Based on NH2Me2[ReX5(DMF)] (X = Cl and Br) Species: Polymorphism and Supramolecular Isomerism in Re(IV) Compounds. Cryst. Growth Des. 2011, 11, 1733–1741. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, J. Towards multifunctional magnetic systems through molecular-programmed self assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289–290, 215–237. [Google Scholar] [CrossRef]
- Woodall, Ch.H.; Craig, G.A.; Prescimone, A.; Misek, M.; Cano, J.; Faus, J.; Probert, M.R.; Parsons, S.; Moggach, S.; Martínez-Lillo, J.; Murrie, M.; Kamenev, K.V.; Brechin, E.K. Pressure induced enhancement of the magnetic ordering temperature in rhenium(IV) monomers. Nat. Commun. 2016, 7, 13870. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Chiozzone, R.; Kremer, C.; Guerra, F.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexahalorhenate(IV) Salts of Ferrocenium Cations [Fe(C5R5)2]2[ReX6] (R = H, CH3; X = Cl, Br, I). Inorg. Chem. 2004, 43, 3013–3019. [Google Scholar] [CrossRef]
- Nelson, C.M.; Boyd, G.E.; Smith, W.T. Magnetochemistry of Technetium and Rhenium. J. Am. Chem. Soc. 1954, 76, 348–352. [Google Scholar] [CrossRef]
- Figgis, B.N.; Lewis, J.; Mabbs, F.E. The Magnetic Properties of Some d3-Complexes. J. Chem. Soc. 1961, 3138–3145. [Google Scholar] [CrossRef]
- Busey, R.; Sonder, E. Magnetic Susceptibility of Potassium Hexachlororhenate (IV) and Potassium Hexabromorhenate (IV) from 5° to 300° K. J. Chem. Phys. 1962, 36, 93–97. [Google Scholar] [CrossRef]
- Rouschias, G.; Wilkinson, G. The chemistry of rhenium–nitrile complexes. J. Chem. Soc. A 1968, 489–496. [Google Scholar] [CrossRef]
- Mroziński, J. Magnetic properties of methylammonium hexachlororhenate (4) salts in the range 1.5-300 K. Bull. Pol. Acad. Sci. Chem. 1978, 26, 789–798. [Google Scholar]
- Mroziński, J. Low temperature magnetic properties of some methylammonium hexabromorhenate (4) salts. Bull. Pol. Acad. Sci. Chem. 1980, 28, 559–567. [Google Scholar]
- Reynolds, P.A.; Moubaraki, B.; Murray, K.S.; Cable, J.W.; Engelhardt, L.M.; Figgis, B.N. Metamagnetism in tetrachlorobis(N-phenylacetamidine)rhenium(IV). J. Chem. Soc., Dalton Trans 1997, 263–268. [Google Scholar] [CrossRef]
- Małecka, J.; Jäger, L.; Wagner, C.; Mroziński, J. Preparation, crystal structure and properties of (Ph4P)2ReCl6·2CH3CN. Pol. J. Chem. 1998, 72, 1879–1885. [Google Scholar]
- Reynolds, P.A.; Figgis, B.N.; Martin y Marero, D.J. Chem. Soc., Dalton Trans. 1999, 945–950. [CrossRef]
- Thornton, P. 13 Manganese, technetium and rhenium. Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem. 2000, 96, 215–228. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef]
- Feng, X.; Liu, J.-L.; Pedersen, K.S.; Nehrkorn, J.; Schnegg, A.; Holldack, K.; Bendix, J.; Sigrist, M.; Mutka, H.; Samohvalov, D.; et al. Multifaceted magnetization dynamics in the mononuclear complex [ReIVCl4(CN)2]2-. Chem. Commun. 2016, 52, 12905–12908. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Sigrist, M.; Sørensen, M.A.; Barra, A.-L.; Weyhermüller, T.; Piligkos, S.; Thuesen, C.Aa.; Vinum, M.G.; Mutka, H.; Weihe, H.; Clérac, R.; Bendix, J. [ReF6]2−: A Robust Module for the Design of Molecule-Based Magnetic Materials. Angew. Chem. Int. Ed. 2014, 53, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Chiozzone, R.; Kremer, C.; De Munno, G.; Nicolò, F.; Lloret, F.; Julve, M.; Faus, J. Magnetic Studies on Hexaiodorhenate(IV) Salts of Univalent Cations. Spin Canting and Magnetic Ordering in K2[ReI6] with Tc = 24 K. Inorg. Chem. 2003, 42, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Kong, J.; Barros, W.P.; Faus, J.; Julve, M.; Brechin, E.K. Metamagnetic behaviour in a new Cu(II)Re(IV) chain based on the hexachlororhenate(IV) anion. Chem. Commun. 2014, 50, 5840–5842. [Google Scholar] [CrossRef]
- Louis-Jean, J.; Balasekaran, S.M.; Lawler, K.V.; Sanchis-Perucho, A.; Martínez-Lillo, J.; Smith, D.; Forster, P.M.; Salamat, A.; Poineau, F. Coexistence of metamagnetism and slow relaxation of magnetization in ammonium hexafluoridorhenate. RSC Adv. 2021, 11, 6353–6360. [Google Scholar] [CrossRef] [PubMed]
- Chiozzone, R.; González, R.; Kremer, C.; Cerdá, M.F.; Armentano, D.; De Munno, G.; Martínez-Lillo, J.; Faus, J. A novel series of rhenium-bipyrimidine complexes: synthesis, crystal structure and electrochemical properties. Dalton Trans. 2007, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lappano, R.; Madeo, A.; Alberto, M.E.; Russo, N.; Maggiolini, M.; De Munno, G. Rhenium(IV) compounds inducing apoptosis in cancer cells. Chem. Commun. 2011, 47, 5283–5285. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Lloret, F.; Julve, M.; Faus, J. Spin canting in Re(IV) complexes: magnetic properties of [ReX4(bpym)] (X = Cl and Br; bpym = 2,2′-bipyrimidine). J. Coord. Chem. 2008, 62, 92–99. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. First Magnetostructural Study on a Heterodinuclear 2,2′-Bipyrimidine-Bridged Complex. Inorg. Chem. 2011, 50, 12405–12407. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Armentano, D.; Fortea-Pérez, F.R.; Stiriba, S.E.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Chiral, Photoluminescent, and Spin-Canted {CuIReIV2}n Branched Chain. Inorg. Chem. 2015, 54, 4594–4596. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dotti, C.; Martínez-Lillo, J. Halogen⋯halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Faus, J. Magneto-structural study on a series of rhenium(IV) complexes containing biimH2, pyim and bipy ligands. Polyhedron 2008, 27, 1447–1454. [Google Scholar] [CrossRef]
- Roy, N.; Sen, U.; Moharana, P.; Babu, L.T.; Kar, B.; Vardhan, S.; Sahoo, S.K.; Bose, B.; Paira, P. 2,2′-Bipyrimidine-based luminescent Ru(II)/Ir(III)–arene monometallic and homo- and hetero-bimetallic complexes for therapy against MDA-MB-468 and caco-2 cells. Dalton Trans. 2021, 50, 11725–11729. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.-Y.; Ou, Y.-L.; Ye, B.-H. Asymmetric Synthesis of Enantiomerically Pure Mono- and Binuclear Bis(cyclometalated) Iridium(III) Complexes. Inorg. Chem. 2016, 55, 6018–6026. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17; University of Western Australia, 2017. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bu, R.; Gou, R.-J.; Zhang, Ch. Hirshfeld Surface Method and Its Application in Energetic Crystals. Cryst. Growth Des. 2021, 21, 6619–6634. [Google Scholar] [CrossRef]
- McAdams, S.G.; Ariciu, A.M.; Kostopoulos, A.K.; Walsh, J.P.S.; Floriana; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Chiozzone, R.; González, R.; Kremer, C.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. Synthesis, Crystal Structure, and Magnetic Properties of Tetraphenylarsonium Tetrachloro(oxalato)rhenate(IV) and Bis(2,2‘-bipyridine)tetrachloro(μ-oxalato)copper(II)rhenium(IV). Inorg. Chem. 1999, 38, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- SHELXTL-2013/4, Bruker Analytical X-ray Instruments, Madison, WI, 2013.
- Diamond 4.5.0, Crystal Impact GbR, CRYSTAL IMPACT, 2018.
- CrystalMaker 8.5.1, CrystalMaker Software Ltd, Oxford, England.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal's Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).