1. Introduction
Geriatric patients represent a common challenge in trauma surgery because of comorbidities and delayed healing processes. Recently, life expectancy has increased by an average of 3 months per year. Boys born in 1964 had an average life expectancy of 68 years, while in 2015, it was 77.9 years. The same effect was observed for girls, who had an average life expectancy of 74 years in 1964 and 82.8 years in 2015. Boysof [
1]. Undisputable, many trails confirm the assumption of a connection between a higher age and comorbidities [
2]. High age and comorbidities, for example Parkinson, are well known for an increased level of suffering low impact fractures by falls [
3]. Furthermore, low activity level, poor nutrition, and osteoporosis decrease the bone mass and increase the risk of fractures [
4]. Comorbidities present a major risk for postoperative and in-patient complications. Next to prolonged hospital stays, a higher risk for inpatient mortality is reported [
5].
In surgical treatment, any foreign material can increase the risk of infection [
6]. Surgical side infection in trauma surgery is reported with a mean of 1%-2%. A range of 0.5% to 8.0% is demonstrated in the most trials [
7,
8]. Concerning side infection, a high risk for mortality (55%) is reported [
9]. therefore, any infection should be prevented. Due to these facts, bone substitutes materials are discussed in the surgical treatment of younger patient.
Evaluating existing data of other complications in geriatrics, for example of pseudarthrosis, less data exists. Barrey et al. 2021 reported increased levels of pseudarthrosis in geriatric patients concerning a dens fracture. Age groups younger than 70 years demonstrated 0% to 12.5% a pseudarthrosis, after 90 years of age 58.6% pseudarthrosis was noticed [
10]. Age groups younger than 70 years demonstrated 0% to 12.5% a pseudarthrosis, after 90 years of age 58.6% pseudarthrosis were noticed [
10]. Pseudarthrosis of the distal forearm is rarely observed [
11].
Although various clinical data for bone substitutes materials in surgical treatment already exist, substitutes in geriatric surgical assessment was never investigated. If one enters a search query in PubMed with the keyword "bone substitutes materials geriatrics" it remains unsuccessful.
This study investigated the use of bone substitutes materials for treating geriatric fractures. In addition to the radiological course of bone healing, complications after surgery were evaluated to determine the safety of the bone graft substitute.
2. Materials and Methods
This retrospective observational trial enrolled patient underwent surgical treatment of acute fractures. Fractures of the distal radius, proximal humerus and proximal tibia were observed. Defining geriatric patients is a current problem in medicine [
12]. Following the most geriatric trials, patients with a minimum age of 65 years were included. Additionally, the inclusion parameter was acute fractures; pathological fractures were excluded.
For comparing bone graft substitutes, the patient files were evaluate. for Demographic data, age, sex, side of fracture, body mass index (BMI), were assessed. To classify comorbidities, the American Association of Anesthesiologists (ASA classification) published in 1941 a categorization which was used in this trial [
13]. Furthermore, the newer Charlson-Comorbidity-Index (CCI classification) differentiate existing comorbidities exactly.
For determining the severity of fracture, two various patterns were noticed. First, the number of open fractures was assessed. Open fractures are frequently associated with severe soft tissue damage and infection by contaminated wounds. Additionally, the Müller AO classification (AO classification) for fracture morphology was recorded.
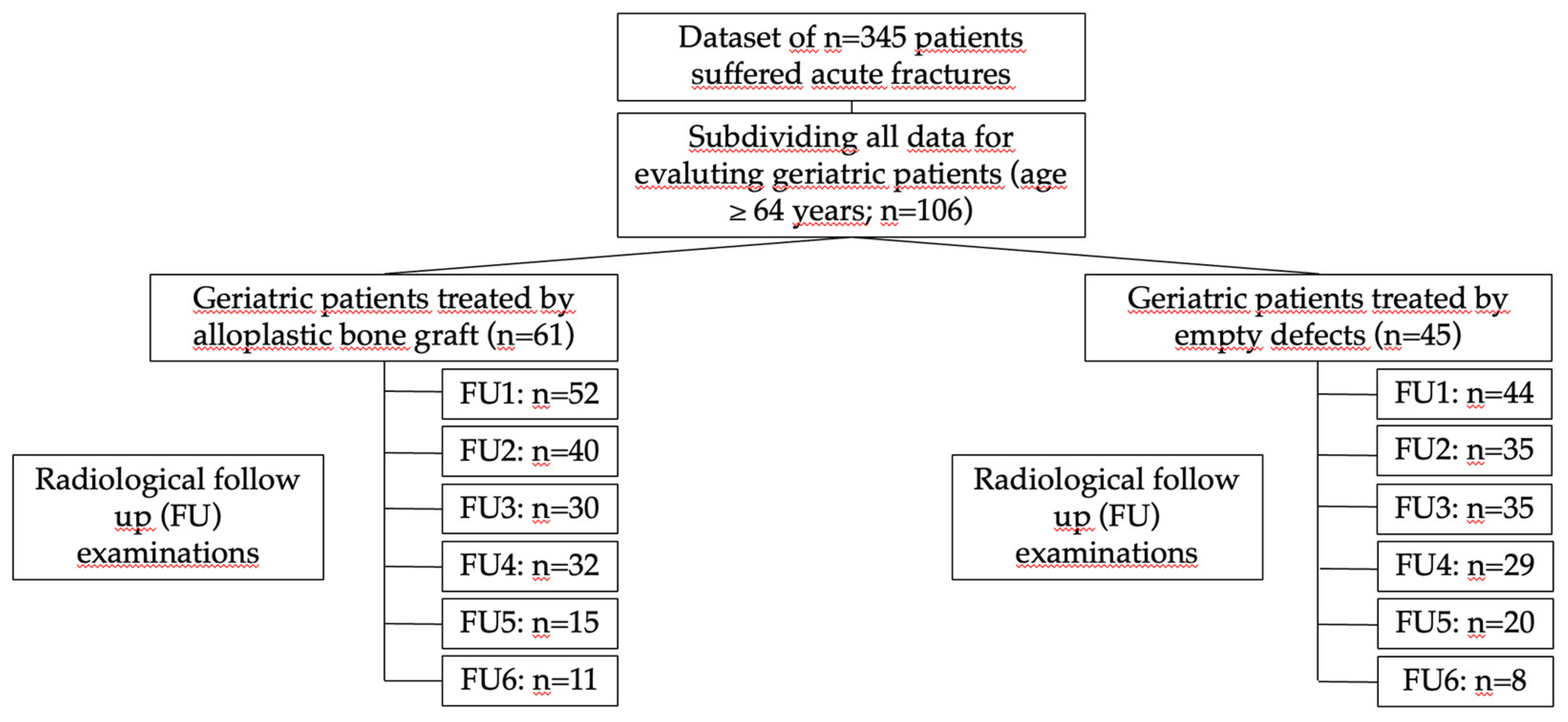
Figure 1.
Overview of the patients treated with bone substitutes materials compared with the empty cavity treatment.
Figure 1.
Overview of the patients treated with bone substitutes materials compared with the empty cavity treatment.
Complications and the radiological performance were evaluated to assess the safety of the medical devices. In this study, each patient record was evaluated with up to 6 follow-up examinations (FU). The radiographs were double blinded examined by two independent evaluators. For determining bone healing, a classification system was developed. based on Bohnhof et al., Freyschmidt et al. and Islam et al. to achieve enhanced evaluation of the fracture edges, fracture gaps, and articular surface [
14,
15,
16]. Regarding the fracture margin, sharp edges (5), partially sharp and partially blurred edges (4), blurred edges (3), faintly visible edges (2), and no visible edges (1) were distinguished. The fracture gap was controlled regarding the bridging process. A visible edge was seen in fractures without consolidation (6).
A reduction in density and a more impressive fracture gap (5) are followed by a blurred fracture line (4), a partially blurred and partially compressed fracture gap (3), and a compression of the fracture gap (2). The best pattern in the fracture gap is complete bridging with any fracture gap (1). Additionally, the implanted osteosynthesis material and local bone density were observed. The radiological assessment was analogous to the German school grading system.
The nature and number of complications were extracted from patient records. Treating injuries by surgery using bone substitute materials is thought to increase the rates of infection. Based on this assumption, bone and soft tissue infections were observed. Furthermore, delayed healing, ligament and muscle defects, CRPS and cartilage damage were evaluated. A delayed bone healing process was evaluated in two ways. According to the FDA definition of pseudarthrosis, non-union for more than 6 months without a healing process for more than 3 months defined pseudarthrosis. Furthermore, mal union was observed. Mal-union was defined as a continuous progression of bone healing criteria without completion within the first 6 months. Mortality rate could not be associated with the augmentation and no deaths were reported until the end of the designated follow-up. To control stability, delayed bone healing and secondary dislocation were under evaluation. Neurologic deficits were assessed to complete the clinic. These included persistent severe pain, paresthesia dysesthesias, and hyperesthesia for more than 6 weeks after surgery.
Data analysis was performed using IBM® SPSS® Statistics. The demographic data and complications were nominally scaled, the radiological pattern ordinally. For comparing post-surgery outcome, Mann–Whitney U test was used. The level of significance was set at ≤5%.
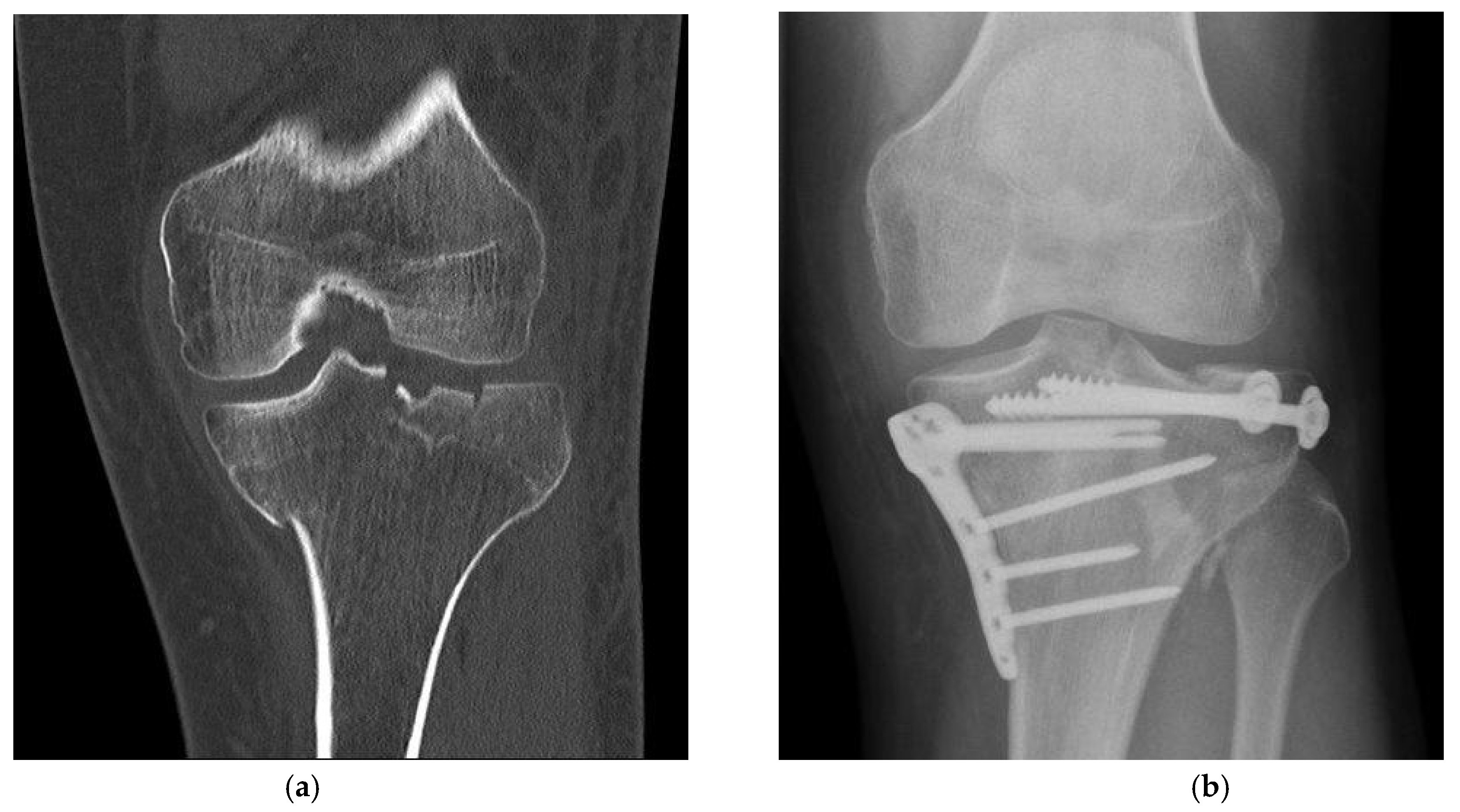
Figure 2.
Fracture treatment of a proximal tibial fracture by surgical bone substitute material augmentation: (a) Preoperative CT scan demonstrates an metaphyseal compression fracture with intra- and extraarticular fracture gaps; (b) Alloplastic bone material substitute augmentation replaces the bone material of the fracture vault defects.
Figure 2.
Fracture treatment of a proximal tibial fracture by surgical bone substitute material augmentation: (a) Preoperative CT scan demonstrates an metaphyseal compression fracture with intra- and extraarticular fracture gaps; (b) Alloplastic bone material substitute augmentation replaces the bone material of the fracture vault defects.
3. Results
We enrolled 106 patients with an age elder than 64 years [Min:Max; Mean±SD] [65:91; 75.31±6.69]. (n=45, 42.5%) patients underwent surgery without bone graft substitutes. Bone substitute material (BSM) was used to treat bone defects in (n=61, 57.5%) patients with a quantity of [1.0:7.5; 2.1±1.51] ml. (n=42, 68.9%) were treated by CP-based bone substitute, (n=19, 31.1%) by NHA augmentation. (n=89, 84.0%) female and (n=17, 16.0%) male patients were under evaluation. The left side was in (n=63, 59.4%) cases affected, in (n=43, 40.6%) the right side. The AO classification of fracture severity demonstrated (n=14, 13.2%) extraarticular, (n=15, 14.2%) partial intraarticular, and (n=73, 68.9%) intraarticular fractures. A loss of (n=4, 3.8%) preoperative radiographs were noticed. Classifying comorbidities, ASA classification showed [1.00:3.00; 2.28±0.56] and CCI [2.00:13.00; 5.41±2.26]. It should be noted, that the CCI classification was evaluated in (n=74, 69.8%).
The BMI over all groups was [17.16:37.80; 26.84±4.49] with a peak for patients with normal weight (BMI≤24.9; n=41, 38.7%) and mild adiposities (BMI≤29.9; n=39, 36.8%). Most injuries were caused by low impact trauma (n=87, 82.1%). High impact injuries were noticed in (n=17, 16.0%), at least (n=2, 1.9%) anamnesis were missing. Subdividing into the type of injury, (n=41, 41.5%) caused the injury by stumbling, followed by (n=26, 24.5%) domestic falls. (n=17, 16.0%) injuries were suffered by falls on stairs. High impact injuries were subdivided into (n=5, 4.7%) falls from a high, (n=2, 5.7%) sports injuries, and (n=10, 9.4%) traffic accidents by car, bike, or motorcycle. Due to the clinical examination, (n=13, 12.3%) patients were initially treated by external fixation. The typical duration of use of the external fixator until the change of procedure was [0:106; 14.92±26.32] days. Antibiotic prophylaxis was administered in accordance with the hospital's internal guidelines (n=104, 98.1%). Mainly, a perioperative single shot antibiotic, Cefuroxime, was used (n=82, 77.4%).
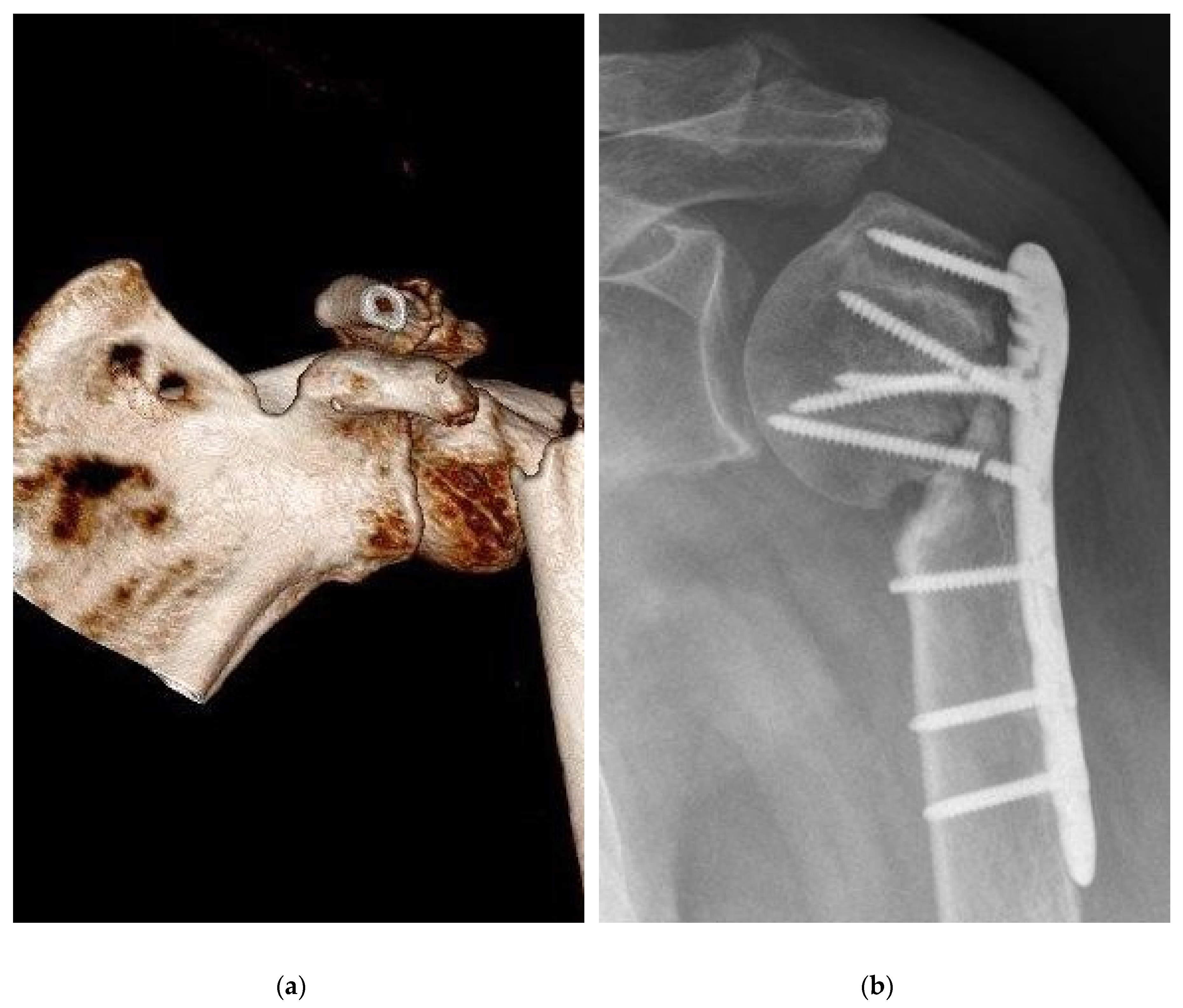
An important criterion in clinical evaluation after surgery is the survey of complications [
Table 2]. There was no relevant difference between the BSM and the ED group. Complications were noticed in the BSM group (n=21, 34.4%) and in the ED (n=23, 51.1%) groups (p=0.11). Regarding the number of complications, a wider range with a non-significant difference (p=0.07) was seen in the BSM group [0.0:4.0; 0.43±0.72] compared with the ED group [0.0:2.0; 0.64±0.71]. Pseudarthrosis decreased in the BSM group (n=3, 4.9%) compared with the ED group (n=7, 15.6%) nonsignificant (p=0.09) [
Figure 3]. Delayed healing, ligamentous and muscular damage, complex regional pain syndrome (CRPS), previous death before follow-up, secondary diseases and neurological diseases showed no significant difference (p>0.05). In the evaluation for the risk of using bone substitutes materials, infections were detected in the ED group (n=1, 2.2%) and in the BSM group (n=2, 3.3%). When comparing mal-union in the BSM and ED groups, a non-significant difference was found (p=0.07). When summarizing the significance of complications, no difference was found. In the clinic, relative (RR) and absolute risk (AR) reductions of complications allow the use of new therapies. Bone graft augmentation resulted in an RR of 32.64%, an ARR of 16.68%, and an NNT of 5.99.
Radiological assessment was used to determine bone healing and complications [
Table 3]. The margin of fracture showed significant differences in some FU examinations (FU3: p=0.002; FU4: <0.001; FU5: <0.001). Furthermore, the fracture gap determined decreased numbers in the BSM group compared with the ED group (p<0.05). The articular surface demonstrated an enhanced pattern in the BSM group (FU3-4: p<0.05). Concerning the osteosynthesis material and the bone substance in the region of fracture, there was no difference between the groups (p>0.05).
4. Discussion
Complex fractures with substance defects in the bone represent an increasing challenge in trauma surgery. Due to a steadily aging population, this situation will continue to worsen in the coming years. In 2006, half of the current traumas (30 to 40%) caused by geriatrics [
17]. The goal of treating fractures in geriatric patient should be to minimize the surgical stress while maintaining exercise or weight-bearing stability of the fracture. Significant progress has been made in this area recently with the use of stable-angle implants. In the osteoporotic bone and larger defects, it is often impossible to achieve satisfactory stability intraoperatively. Additional defect augmentation with autologous bone graft is often out of the question in geriatric patients because of the additional risks with a donor side morbidity of 15% [
18]. As an alternative to allogeneic bone grafting, bone substitute materials have been insufficiently tested clinically to date. In our work, we could demonstrate better performance of augmented fractures, particularly in the intermediate FU examinations (FU3 and FU4). As a hypothesis, already better stability could be interpreted here, which would represent an advantage in therapy, especially with regard to secondary dislocations or delayed bone healing.
Concerning complication, infection is well discussed in some case reports and trials [
19]. Although the absolute number of infections was increased in the ABP group, there was equality in comparing the groups of treatment (p=1.0). Further on, one of the most serious complications, pseudarthrosis decreased (p=0.09). If bone grafts are used in case of pseudarthrosis, various trials demonstrate well healing processes [
20]. Connecting these trials with the results of our study, bone graft substitute augmentation may prevent the risk of suffering a non-union.
Since our study is a retrospective data set, which was compared with a homogeneous comparison group, further investigations would have followed to verify this hypothesis. Regarding the questionable increased risk of infection when using bone substitutes materials, we could not find any increased risk in this work. However, the statement about the risk of infection cannot be generalized. Due to the large quantity of bone substitute materials, this risk must probably continue to be assessed for the respective materials depending on their composition in clinical investigations.
5. Conclusions
Summarizing the presented data, bone graft substitutes are a safe alternative to autologous bone grafts. Multimorbid patients may benefit from bone substitutes materials because of neither infection and a low NNT of 5.99 for preventing complications.
Author Contributions
Conceptualization, G.K. and J.P.; methodology, J.P.; validation, C.H., T.E.K. and L.E.; formal analysis, J.P. and V.V.; investigation, V.V. and J.P.; resources, C.H and T.E.K.; data curation, J.P.; writing—original draft preparation, G.K.; writing—review and editing, T.E.K. and L.E; visualization, J.P.; supervision, C.H.; project administration, T.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the University Hospital Giessen (AZ225/20 of 22/29/2021) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to a retrospective data analysis with local ethical approval.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rabast, U. Permanenter Anstieg unserer Lebenserwartung. In Gesunde Ernährung, gesunder Lebensstil; Springer, 2018; pp. 3–28. [Google Scholar]
- Schoenborn, N.L.; Blackford, A.L.; Joshu, C.E.; Boyd, C.M.; Varadhan, R. Life expectancy estimates based on comorbidities and frailty to inform preventive care. Journal of the American Geriatrics Society 2022, 70, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Aithal, S.; Sequeira, R.; Edwards, C.; Singh, I. Fragility Fractures and Parkinsonism: Relationship of Fractures with Demography, Severity and Predictors of Adverse Outcomes. Geriatrics 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Huffman, F.G.; Vaccaro, J.A.; Zarini, G.G.; Vieira, E.R. Osteoporosis, Activities of Daily Living Skills, Quality of Life, and Dietary Adequacy of Congregate Meal Participants. Geriatrics 2018, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Z.; Huo, H.; Zhao, P. The Impact of Frailty on Adverse Outcomes in Geriatric Hip Fracture Patients: A Systematic Review and Meta-Analysis. Front Public Health 2022, 10, 890652. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.J.; Jackowski, A.; Jamal, A.; Vaz, J.; Rodrigues, J.N.; Tyler, M.; Murray, A.; Wormald, J.C.R. Risk of surgical site infection in hand trauma, and the impact of the SARS-CoV-2 pandemic: A cohort study. J Plast Reconstr Aesthet Surg 2021, 74, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Warnock, M.; Ogonda, L.; Yew, P.; McIlvenny, G. Antibiotic Prophylaxis Protocols and Surgical Site Infection Rates in Trauma Surgery: A Prospective Regional Study of 26,849 Procedures. Ulster Med J 2019, 88, 111–114. [Google Scholar] [PubMed]
- Tunthanathip, T.; Tepaamondej, N. Factors associated with surgical site infection in blast-induced traumatic brain injury. Chin Med J (Engl) 2019, 132, 2514–2515. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, C.T.; Dahl, F.A.; Rotterud, J.H.M.; Gjertsen, J.E.; Aroen, A. Surgical site infection after hip fracture - mortality and risk factors: an observational cohort study of 1,709 patients. Acta Orthop 2020, 91, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Barrey, C.Y.; di Bartolomeo, A.; Barresi, L.; Bronsard, N.; Allia, J.; Blondel, B.; Fuentes, S.; Nicot, B.; Challier, V.; Godard, J.; et al. C1-C2 Injury: Factors influencing mortality, outcome, and fracture healing. Eur Spine J 2021, 30, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Prommersberger, K.J.; van Schoonhoven, J.; Laubach, S. [Pseudarthroses after distal radius fractures. What is the role of the distal radioulnar joint?]. Handchir Mikrochir Plast Chir 2000, 32, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Der ältere Patient–wer ist das? Der Internist 2007, 48, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J.; Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Classification. In StatPearls; Treasure Island (FL), 2022.
- Freyschmidt, J. Klinisch-radiologische Diagnose und Differentialdiagnose In Skeletterkankungen; Springer Verlag: 2016.
- Islam, O.; Soboleski, D.; Symons, S.; Davidson, L.K.; Ashworth, M.A.; Babyn, P. Development and duration of radiographic signs of bone healing in children. AJR Am J Roentgenol 2000, 175, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Klaus Bohnhof, H.I. , Wolfgang Fischer. Frakturheilung. In Radiologische Diagnostik der Knochen und Gelenke; Georg Thieme Verlag KG: 2006.
- Lau, L.; Ajzenberg, H.; Haas, B.; Wong, C.L. Trauma in the Aging Population: Geriatric Trauma Pearls. Emerg Med Clin North Am 2023, 41, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.T.; Dowd, T.C.; Giza, E. Surgical Treatment for Osteochondral Lesions of the Talus. Arthroscopy 2021, 37, 3393–3396. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yoshinuma, N.; Kishida, O.; Fujisaki, Y.; Ito, K. Removal of infected nonresorbable hydroxyapatite graft material in recurrent periodontitis: a report of two cases. J Oral Sci 2009, 51, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Chmali, K.; ElIdrissi, M.; Abid, H.; ElIbrahimi, A.; Berraho, M.; A, E.L. Aseptic nonunion of the tibia treated by plating and bone grafting: retrospective study about 40 cases. J Orthop Surg Res 2022, 17, 321. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).