1. Introduction
The ongoing renewable energy transition includes new energy carriers such as hydrogen [
1,
2,
3,
4], where production through water electrolysis yields green hydrogen. Key to this process is the hydrogen evolution reaction (HER), which is one of the two half-cell reactions in water electrolysis [
5]. The advent of the novel anion exchange membrane (AEM) [
1,
2,
3,
4] has caused a shift within water electrolysis creating a greater emphasis on alkaline water electrolysis utilising AEM technology. The alkaline HER is currently under profound scrutiny, as there is considerable difficulty in finding stable catalyst materials capable of replacing platinum group metals (PGMs) [
5]. As such, methods of improving the performance of non-PGM catalyst materials is subject to ample R&D efforts.
Chief among such methods is acid washing, where the current trend in technologies related to both water electrolysis, batteries, and general electrochemistry for electrode preparations includes this step [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. The utility of acid washing is great with respect to decreasing the influence of organic contaminants and reducing the surface oxide layer. The benefits include a notable reduction in series resistance, thus enabling greater performance and tenacity. However, there is great disparity in the manner in which this step is carried out, with respect to acid type and concentration.
There are reports utilising hydrochloric acid, sulphuric acid, perchloric acid, nitric acid [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19] and likely several other options, where the concentration varies from weak dilute acids around 0.05 M [
6,
7] to undiluted acids [
8,
9,
10,
11,
12] and everything in between [
13,
14,
15,
16,
17,
18,
19]. The influence of acid washing has been characterised for use in batteries and supercapacitors [
20], medical implants [
21] and the alkaline oxygen evolution reaction [
22]. The implications of corrosion during hydrothermal processes for direct catalyst growth onto nickel foam support was investigated [
23]. It was determined that the nickel foam may indeed supply nickel ions which may alter the catalyst composition, proving that catalytic influence of the support cannot be neglected during processes such as electrodeposition.
However, the effects of acid washing nickel foam supports for the alkaline hydrogen evolution reaction remain largely unreported. Hitherto unpublished work displays a notable improvement in both series resistance and charge transfer resistance in bare nickel foam substrates as alkaline HER electrodes from utilising both hydrochloric and sulphuric acid, where the latter outperformed the former [
24]. A peak performance was achieved by acid washing with 0.50 M sulphuric acid, yielding a notable decline in series resistance []. It is highly common to apply a catalyst ink to a substrate through spray-coating, though degree to which the aforementioned benefits of acid washing persist after spray-coating is currently unknown, where the same applies to the lifetime of such benefits. Herein, we seek to fill this void in literature by testing multiple catalyst coated nickel foam substrates in an efficient three-electrode setup both with and without the initial step of acid washing.
2. Experimental
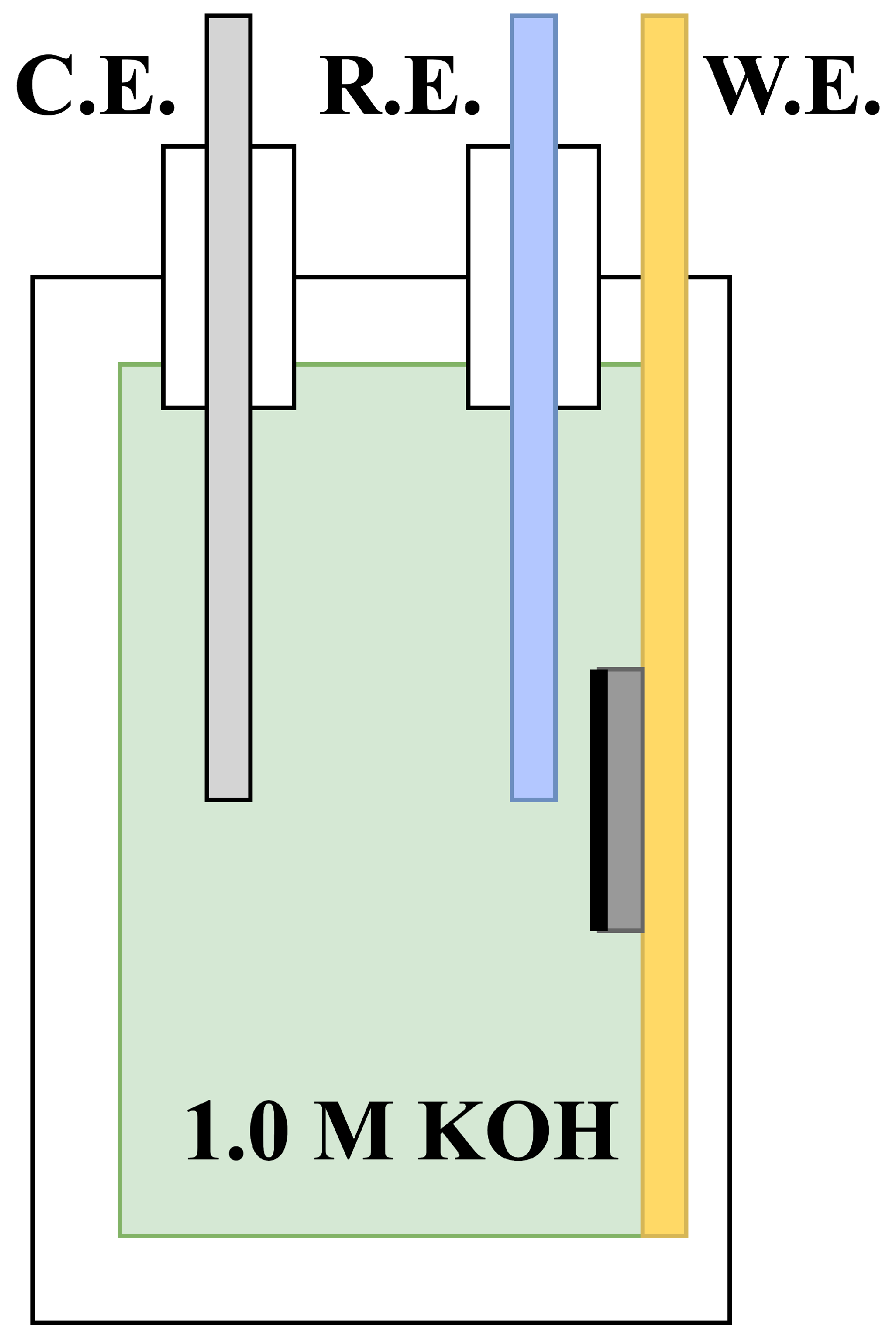
A three electrode setup was utilised, consisting of a nickel foam working electrode, a platinum wire counter electrode and an extended Luggin probe (agar salt bridge) connected with a Ag/AgCl reference electrode. The nickel foam electrode was pressed against a gold current collector to reduce ohmic resistance as shown in
Figure 1. The small-scale setup with fixed distance between the three electrodes allowed for a high degree of reproducibility.
The use of ionic bridges (extended Luggin probes) was essential to allow the reference electrodes to fit into the half-cell shown in
Figure 1. These ionic bridges were produced by mixing 22.37 g KCl (Sigma Aldrich) into 100 mL deionised water and heating until the boiling point was reached. From here, 1.5 g (1.5 wt.%) of Difco Agar (Fischer Scientific) was carefully added, thereby creating a 3.0 M KCl solution covered in Agar gel in liquid form. While the mixture was boiling, a tube attached to a syringe was employed to suck the solution into the tubing, thus filling the entire volume of both tubing and syringe. This was left overnight to settle, creating an ionic bridge ready for the insertion of Ag/AgCl (3.0 M KCl) reference electrodes the next day.
Deionised water and an appropriate quantity of potassium hydroxide (powder) for synthesis (Sigma Aldrich) were combined to create the 1.0 M KOH electrolyte, which was thoroughly hand-stirred to ensure a homogeneous concentration. The three-electrode setup was left to settle to establish steady state, by allowing the electrolyte to completely permeate all cavities of the nickel foam electrode.
The non-PGM inks were created by mixing 11 mg of MoS powder (US Nano) with 1.5 mL deionised water, 0.5 mL isopropanol and 22 mg of Sustainion® XB-7 alkaline ionomer. The PGM ink was created by distributing 3 mg 10 wt.% PtIr(1:3)/C (fuelcellstore.com) in 1 mL deionised water, 1.0 mL isopropanol and 6 mg of Sustainion® XB-7 alkaline ionomer (DiOxide Materials). These dispersions were ultrasonicated for 15 minutes, where the resulting inks were spray-coated onto four 1 cm nickel foam electrodes. These electrodes were attached to an aluminium plate heated to approximately 80C. These four electrodes comprise two MoS electrodes and two PtIr(1:3)/C electrodes, one acid-treated and one regular for both types of electrode. Several sets of electrodes were created to allow additional control experiments to be executed.
The electrochemical results were procured utilising an Iviumstat electrochemical workstation. Electrochemical impedance spectroscopy (EIS) spectra at open circuit were initially collected at the frequencies 10–10 Hz with a sinusoidal perturbation of 10 mV and 10 points per decade. This was followed by polarised EIS spectra at −50, −100, −150 and −200 mV relative to the hydrogen evolution reaction (HER). Linear sweep voltammetry (LSV) curves were subsequently recorded between 0.3 to -0.3 V at 10 mV s, and repeated to guarantee repeatability. This data was utilised to determine Tafel slopes. The electrochemical double layer was determined through cyclic voltammetry (CV) between 0.346 to 0.446 V at scan speeds of 10 to 20 mV s with 2 mV increments. To ensure stability, the initial scan at 10 mV s was repeated until all scans were overlapping. Stable CV results were obtained once scans at the slowest rate (10 mV s) were stable. Chronopotentiometry was performed at 10 mA cm for 12 h in 1.0 M KOH at room temperature to ascertain stability. The degradation rates were determined based off the same starting point at -0.20 V. This sequence was repeated for all electrodes to improve the basis for comparative work, and multiple versions of each electrode type were tested to ensure stability and repeatability.
The surface morphology of the nickel foam electrodes was analysed in a Jeol JSM-7200F field emission scanning electron microscope (SEM). Multiple points were analysed to ensure a solid foundation for determining the atomic composition of the surface. X-ray diffraction (XRD) was performed with a Bruker D8 Advance where all Bragg Brentano measurements were made in reflection mode. The X-ray source was a Cu k (1.5406 Å), operated at 40 kV, 40 mA (1600 W). The peaks were fitted utilising Fityk with a pseudo-voigt function utilising the Rietveld refinement method.
3. Results & discussion
3.1. Microstructural characterisation
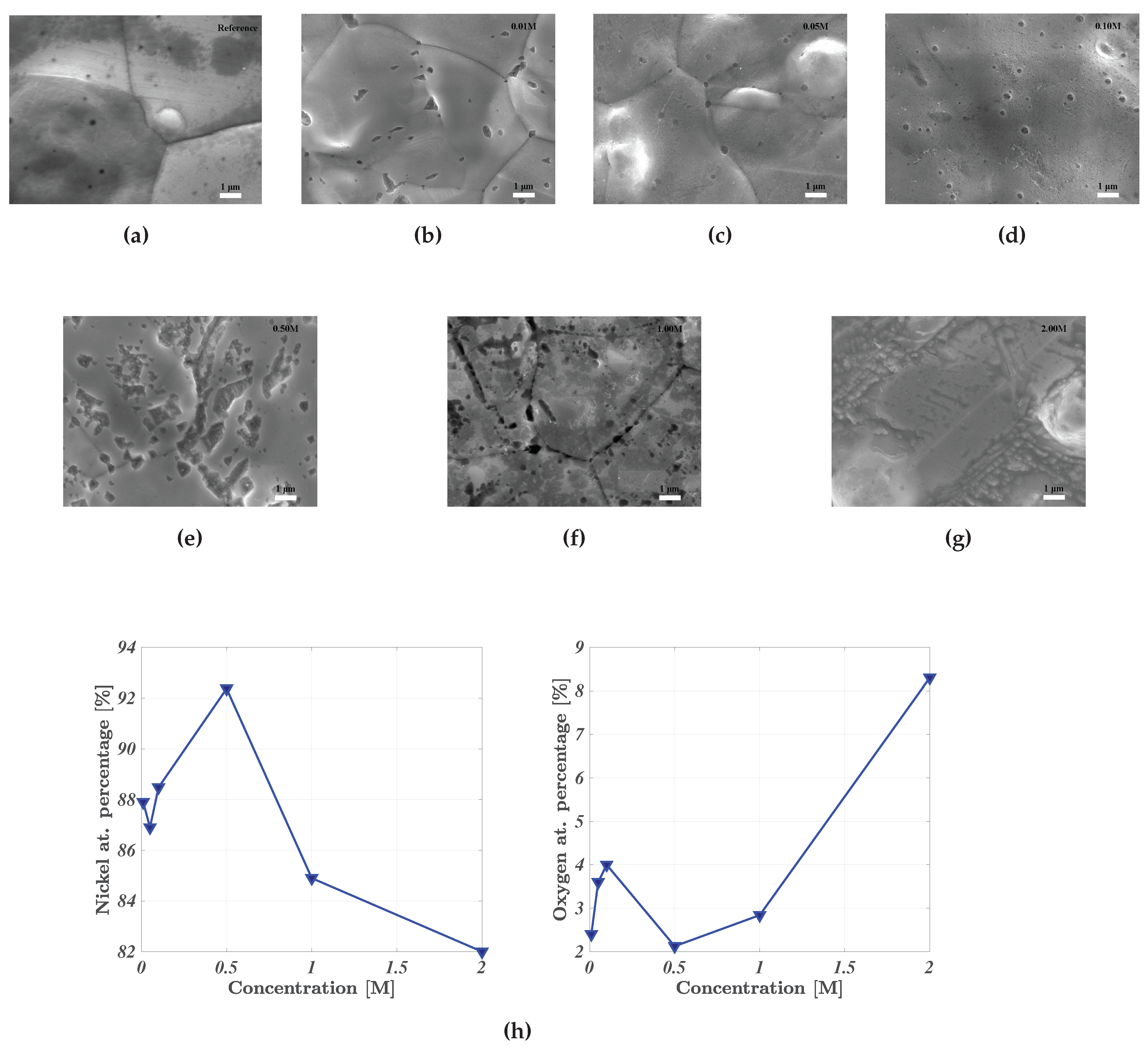
The effect of acid washing was ascertained through scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD). The effect of acid washing for increasingly potent concentrations of sulphuric acid is illustrated in
Figure 2. Change in surface morphology is clear, where comparing the pristine reference nickel foam in
Figure 2a to the surfaces in
Figure 2b-2g. The degree of uniform corrosion increases together with the influence of pitting corrosion. The reference nickel foam in
Figure 2a exhibits a clean, untainted surface, whereas the surface treated with 2.0 M H
SO
bears little resemblance. The degree of uniform surface corrosion has decreased the influence of pitting corrosion due to its severity.
EDS results indicate a nadir/peak in the atomic percentage of oxygen/nickel respectively for the nickel foam electrode treated with 0.50 M sulphuric acid. This affirms previous work showing a nadir for the EIS-determined series resistance for the same acid concentration. Greater acid concentrations resulted in greater/lower concentrations of oxygen/nickel as shown in
Figure 2h, thus agreeing with previous electrochemical evidence indicating decreased influence of a surface oxide layer. For great acid concentrations, the uniformity of the surface decreased simultaneously with a greater influence of oxygen. This is contradictory to the intention of acid washing, namely to reduced the effect of surface oxides. The electrodes were exposed to air during sample mounting for SEM/EDS analysis. Thus the increase in oxygen surface concentration after 0.50 M sulphuric acid may be related to an increased propensity to form an oxide layer once exposed to air after acid washing with great concentrations (>0.50 M H
SO
).
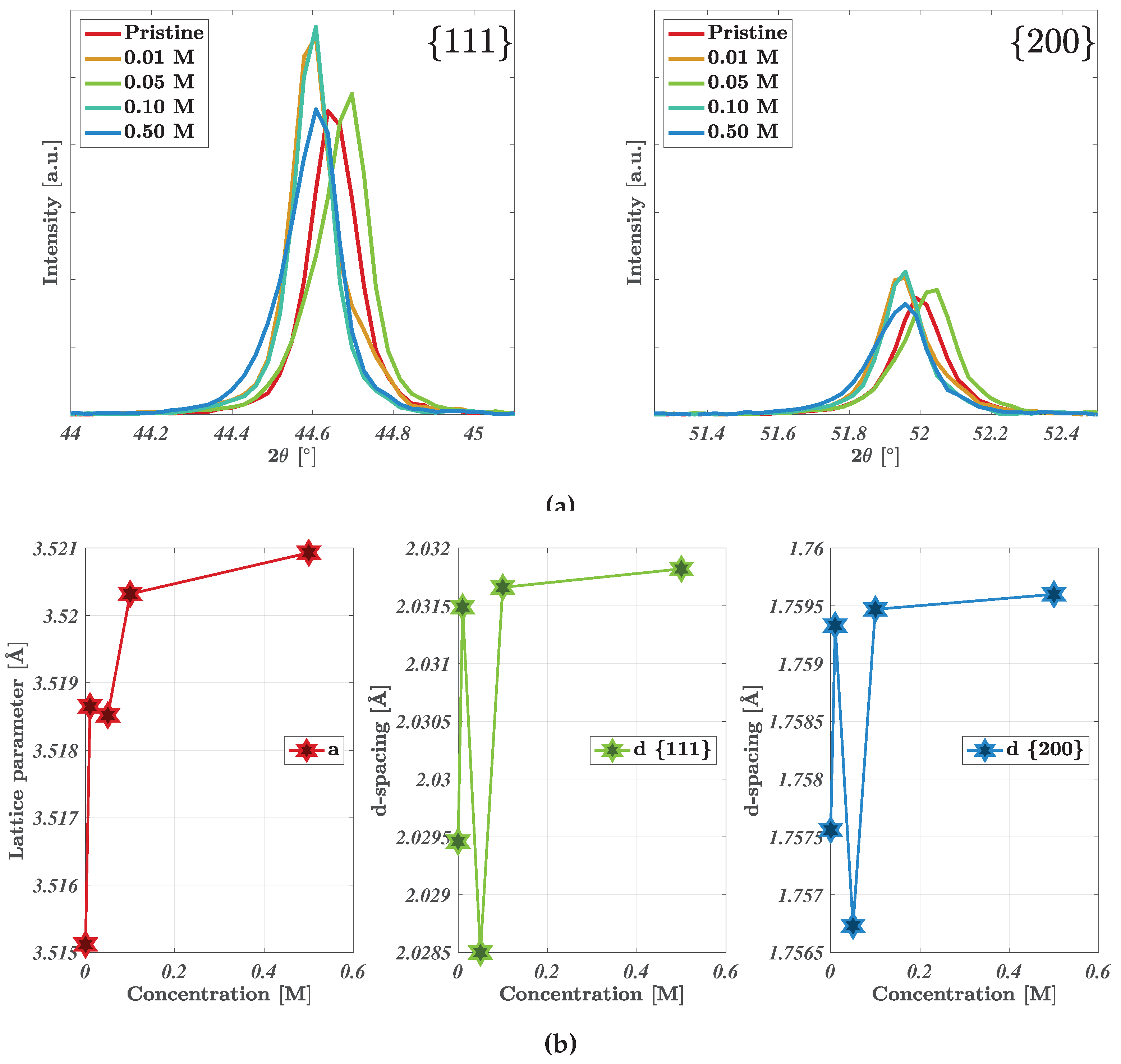
Shown in
Figure 3a is the XRD spectra for select concentrations of the acid-treated nickel foam electrodes. The electrodes presented a singular cubic phase fm-3m, where the highlighted peaks are affiliated with the 111- and 200 facets of nickel foam at 44.61-44.69
and 51.96-52.07
respectively (JCPDS No. 87-0712) [
25]. The minor variation in peak position (0.08
) for the 0.05 M sample originates most likely from height variations during sample mounting due to the porous nature of the nickel foam electrodes. The peaks were fitted allowing the extraction of the lattice parameters and d-spaces for the two peaks as shown in
Figure 3b. Both the lattice parameter
a and the d-spacing increases slightly with increasing acid concentration. The increase in lattice parameter
a follows the same trend as the nickel at.% . The change in lattice parameter implies increasing lattice distortion with increasing acid concentration, where this would likely increase further as the acid concentration exceeds 0.50 M [
26].
3.2. Electrochemical Characterisation
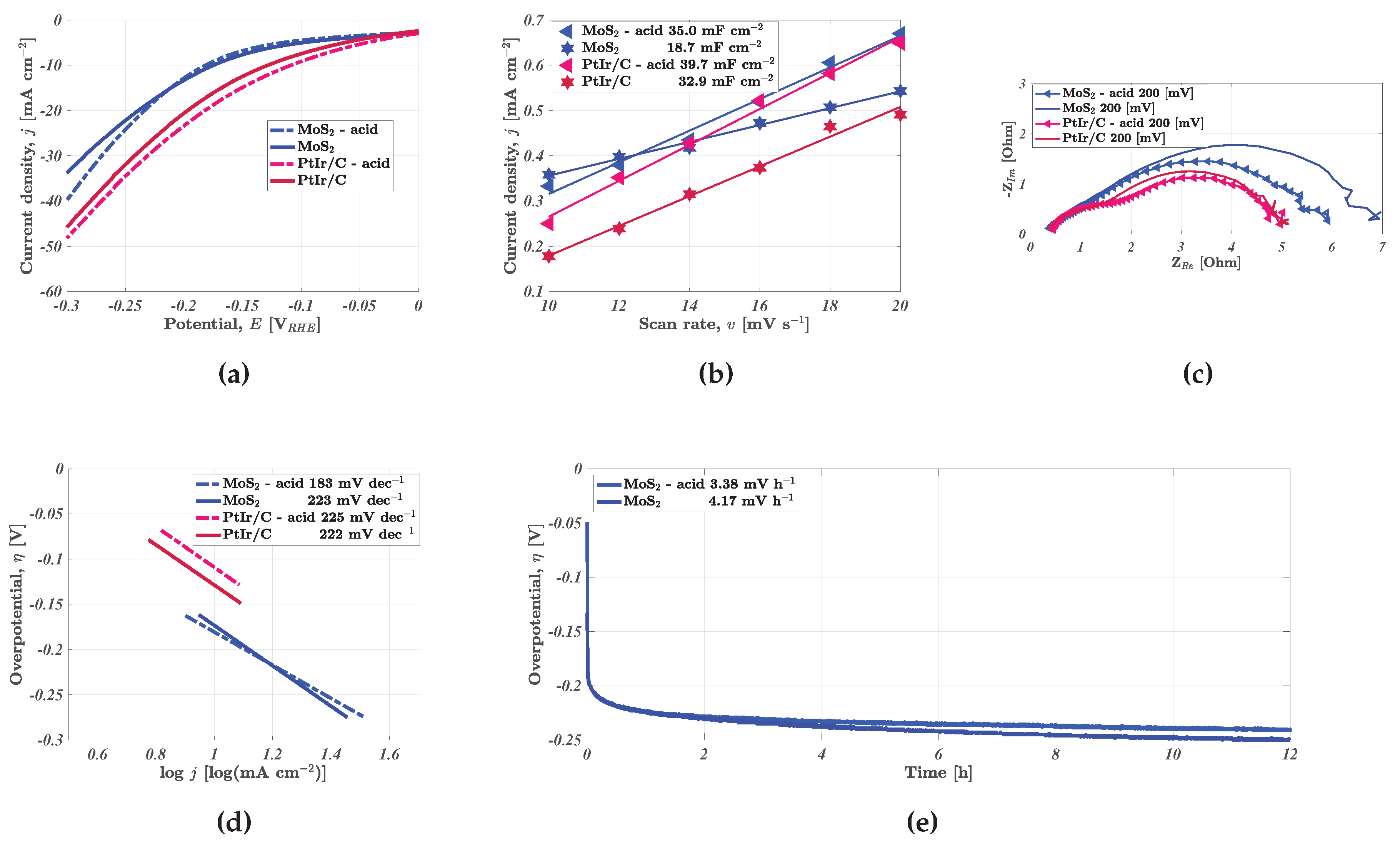
The following section details the results from the electrochemical characterisation. These results were procured following the protocol detailed in the experimental section. The effect of spray-coating acid treated nickel foam electrodes was tested for two different types of catalyst ink, specifically non-PGM MoS
and PGM PtIr(1:3)/C. LSVs were recorded for the four types of electrodes between 0 to -0.3 V
as shown in
Figure 4a. The acid-treated MoS
and PtIr(1:3)/C electrodes outperform the untreated electrodes by 17.9% and 5.19% respectively. Predictably enough, the PGM electrode surpassed the performance of the non-PGM MoS
-coated electrode. The rationale for this performance increment was dissected through determining the electrochemical double layer C
(
Figure 4b), which scales linearly with the electrochemically active surface area (ECSA). Both the acid-treated, spray coated electrodes display fair improvement relative to the regular spray coated electrodes. However, the improvement was notably greater for the MoS
-coated, acid treated electrode where the ECSA increased with 87.2%. The ECSA of the acid treated, PtIr(1:3)/C-coated electrode improved by 20.7%. Acid treatment with 0.50 M H
SO
caused notable changes to the surface as shown in the SEM images in
Figure 2, where this increased inhomogeneity was likely a great contributor to the augmented surface.
These are similar trends to that seen in the polarised EIS spectra in
Figure 4c. The MoS
-coated, acid treated electrode displays a fair improvement with respect to the charge transfer resistance, amounting to a 14.3% reduction. This displays that while the series resistance is similar, a performance increment is still achieved through reducing the charge transfer resistance. This adheres to established trends in unpublished research [
24] displaying that while acid treatment reduced the series resistance, the decline in charge transfer resistance is greater. Both versions of the PtIr(1:3)/C coated electrodes display a similar total impedance spectra, though there was a slight change in capacitance which correlates with the 20.7% improvement in C
.
Tafel curves in
Figure 4d indicate that the MoS
-coated, acid treated electrode displays a reduction in Tafel slope, while the affiliated slopes of both versions of the PtIr(1:3)/C were largely similar. Moreover, the MoS
-coated, untreated electrode displays a Tafel slope very similar to the PtIr(1:3)/C-coated electrodes. The reduction in Tafel slope for the MoS
-coated, acid treated electrode indicates an improved pathway for hydrogen evolution. While both slopes indicate that the Volmer step (water dissociation) is rate limiting, the use of acid treatment would appear to improve the efficiency of this step. The stability of these improvements was tested by performing CP as shown in
Figure 4e, where the degradation rate of the MoS
-coated, acid treated electrode was lower than the untreated version of the non-PGM HER electrode. The 18.9% lower degradation rate (3.38 mV h
) shows the improvements induced by the acid treatment do not immediately abate during continuous operation. This indicates the possibility of greater longevity when including the initial acid washing step prior to catalyst coating.
4. Conclusions
This paper investigates how acid washing nickel foam substrates affects the performance and stability of the electrodes after they have been spray coated with a catalytic ink. The electrochemical performance of catalyst-coated nickel foam was evaluated for both acid-treated and untreated nickel foam for two types of catalytic ink. By employing MoS- or PtIr(1:3)/C-coated nickel foam as a hydrogen evolution reaction electrode in 1.0 M KOH, it was clear that the acid washing positively affected the performance of both types of HER-electrode. The LSV performance of the acid-treated, MoS- and PtIr(1:3)/C-coated electrodes increased by 17.9% and 5.19% respectively. This was correlated with an augmented ECSA as shown by CV and EIS spectra. A great ECSA improvement was noted for the MoS-coated electrode which increased by 87.2%. Tafel analysis indicates an improvement in the water dissociation step which likely contributed to the enhanced LSV performance. Stability also appeared to increase after acid washing, where the degradation rate was lowered by 18.9%. These results demonstrate the utility of acid washing substrates prior to the application of a catalyst layer.
Author Contributions
Conceptualisation, T.B.F. and S.N.S.; methodology, T.B.F. and S.N.S.; software, T.B.F.; validation, T.B.F. and S.N.S.; formal analysis, T.B.F. and S.N.S.; investigation, T.B.F.; resources, P.H.M., J.V.H., M.L.K.; data curation, T.B.F.; writing—original draft preparation, T.B.F.; writing—review and editing, T.B.F. and S.N.S.; visualisation, T.B.F.; supervision, P.H.M., J.V.H., M.L.K.; project administration, P.H.M., J.V.H., M.L.K.; funding acquisition, P.H.M., J.V.H., M.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferriday, T.; Middleton, P. Alkaline fuel cell technology - A review. International Journal of Hydrogen Energy 2021, 46, 18489–510. [Google Scholar] [CrossRef]
- Ferriday, T.; Middleton, P. 4.07 - Alkaline Fuel Cells, Theory and Applications. In Comprehensive Renewable Energy (Second Edition), Second ed.; Letcher, T., Ed.; Elsevier: Oxford, 2022; pp. 166–231. [Google Scholar]
- Vincent, I.; Lee, E.C.; Kim, H.M. Comprehensive impedance investigation of low-cost anion exchange membrane electrolysis for large-scale hydrogen production. Scientific Reports 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, J.; Atanassov, P.; Dekel, D.; Herring, A.; Hickner, M.; Kohl, P.; Kucernak, A.; Mustain, W.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy & environmental science 2014, 7, 3135–91. [Google Scholar]
- Ferriday, T.; Middleton, P.; Kolhe, M. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Akhtar, N.; El-Safty, S.; Khairy, M.; El-Said, W. Fabrication of a highly selective nonenzymatic amperometric sensor for hydrogen peroxide based on nickel foam/cytochrome c modified electrode. Sensors and Actuators B: Chemical 2015, 207, 158–66. [Google Scholar] [CrossRef]
- Dong, S.; Ji, X.; Yu, M.; Xie, Y.; Zhang, D.; He, X. Direct synthesis of interconnected porous carbon nanosheet/nickel foam composite for high-performance supercapacitors by microwave-assisted heating. Journal of Porous Materials 2018, 25, 923–33. [Google Scholar] [CrossRef]
- Chaudhari, N.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, X.; Wang, Y.; Han, X.; Zhang, X. NiSe2@ NixSy nanorod on nickel foam as efficient bifunctional electrocatalyst for overall water splitting. International Journal of Hydrogen Energy 2021, 46, 34713–26. [Google Scholar] [CrossRef]
- Lu, L.; Hou, D.; Fang, Y.; Huang, Y.; Ren, Z. Nickel based catalysts for highly efficient H2 evolution from wastewater in microbial electrolysis cells. Electrochimica Acta 2016, 206, 381–7. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, W.; Duan, J.; Fu, L.; Ma, L.; Yang, Z. Immobilized Ag3PO4/GO on 3D nickel foam and its photocatalytic degradation of norfloxacin antibiotic under visible light. RSC advances 2020, 10, 4427–35. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Kowalski, I. Hydrogen evolution at catalytically-modified nickel foam in alkaline solution. Journal of Power Sources 2014, 271, 231–8. [Google Scholar] [CrossRef]
- He, Z.; Sun, J.; Wei, J.; Wang, Q.; Huang, C.; Chen, J.; Song, S. Effect of silver or copper middle layer on the performance of palladium modified nickel foam electrodes in the 2-chlorobiphenyl dechlorination. Journal of Hazardous Materials 2013, 250-251, 181–89. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.J.; Wu, Z.L.; Huang, Y.H.; Huang, C.P. Electrochemical nitrate reduction as affected by the crystal morphology and facet of copper nanoparticles supported on nickel foam electrodes (Cu/Ni). Chemical Engineering Journal 2020, 383, 123157. [Google Scholar] [CrossRef]
- Sarawutanukul, S.; Phattharasupakun, N.; Wutthiprom, J.; Sawangphruk, M. 3D CVD graphene oxide on Ni foam towards hydrogen evolution reaction in acid electrolytes at different concentrations. ECS Transactions 2018, 85, 49. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.; Ng, Y.; Amal, R. A sea-change: manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy & Environmental Science 2018, 11, 1898–1910. [Google Scholar]
- Li, X.; Zhang, Z.; Xiang, Q.; Chen, R.; Wu, D.; Li, G.; Wang, L. A three-dimensional flower-like NiCo-layered double hydroxide grown on nickel foam with an MXene coating for enhanced oxygen evolution reaction electrocatalysis. RSC advances 2021, 11, 12392–97. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Sun, Q.; Wang, Z.; Zhao, C. Electrodeposition of cobalt nickel hydroxide composite as a high-efficiency catalyst for hydrogen evolution reactions. Journal of The Electrochemical Society 2017, 164, H587. [Google Scholar] [CrossRef]
- Dastafkan, K.; Li, Y.; Zeng, Y.; Han, L.; Zhao, C. Enhanced surface wettability and innate activity of an iron borate catalyst for efficient oxygen evolution and gas bubble detachment. Journal of Materials Chemistry A 2019, 7, 15252–61. [Google Scholar] [CrossRef]
- Ansari, S.; Parveen, N.; Al-Othoum, M.; Ansari, M. Effect of washing on the electrochemical performance of a three-dimensional current collector for energy storage applications. Nanomaterials 2021, 11, 1596. [Google Scholar] [CrossRef]
- Hung, K.Y.; Lin, Y.C.; Feng, H.P. The Effects of Acid Etching on the Nanomorphological Surface Characteristics and Activation Energy of Titanium Medical Materials. Materials 2017, 10, 1164. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Qin, L.; Mathew, S.; Han, Y.; Li, O.L. Gas-Liquid Interfacial Plasma engineering under dilute nitric acid to improve hydrophilicity and OER performance of nickel foam. Progress in Natural Science: Materials International 2022, 32, 608–616. [Google Scholar] [CrossRef]
- Bu, X.; Wei, R.; Cai, Z.; Quan, Q.; Zhang, H.; Wang, W.; Li, F.; Yip, S.; Meng, Y.; Chan, K.; et al. More than physical support: The effect of nickel foam corrosion on electrocatalytic performance. Applied Surface Science 2021, 538, 147977. [Google Scholar] [CrossRef]
- T.B. Ferriday, S.N. Sampathkumar, P.H. Middleton, J. Van Herle. Investigation of Wet-Preparation Methods Of Nickel Foam For Alkaline Water Electrolysis. Journal of Physics 2023. [CrossRef]
- Long, L.; Yao, Y.; Yan, M.; Wang, H.; Zhang, G.; Kong, M.; Yang, L.; Liao, X.; Yin, G.; Huang, Z. Ni3S2@polypyrrole composite supported on nickel foam with improved rate capability and cycling durability for asymmetric supercapacitor device applications. Journal of Materials Science 2017, 52, 3642–3656. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xia, K.; Xu, L.; Xu, Y.; Fang, X.; Lu, W. Effect of electrodeposition parameters on the morphology of three-dimensional porous copper foams. Int. J. Electrochem. Sci 2015, 10, 7331–7340. [Google Scholar] [CrossRef]
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).