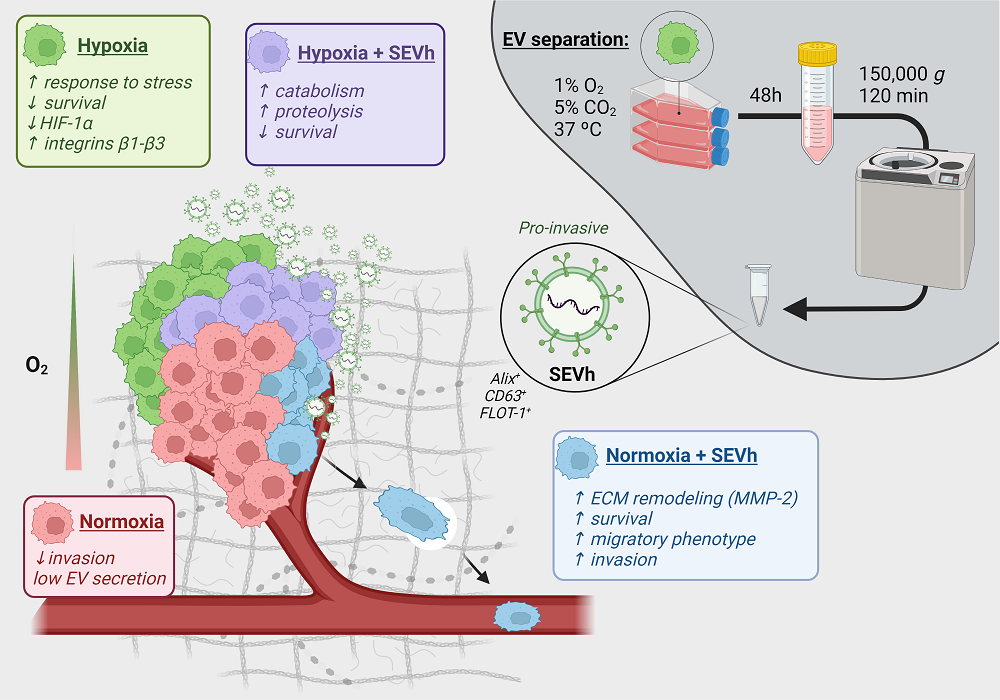
Hypoxia, a condition of low oxygenation frequently found in triple-negative breast tumors (TNBC), promotes extracellular vesicle (EV) secretion and favors cell invasion, a complex process in which cell morphology is altered, dynamic focal adhesion spots are created, and ECM is re-modeled. Here, we investigated the invasive properties triggered by TNBC-derived hypoxic small EV (SEVh) in vitro in cells cultured under hypoxic and normoxic conditions, using pheno-typical and proteomic approaches. SEVh characterization demonstrated increased protein abundance and diversity over normoxic SEV (SEVn), with enrichment in pro-invasive pathways. In normoxic cells, SEVh promotes invasive behavior through pro-migratory morphology, in-vadopodia development, ECM degradation and matrix metalloprotease (MMP) secretion. Pro-teome profiling of normoxic cells exposed to SEVh determined enrichment in metabolic processes and cell cycle, modulating cell health to escape apoptotic pathways. In hypoxia, SEVh was re-sponsible for proteolytic and catabolic pathway inducement, interfering with integrin availabil-ity and gelatinase expression. Overall, our results demonstrate the importance of hypoxic signal-ing via SEV in tumors for the early establishment of metastasis.