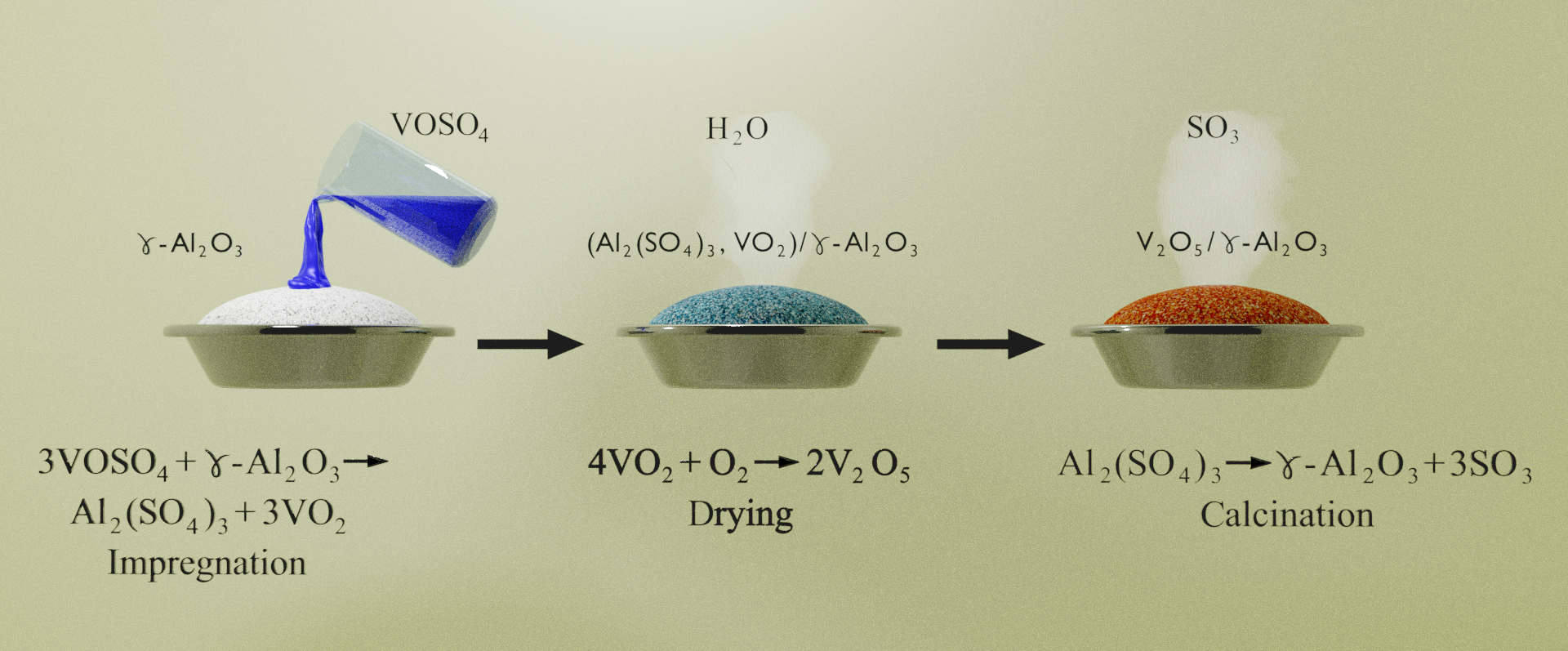
The catalytically active vanadium-containing system on γ-Al2O3 was studied using a wide range of physical and chemical methods depending on the synthesis conditions. It is shown that the vanadium-containing system includes several complexes with different thermal stability and catalytic activity. Low-active complexes are destroyed with the formation of more active ones based on V2O5 oxide as the temperature of heat treatment increases. It can be assumed that the V2O5 oxide has the decisive role in its catalytic activity. It was concluded that the vanadium-containing catalytic system on aluminum oxide, in the studied temperature range, is thermal stable and shows high activity not only in the reduction of nitrogen oxides but also in the oxidation of hydrocarbons (even of the most difficult ones, such as oxidizable methane). These properties of the system make it quite promising in the field of application for purification of exhaust gases of motor transport and industrial enterprises from environmentally harmful components as well as for understanding the mechanism of the action of catalysts in these processes, which is very important for solving the problems of decarbonization and achieving carbon neutrality.