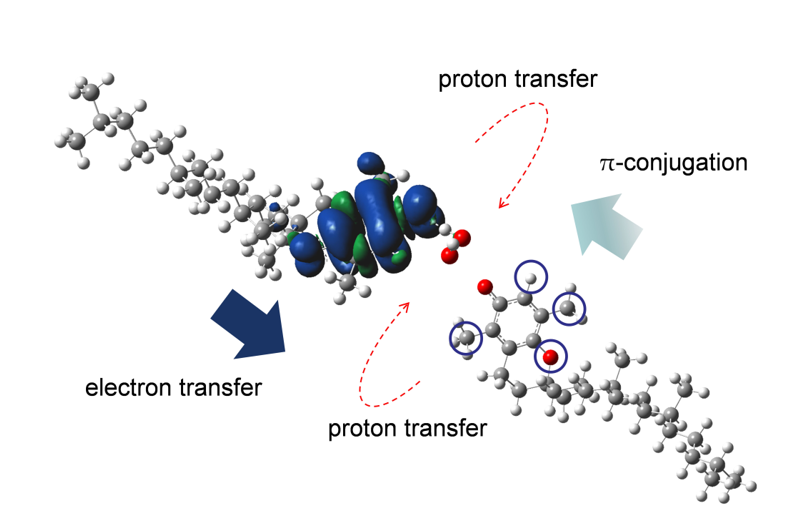
Abstract: Elimination of superoxide radical anion (O2•−) by tocopherols (TOH), and related compounds was investigated on the basis of cyclic voltammetry and in situ electrolytic electron spin resonance spectral measurements in N,N-dimethylformamide (DMF) with the aid of density functional theory (DFT) calculations. Quasi-reversible O2/O2•− redox was modified by the presence of TOHs, suggesting that the electrogenerated O2•− was eliminated by α-, β-, γ-TOH through proton-coupled electron transfer (PCET), but not by δ-TOH. The structure–activity correlation of α-, β-, γ-, and δ-TOH characterized by methyl group on the 6-chromanol ring was experimentally confirmed, where the methyl group promotes the PCET mechanism. Furthermore, comparative analyses using some related chemical analogues suggested that methoxyl group of the 6-chromanol ring is required for a successful electron transfer (ET) to O2•− through the PCET. The electrochemical and DFT results in dehydrated DMF suggested that the PCET mechanism involves preceding proton transfer (PT) forming hydroperoxyl radical followed by a concerted PCET (ET–PT). The O2•− elimination by TOH proceeds efficiently along the net PCET mechanism involving one ET and two PTs.