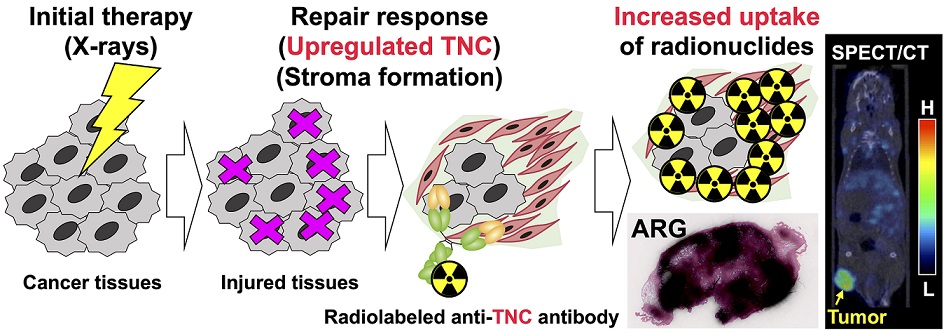
Background: In treatment-refractory cancers, tumor tissues damaged by therapy initiate the repair response; therefore, tumor tissues must be exposed to an additional burden before successful repair. We hypothesized that an agent recognizing a molecule that responds to anticancer treatment–induced tissue injury could deliver an additional antitumor agent including a radionuclide to damaged cancer tissues during repair. We selected the extracellular matrix glycoprotein tenascin-C (TNC) as such a molecule, and three antibodies recognizing human and murine TNC were employed to evaluate X-irradiation–induced changes in TNC uptake by subcutaneous tumors. Methods: TNC expression was assessed by immunohistochemical staining of BxPC-3 tumors treated with or without X-irradiation (30 Gy) for 7 days. Antibodies against TNC (3-6, 12-2-7, TDEAR) and a control antibody were radiolabeled with 111In and injected into nude mice having BxPC-3 tumors 7 days after X-irradiation, and temporal uptake was monitored for an additional 4 days by biodistribution and single-photon emission computed tomography with computed tomography (SPECT/CT) studies. Intratumoral distribution was analyzed by autoradiography. Results: The immunohistochemical signal for TNC expression was faint in nontreated tumors but increased and expanded with time until day 7 after X-irradiation. Biodistribution studies revealed increased tumor uptake of all three 111In-labeled antibodies and the control antibody. However, a statistically significant increase in uptake was evident only for 111In-labeled 3-6 (35%ID/g for 30 Gy vs. 15%ID/g for 0 Gy at day 1, P < 0.01; ID, injected dose), whereas limited changes in 111In-labeled TDEAR2, 12-2-27, and control antibody were observed (several %ID/g for 0 and 30 Gy). Serial SPECT/CT imaging with 111In-labeled 3-6 or control antibody provided consistent results. Autoradiography revealed noticeably stronger signals in irradiated tumors injected with 111In-labeled 3-6 compared with each of nonirradiated tumors and the control antibody. The signals were observed in TNC-expressing stroma. Conclusion: Markedly increased uptake of 111In-labeled 3-6 in irradiated tumors supports our concept that an agent, such as an antibody, that recognizes a molecule, such as TNC, involved in tissue-injury repair could enhance drug delivery to therapy-experienced tumor tissues. The combination of antibody 3-6 coupled to a tumoricidal drug and conventional therapy has the potential to achieve better outcomes for patients with refractory cancer.