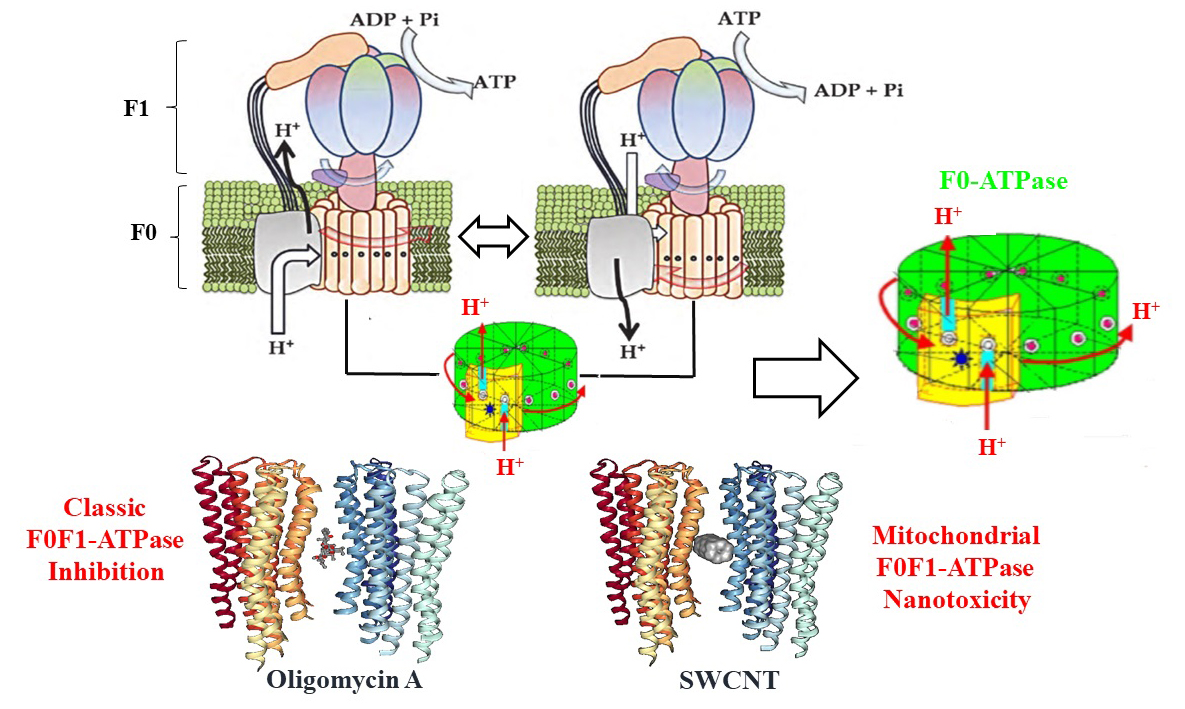
Herein, we present a combination of experimental and computational study on the mitochondrial F0F1-ATPase nanotoxicity inhibition induced by single-walled carbon nanotubes (SWCNT-pristine, SWCNT-COOH). To this end, the in vitro inhibition responses in submitochondrial particles (SMP) as F0F1-ATPase enzyme were strongly dependent on the concentration assay (from 3 to 5 µg/ml) for both types of carbon nanotubes. Besides, both SWCNTs show an interaction inhibition pattern like the oligomycin A (the specific mitochondria F0F1-ATPase inhibitor). Furthermore, the best crystallography binding pose obtained for the docking complexes based on the free energy of binding (FEB), fit well with the previous in vitro evidences from the thermodynamics point of view. Following an affinity order as: FEB (oligomycin A/F0-ATPase complex) = -9.8 kcal/mol > FEB (SWCNT-COOH/F0-ATPase complex) = - 6.8 kcal/mol ~ FEB (SWCNT-pristine complex) = -5.9 kcal/mol. With predominance of van der Waals hydrophobic nanointeractions with key F0-ATPase binding site residues (Phe 55 and Phe 64). By the other hand, results on elastic network models, and fractal-surface analysis suggest that SWCNTs induce significant perturbations by triggering abnormal allosteric responses and signals propagation in the inter-residue network which could affect the substrate recognition ligand geometrical specificity of the F0F1-ATPase enzyme in order (SWCNT-pristine > SWCNT-COOH). Besides, the performed Nano-QSTR models for both SWCNTs show that this method may be used for the prediction of the nanotoxicity induced by SWCNT. Overall, the obtained results may open new avenues toward to the better understanding and prediction of new nanotoxicity mechanisms, rational drug-design based nanotechnology, and potential biomedical application in precision nanomedicine.