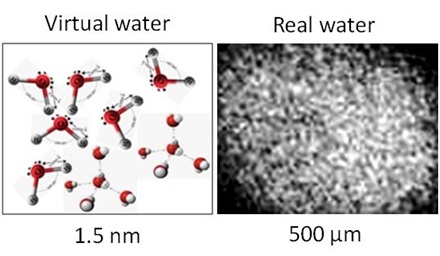
The paper provides information on significant contamination of real laboratory water with hydrophilic microimpurities. This fact suggests that researchers are practically dealing with microdispersed systems. However, this fact is usually neglected in the discussions of the causes of the anomalous properties of water. We will show that, when exposed to various factors of physical nature, water demonstrates reactions of the same type, namely, increased pH and electrical conductivity, reduced redox potential and viscosity, and enhanced bioavailability. Each exposure is accompanied by the destruction of aggregates of the dispersed phase and its transition to a fine-dispersed state. The relationship between this phenomenon and the change in the physicochemical properties of water is discussed.