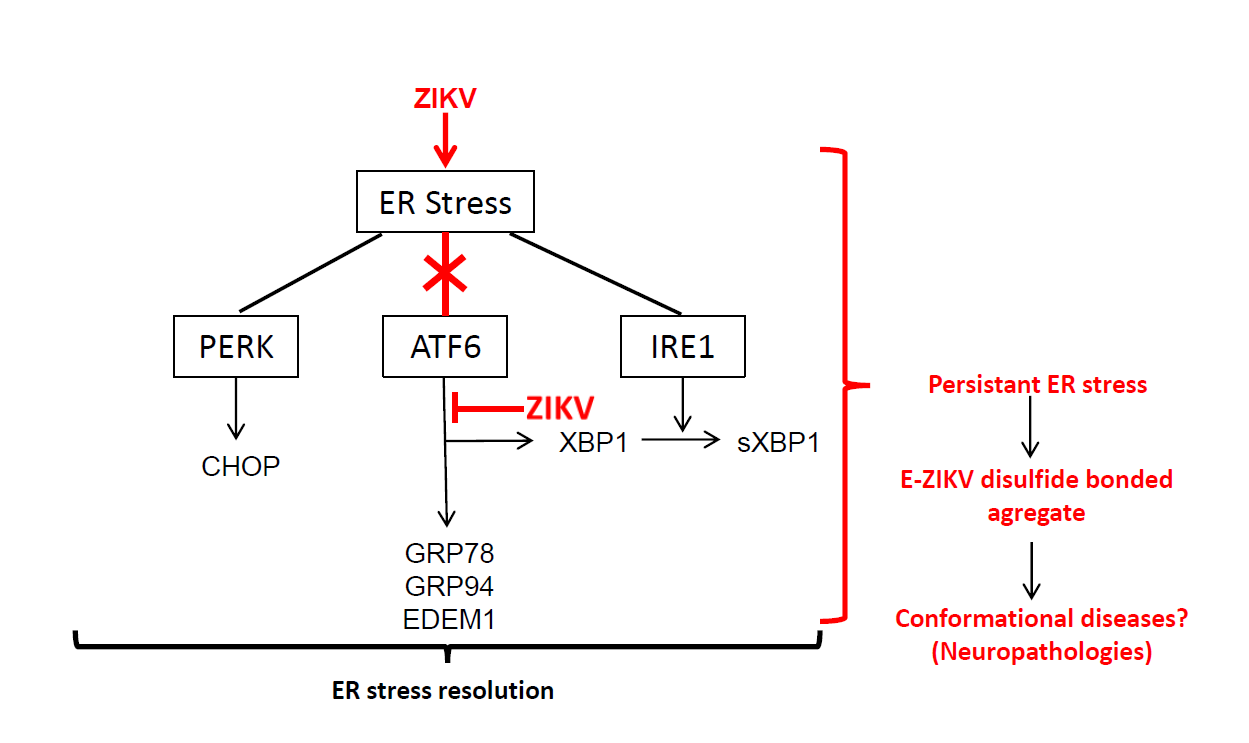
Flaviviruses replicate in membranous factories associated with the endoplasmic reticulum (ER). Significant levels of flavivirus polyprotein integration contribute to ER stress and the host cell may exhibit an Unfolded Protein Response (UPR) to this protein accumulation, stimulating appropriate cellular responses such as adaptation, autophagy or cell death. These different stress responses support other antiviral strategies initiated by infected cells and can help to overcome viral infection. In epithelial A549 cells, a model currently used to study the flavivirus infection cycle and the host cell responses, all three pathways leading to UPR are activated during infection by Dengue virus (DENV), Yellow Fever virus (YFV) or West Nile virus (WNV). In the present study, we investigated the capacity of ZIKA virus (ZIKV) to induce ER stress in A549 cells. We observed that the cells respond to ZIKV infection by implementing an UPR through activation of the IRE1 and PERK pathway without activation of the ATF6 branch. By modulating the ER stress response, we found that UPR inducers significantly inhibit ZIKV replication. Interestingly, our findings provide evidence that ZIKV could manipulate the UPR to escape this host cell defence system. Since incomplete UPR could lead to unresolved and persistent ER stress, we found that ZIKV infection was associated with an abnormal accumulation of viral envelope proteins, which were aggregated with non-native disulfide bridges. As the presence of these “amyloid like” protein polymers may be cytopathic, our observations provide new insights into specific neuropathologies associated with ZIKA virus infections.