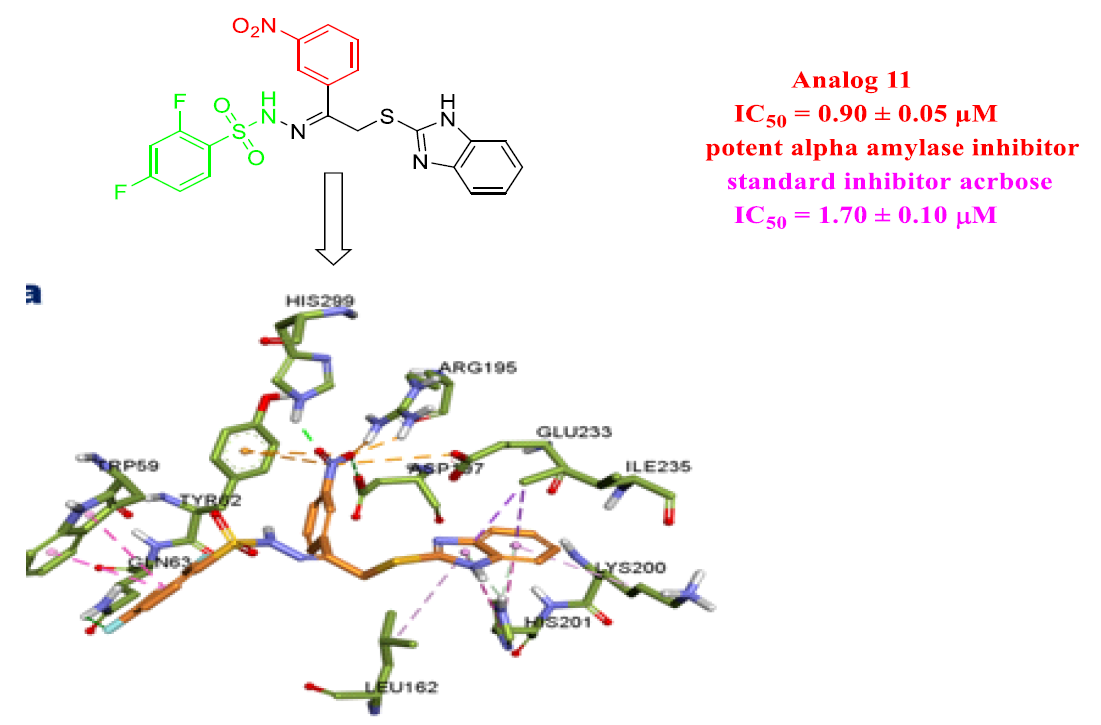
In the search of potent α-amylase inhibitors, we have synthesized seventeen derivatives of 2-mercaptobenzimidazole bearing sulfonamide (1-17) and evaluated for their α-amylase inhibitory potential. All compounds display a variable degree of α-amylase activity having IC50 values ranging between 0.90 ± 0.05 to 11.20 ± 0.30 µM when compared with the standard drug acarbose having IC50 value 1.70 ± 0.10 µM. Compound 1, 2, 11, 12 and 14 having IC50 values 1.40 ± 0.10, 1.30 ± 0.05, 0.90 ± 0.05, 1.60 ± 0.05 and 1.60 ± 0.10 µM respectively were found many folds better than the standard drug acarbose. The remaining analogs showed good inhibitory potentials. All the synthesized compounds were characterized by HREI-MS, 1H and 13C-NMR. Structure activity relationship (SAR) has been recognized for all newly synthesized analogs. Through molecular docking study, binding mode of active analogs with α-amylase enzyme was confirmed.