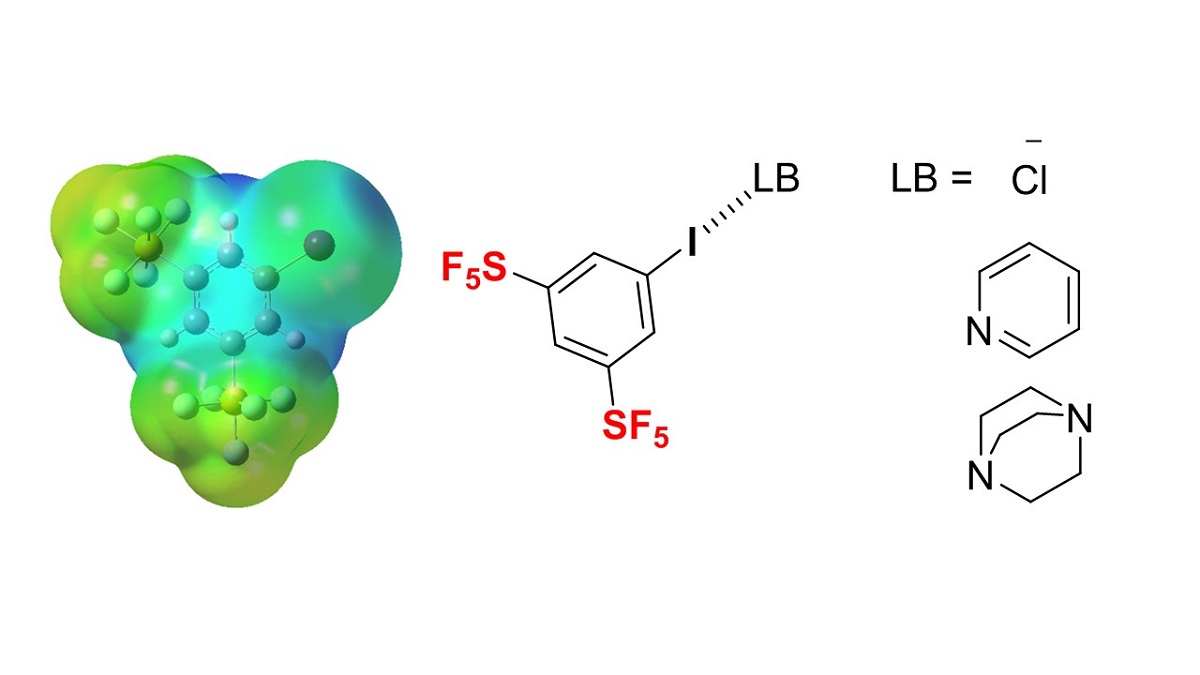
The activation of halogen bonding by the substitution of the pentafluorosulfanyl (SF5) group was studied using a series of SF5-substituted iodobenzenes. The simulated electrostatic potential values of SF5-substituted iodobenzenes, ab initio molecular orbital calculations of intermolecular interactions of SF5-substituted iodobenzenes with pyridine, and the 13C NMR titration experiments of SF5-substituted iodobenzenes in the presence of pyridine or tetra (n-butyl) ammonium chloride (TBAC) indicated the obvious activation of halogen bonding, although this was highly dependent on the position of SF5-substitution on the benzene ring. 3,5-Bis-SF5-iodobenzene was the most effective halogen bond donor followed by o-SF5-substituted iodobenzene, while the m- and p-SF5 substitutions did not activate the halogen bonding of iodobenzenes. The 2:1 halogen bonding complex of 3,5-bis-SF5-iodobenzene and 1,4-diazabicyclo[2.2.2]octane (DABCO) was also confirmed. Since SF5-containing compounds have emerged as promising novel pharmaceutical and agrochemical candidates, the 3,5-bis-SF5-iodobenzene unit should be an attractive fragment of rational drug design capable of halogen bonding with biomolecules.