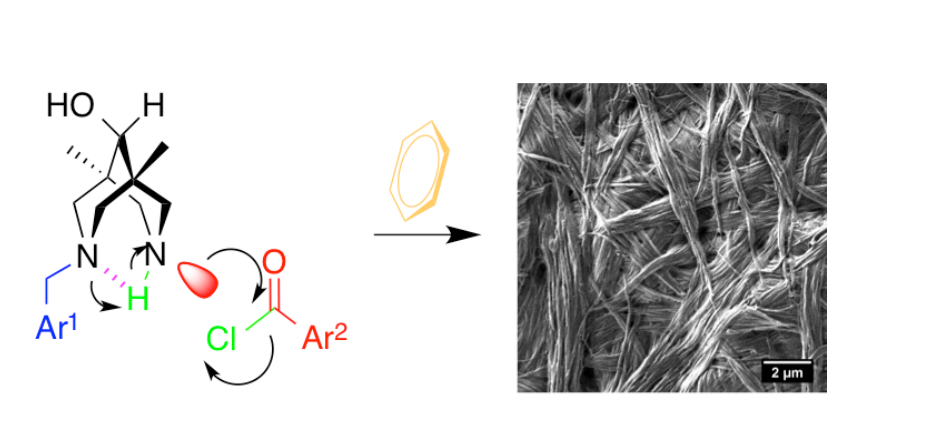
The acylation of unsymmetrical N-benzylbispidinols in aromatic solvents without external base led to formation of supramolecular gels, which possess different thickness and stability depending on the substituents in para-positions of benzylic group and nature of acylating agent as well as on the nature of the solvent used. Structural features of the native gels as well as of their dried forms were studied by complementary techniques including FT IR- and ATR-spectroscopy, AFM, TEM, SEM, SAXS. Structures of the key crystalline compounds were established by X-ray diffraction. Analysis of obtained data allowed speculating on the crucial structural and condition factors that governed the gel formation. The most important factors were: (i) absence of base, either external or internal; (ii) presence of HCl; (iii) presence of carbonyl and hydroxyl groups to allow hydrogen bonding; (iv) presence of two (hetero)aromatic rings at both sides of the molecule. The hydrogen bonding involving amide carbonyl, hydroxyl at 9th position and, very probably, ammonium N-H+ and Cl- anion appear to be responsible for the formation of infinite molecular chains required for the first step of gel formation. Subsequent lateral cooperation of molecular chains into fibers occured, presumably, due to the aromatic pi-pi-stacking interactions. sc-CO2 drying of the gels gave rise to aerogels morphology different from that of air dried samples.