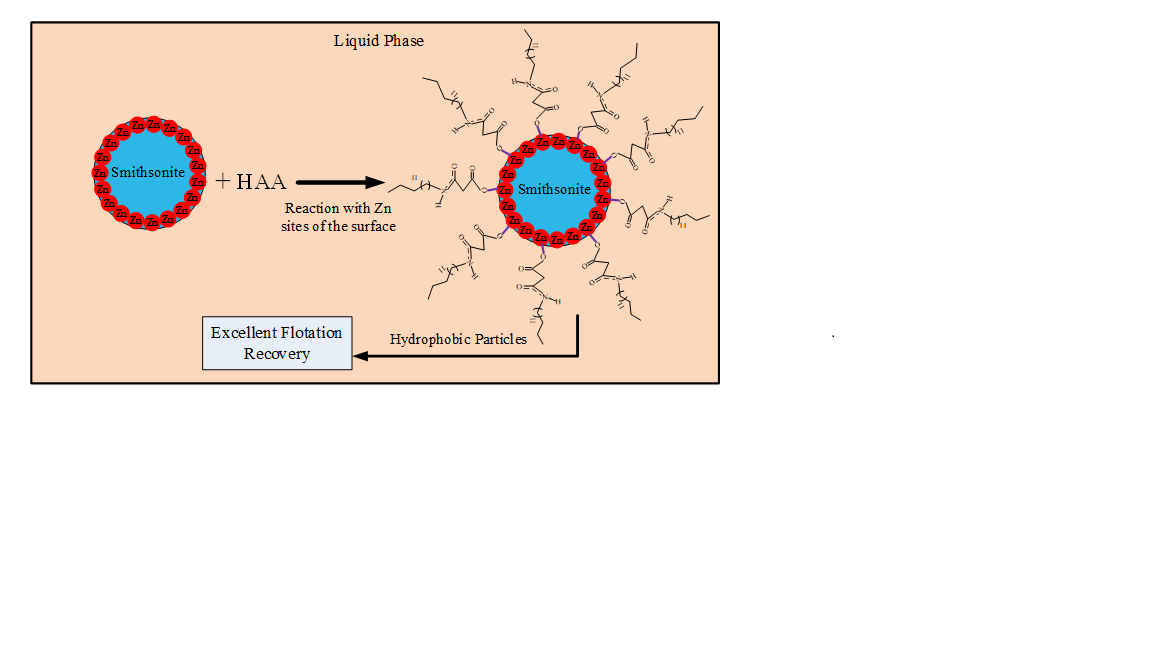
Zinc is mostly extracted from zinc oxide and sulfide minerals, and this process involves flotation as a key step. While it is easier to float the sulfide mineral, its consumption and depletion has led to an increased reliance on zinc oxide minerals, including smithsonite; hence the development of efficient ways of collecting smithsonite by flotation is an important objective. Herein, we describe the use of 2-(hexadecanoylamino)acetic acid (HAA), a novel surfactant, as a collector during smithsonite flotation. The mechanism and flotation performance of HAA during smithsonite flotation were investigated by total organic carbon (TOC) content studies, zeta potential measurements, FTIR spectroscopy, and XPS analyses, combined with micro-flotation experiments. The flotation results revealed that HAA is an excellent collector in pulp over a wide pH range (9–12) and at a relatively low concentration (2 × 10‒4 mol/L), at which a recovery of close to 90% of the smithsonite mineral was obtained. TOC-content studies reveal that the good flotation recovery is ascribable to large amounts of collector molecule adsorbed on the smithsonite surface, while zeta potential measurements show that the HAA is chemically adsorbed onto the smithsonite. FTIR and XPS analyses reveal that the HAA-collector molecules adsorb onto the smithsonite surface as zinc-HAA complexes involving carboxylate moieties and Zn sites on the smithsonite surface in alkaline solution.