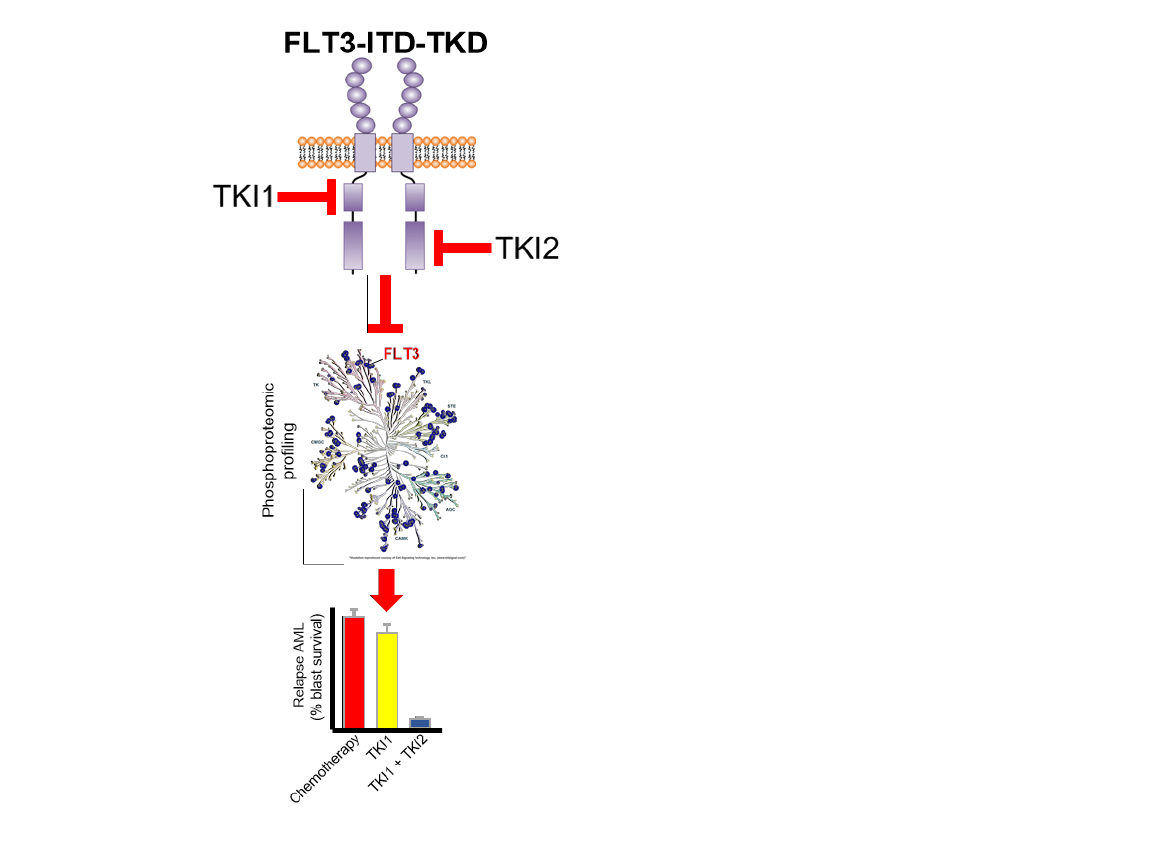
Identification of recurrent driver mutations in genes encoding tyrosine kinases has resulted in the development of molecularly targeted strategies designed to improve the outcomes for patients diagnosed with acute myeloid leukemia (AML). The receptor tyrosine kinase FLT3, is the most commonly mutated gene in AML, with internal tandem duplications within the juxtamembrane domain (FLT3-ITD) or missense mutations in the tyrosine kinase domain (FLT3-TKD), present in 30%-35% of AML patients at diagnosis. An established driver mutation and marker of poor prognosis, the FLT3 tyrosine kinase has emerged as an attractive therapeutic target, and thus has encouraged the development of FLT3 tyrosine kinase inhibitors (TKIs). However, the therapeutic benefit of FLT3 inhibition, particularly as monotherapy, frequently results in the development of treatment resistance and disease relapse. Commonly, FLT3 inhibitor resistance is induced by the emergence of secondary lesions in the FLT3 gene, particularly in the second tyrosine kinase domain at residue Asp835 (D835) to form a ‘dual mutation’ (ITD-D835). Individual FLT3-ITD and FLT3-TKD mutations influence independent signaling cascades however, currently little is known which divergent signaling pathways are controlled by each of these FLT3 specific mutations, particularly in the context of patients harboring dual ITD-D835 mutations. This review provides a comprehensive analysis of the known discrete and cooperative signaling pathways regulated by each of the FLT3 specific mutations, as well as the therapeutic approaches that hold the most promise for development of more durable and personalized therapeutic approaches targeting mutant FLT3, to improve the treatment of AML.