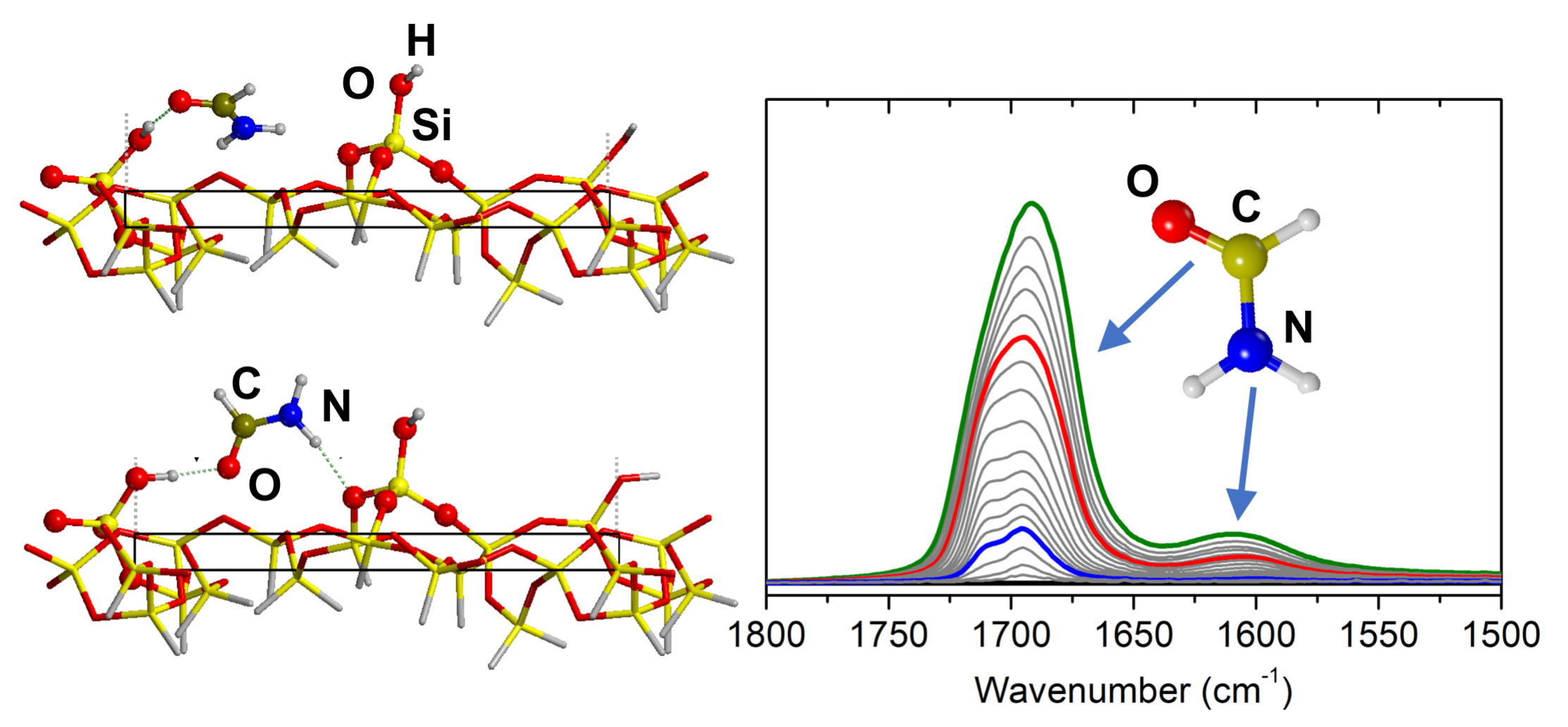
Mineral surfaces have been demonstrated to play a central role in prebiotic reaction, which are supposed to be at the basis of the origin of life. Among the various molecules proposed as precursors for these reactions, one of the most interesting is formamide. Formamide has been shown to be a pluripotent molecule, generating a wide distribution of relevant pre-biotic products. In particular, the outcomes of its reactivity are strongly related to the presence of mineral phases, acting as catalysts toward specific reaction pathways. Even if the mineral-products relationship has been deeply studied for a large pool of materials, the fundamental description of the formamide reactivity over the mineral surface at a microscopic level is missing in the literature. In particular, a key step of formamide chemistry at surfaces is its adsorption on the available interaction sites. This report aims to investigate the adsorption of formamide over a well-defined amorphous silica, chosen as model mineral surface. An experimental IR investigation of formamide adsorption has been carried out and its outcomes have been interpreted on the basis of first principles simulation of the process adopting a realistic model of the amorphous silica.