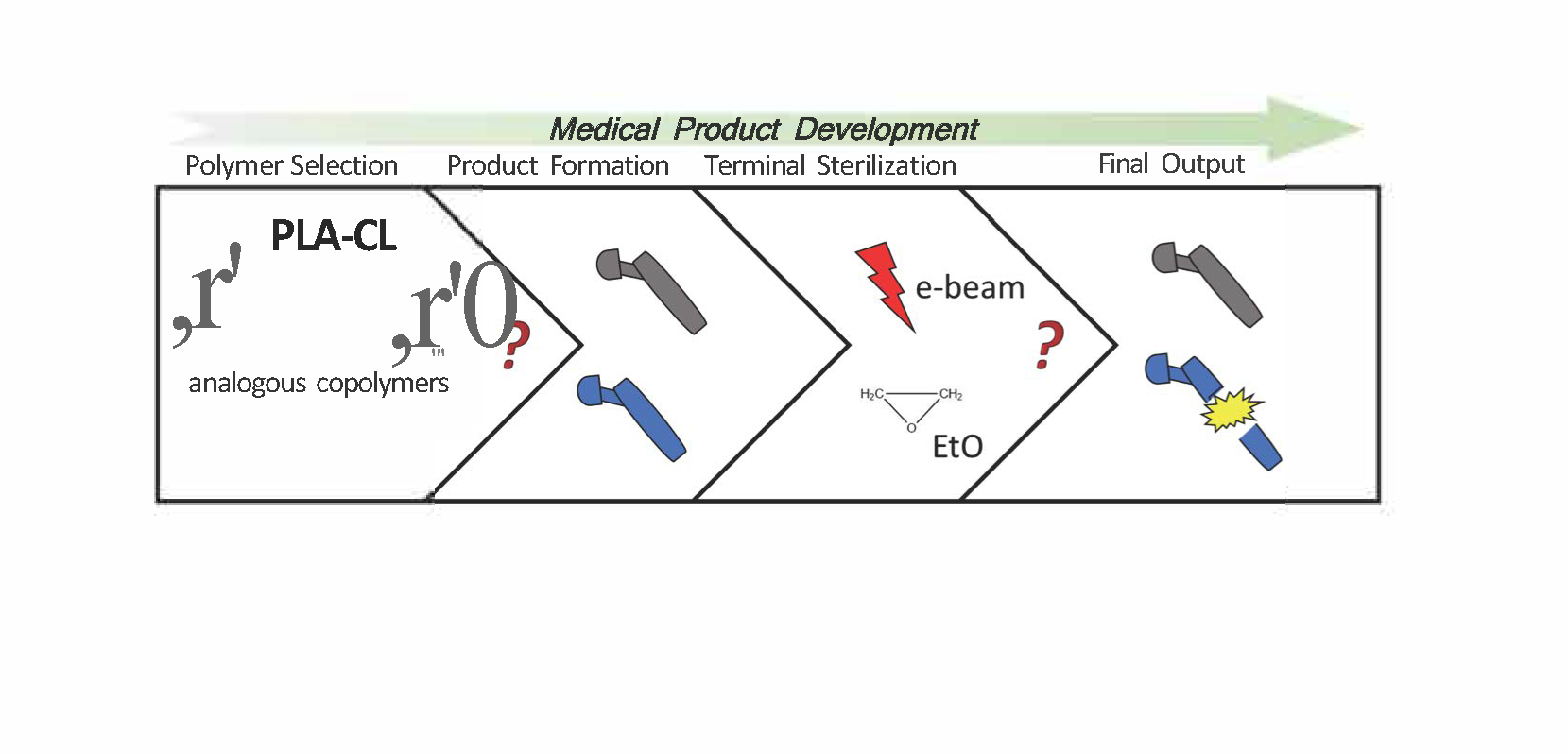
In the last decades bioresorbable and biodegradable polymers have gained a very good reputation both in research and in industry thanks to their unique characteristics. They are, indeed, able to ensure high performances and biocompatibility, at the same time avoiding post-healing surgical interventions for devices removal. In the medical device industrial use of such biopolymers, it is widely known that product formulation and manufacturing need to follow specific procedures in order to ensure both proper mechanical properties and desired degradation profile. Moreover, also the sterilization method is crucial and its impact on physical properties is generally underestimated. In this work we focused our attention on the effect of different terminal sterilization methods on two commercially available poly(L-lactide-co-ε-caprolactone) with equivalent chemical composition (70% PLA and 30% PCL) and relatively similar initial molecular weight, but different chains arrangement and crystallinity. Results obtained show that crystallinity plays a key role, helping in preserving the narrow distribution of chains and, as a consequence, defined physical properties. These statements can be used as guidelines for a better choice of the most adequate biodegradable polymers in the production of resorbable medical devices.