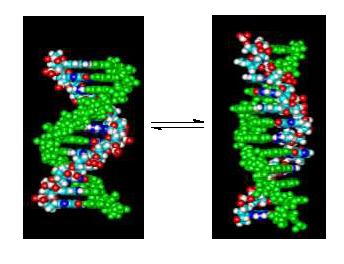
High concentrations of Na+ or [Co(NH3)6]3+ can induce the B to Z conformational transition in alternating (dC-dG) oligo and polynucleotides. The use of short DNA oligomers (dC-dG)4 and (dm5C-dG)4 as models can allow a thermodynamic characterization of the transition. Both form right handed double helical structures (B-DNA) in standard phosphate buffer with 115 mM Na+ at 25 oC. However, at 2.0 M Na+ or 200 mM [Co(NH3)6]3+, (dm5C-dG)4 assumes a left handed double helical structure (Z-DNA) while the unmethylated (dC-dG)4 analogue remains right handed under those conditions. We have previously demonstrated that the enthalpy of the transition at 25 oC for either inducer can be determined using isothermal titration calorimetry (ITC) [Ferreira, J. M. & Sheardy, R. D., Biophys. J. 2006, 91, 1–7]. Here, ITC is used to investigate the linkages between temperature, water activity and DNA conformation. We found that the determined enthalpy for each titration varied linearly with temperature allowing determination of the heat capacity change (DCp) between the initial and final states. As expected, the DCp values were dependent upon the cation (i.e. Na+ vs [Co(NH3)6]3+) as well as the sequence of the DNA oligomer (i. e., methylated vs unmethylated). Osmotic stress experiments were carried out to determine the gain or loss of water by the oligomer induced by the titration. The results are discussed in terms of solvent accessible surface areas, electrostatic interactions and the role of water.