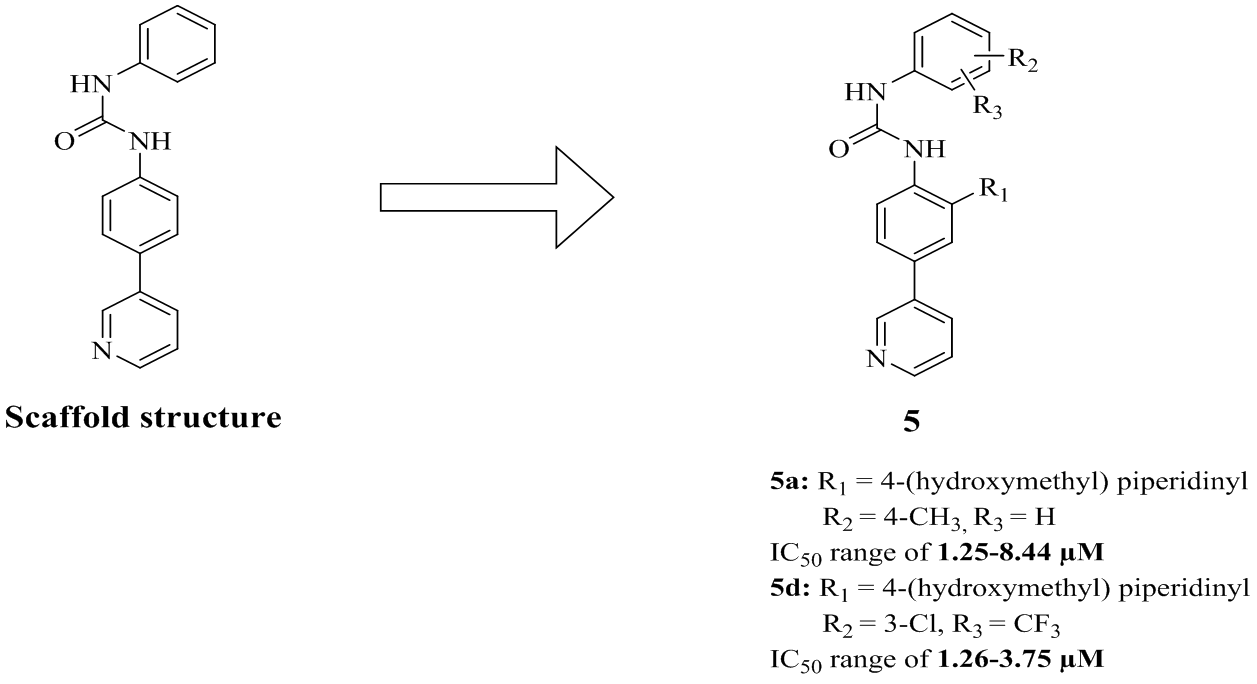
Synthesis of new series of 1-phenyl-3-(4-(pyridin-3-yl) phenyl) urea derivatives and its in vitro antiproliferative activities against NCI-60 human cancer cell lines of nine different cancer types are described. Fourteen compounds 5a-n have been synthesized with three different hydrogen bondable moieties (4-hydroxylmethylpiperidinyl and trimethoxyphenyloxy and 4-hydroxyethylpiperazine) attached to the core structure 1-phenyl-3-(4-(pyridin-3-yl) phenyl) urea. Different substituents with different π and σ values were added on the terminal phenyl group. Compounds with 4-hydroxymethylpiperidine moiety showed higher mean percentage inhibition values over the 60-cell line panel at 10-µM concentration. They showed broad-spectrum antiproliferative activity over many cell lines of different cancer types. For instance, compound 5a elicited some lethal rather than inhibition effects on SK-MEL-5 melanoma cell line, 786-0, A498, RXF 393 renal cancer cell lines, and MDA-MB-468 breast cancer cell line by 146.1, 108.7, 136.2, 134.8, 116.6 % at 10 µM, respectively. Compounds 5a-e exhibited superior antiproliferative activity than Paclitaxel and Gefitinib against the most sensitive cell lines. Two compounds, 5a and 5d showed promising mean growth inhibitions and thus were further tested at five-dose testing mode to determine their IC50 values. The data revealed that 5a and 5d urea compounds are the most active derivatives with significant efficacies and superior potencies than Paclitaxel in 21 different cancer cell lines, belonging particularly to renal cancer and melanoma cell lines. Moreover, 5a and 5d had superior potencies than Gefitinib in 38 and 34 cancer cell lines, respectively; belonging particularly to colon cancer, breast cancer and melanoma cell lines.