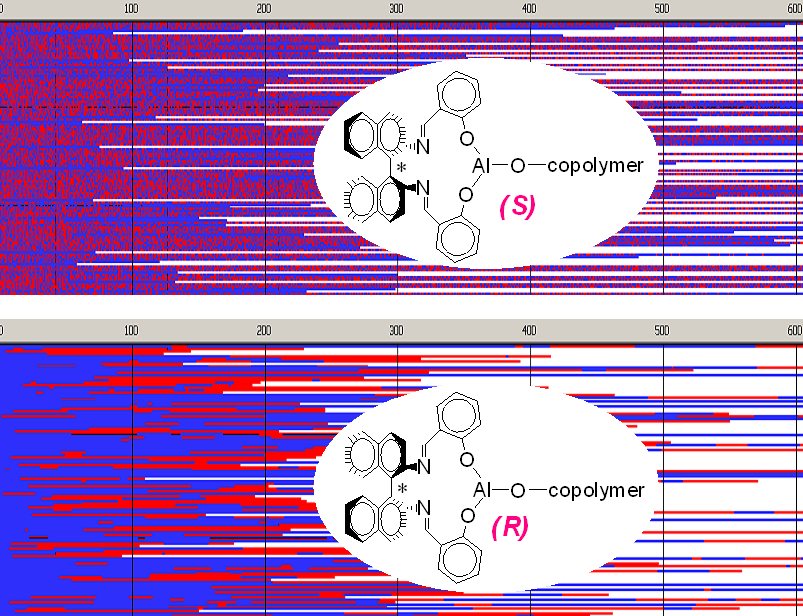
The effect of configuration of an asymmetric bulky initiator 2,2’-[1,1’-binaphtyl-2,2’-diyl- bis-(nitrylomethilidyne)]diphenoxy aluminum isopropoxide (Ini) on structure of copolymer of asymmetric monomer L,L-lactide (Lac) with symmetric comonomer trimethylene carbonate (Tmc) was studied using polarimetry, dilatometry, SEC and 13C NMR. When the S-enantiomer of Ini was used the distribution in copolymer chains at the beginning of polymerization is statistical, with alternacy tendency, changing next through a gradient region to homoblocks of Tmc. When, however, R-Ini was used, the product formed was a gradient oligoblock one, with Tmc blocks prevailing at the beginning, changing to Lac blocks dominating at end part of chains. Initiation of copolymerization with the mixture of both initiator enantiomers (S:R = 6:94) gave multiblock copolymer, of similar features but shorter blocks. Analysis of copolymerization progress required complex analysis of dilatometric data, assuming different contraction coefficients for units located in different triads. Comonomer reactivity ratios of studied copolymerizations were determined.