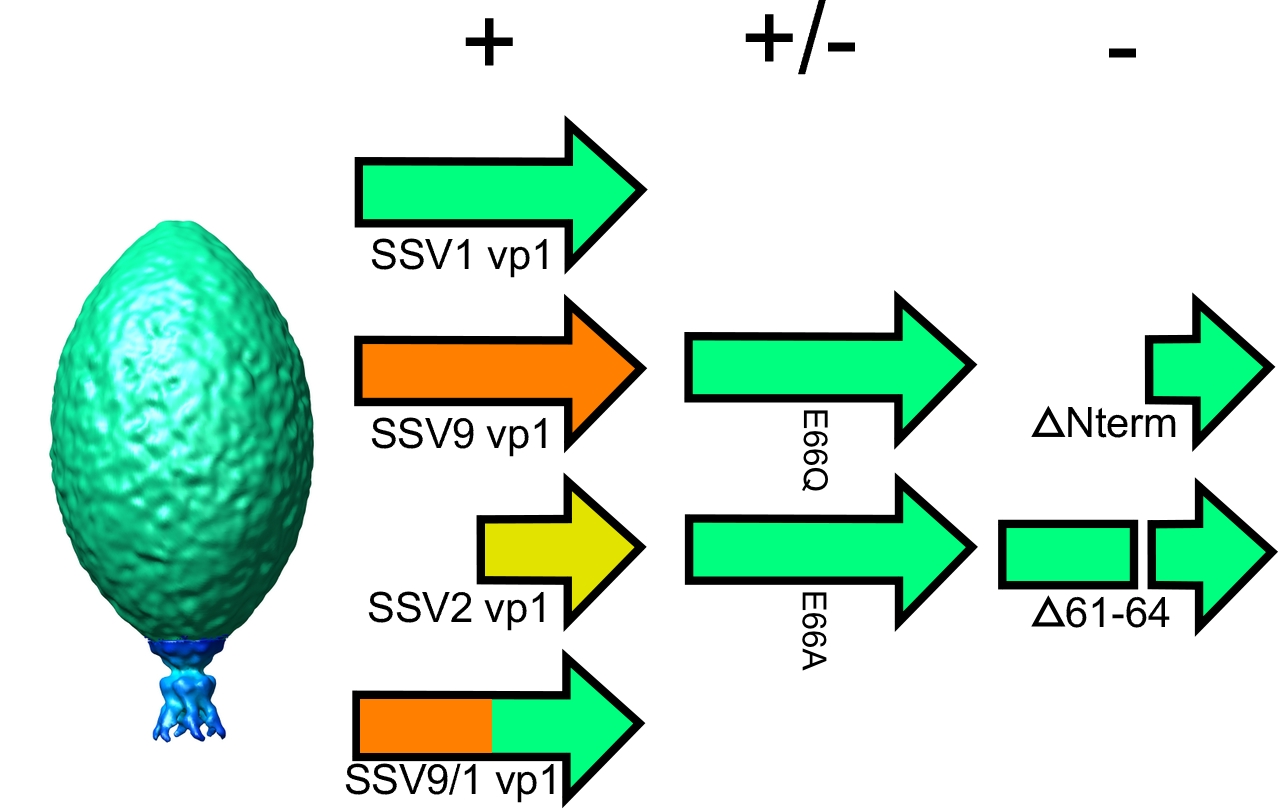
Viruses with spindle or lemon-shaped virions are rare in the world of viruses, but are common in viruses of archaeal extremophiles, possibly due to the extreme conditions in which they thrive. However, the structural and genetic basis for the unique spindle shape is unknown. The best-studied spindle-shaped virus, SSV1, is composed mostly of the major capsid protein VP1. Similar to many other viruses, proteolytic cleavage of VP1 is thought to be critical for virion formation. Unlike half of the genes in SSV1, including the minor capsid protein gene vp3, the vp1 gene does not tolerate deletion or transposon insertion. In order determine the role of the vp1 gene and its proteolysis for virus function, we developed techniques for site-directed mutagenesis of the SSV1 genome and complemented deletion mutants with vp1 genes from other SSVs. By analyzing these mutants we demonstrate that the N-terminus of the VP1 protein is required, but the N-terminus, or entire SSV1 VP1 protein, can be exchanged with VP1s from other SSVs. However, the conserved glutamate at the cleavage site is not essential for infectivity. Interestingly, viruses containing point mutations at this position generate mostly abnormal virions.