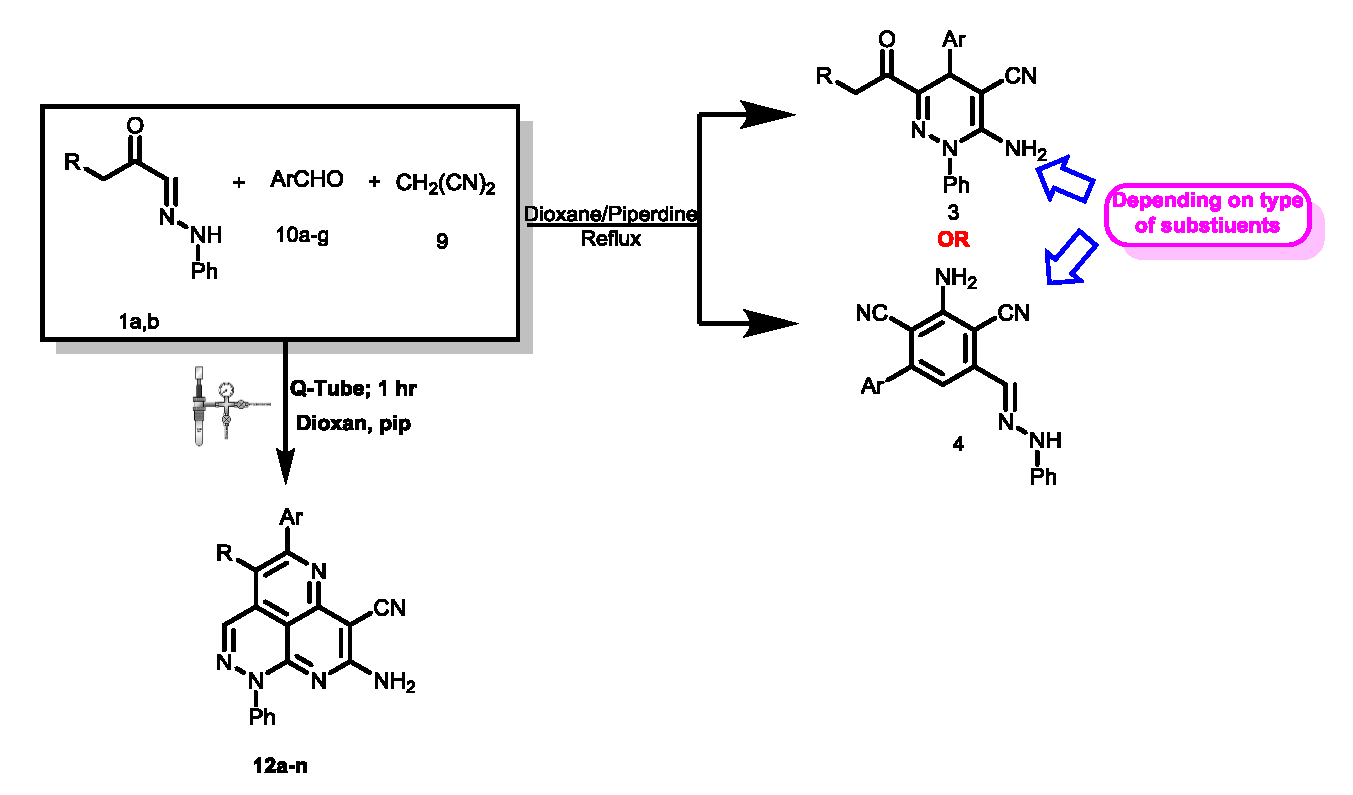
The considerable biological and medicinal activities of pyridazines has stimulated considerable research on efficient syntheses of these derivatives. In the last decade, microwave irradiation has generally been used for the energy source. As demonstrated in recent studies, pressure reactor “Q-tubes” may be used to accelerate several of these reactions in a more optimal and safer manner (compared to microwaves). In these studies there has been postulated a pathway for the formation of pyridazino[5,4,3-de][1,6]naphthyridine derivatives . In this paper we consider this pathway, and an alternate pathway, for several reactions. Contrary to the suggestion in these studies the pathway in which initial dimerization of malononitrile was postulated could be excluded based on chemical evidence. The reactions performed were the reaction of arylhydrazonals 1a,b with benzylidinemalononitrile which afforded in Q-tube the 3-acyl-4-aryl-1-phenyl-6-amino-1,4-dihydropyridazines, and the reaction of arylhydrazonals 1a,b, malononitrile 9 and aromatic aldehydes 10a-g in Q-tubes which afforded the tricyclic systems 12a-n whose structure could be established by X-ray crystal structure determination. In conclusion, we have added to the work of the recent studies by excluding a reaction pathway for one of their reaction products.