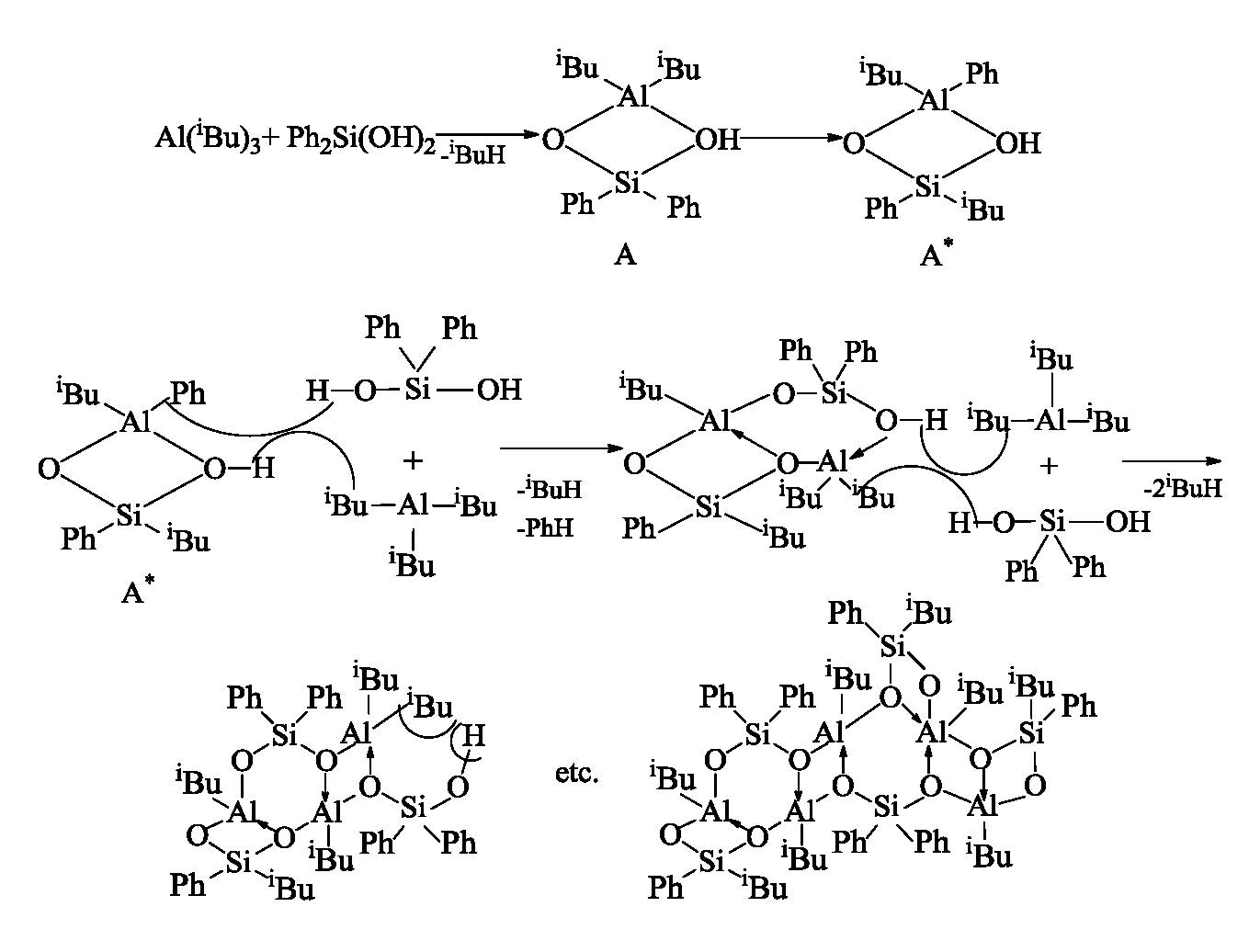
We have drawn a few interesting conclusions while studying reaction products of Ph2Si(OH)2 with Al(iBu)3 and tetraisobutyl alumoxane. In the first place, this is the production (at Ph2Si(OH)2 and Al(iBu)3 equimolar ratio) of oligomer alumoxanesiloxane structure with alternating four- and six-member rings, as well as isobutyl and phenyl groups migration between aluminum and silicon due to formation of intramolecular four-member cyclic alumosiloxane complex [Ph2(OH)SiO]Al(iBu)2 → [(iBu)Ph(OH)SiO]Al(iBu)Ph. Ph2Si(OH)2 interaction with Al(iBu)3 not only starts from intramolecular alumosiloxane complex production, but the chain is terminated for the same reason, which in the case of Ph2Si(OH)2 reaction with tetraisobutylalumoxane results in failure of obtaining high-polymer alumosiloxane compounds. When Al(iBu)3 interacts with α- and γ-diols, no oligomer compounds are produced. Al(iBu)3 reaction with α, γ-diols results in monomer compounds that are likely to have cyclic structure. Notably at Al(iBu)3 interaction with α-diol only double excess of Al(iBu)3 allows full replacement of hydrogen in α-diol hydroxyl groups by aluminum alkyl residue with 1,3-bis(diisobutylalumoxymethyl)-1,1,3,3-tetramethyldisiloxane production. At equimolar ratio of initial reagents the second isobutyl radical at Al does not interact with the second hydroxyl group of α-diol, apparently due to steric hindrance and 1-(diisobutylalumoxymethyl)-3-(hydroxymethyl)-1,1,3,3-tetramethyl-disiloxane is produced. Al(iBu)3 reactions with γ-diol also result in monomer compounds but the presence of a chain consisting of three СН2-groups between Si and hydroxyl group facilitates interaction between the second hydroxyl group of γ-diol and the second isobutyl radical Al(iBu)3. Tetraisobutylalumoxane reactions with α- and γ-diols results in oligomer compounds.