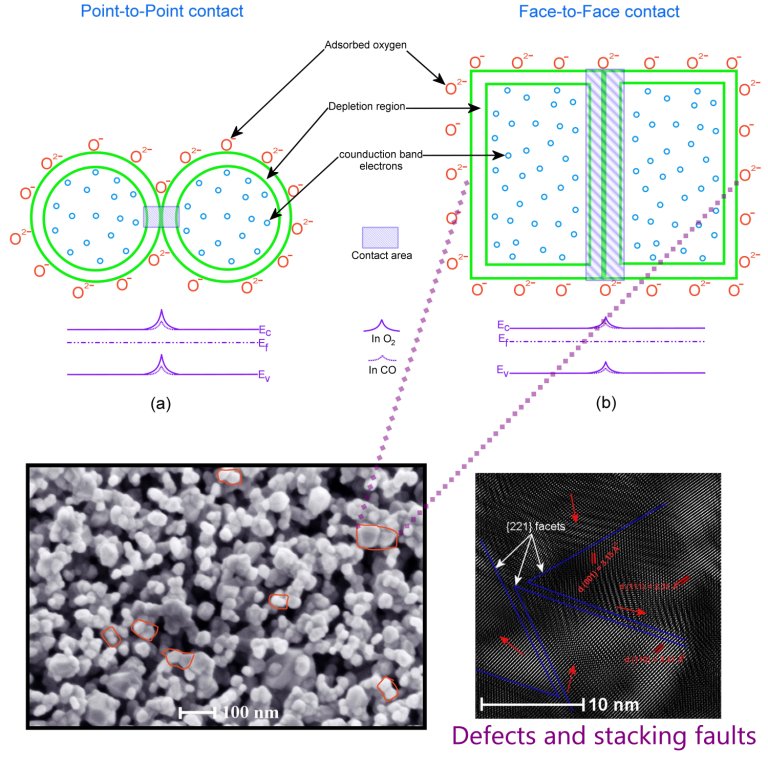
In this work, we report synthesis of Cu, Pt and Pd doped SnO2 powders and their comparative CO gas sensing studies. Dopants were incorporated into SnO2 nanostructures using chemical and impregnation methods by using urea and ammonia as precipitation agents. The synthesized samples were characterized using X-ray diffraction (XRD), Raman spectroscopy, Scanning electron microscopy (SEM) and High resolution transmission electron microscopy (HR-TEM). The presence of dopants within the SnO2 nanostructures was evidenced from HR-TEM. Doped powders utilizing chemical methods with urea as precipitation agent presented higher sensitivities compared to the remaining, which is due to the formation of uniform and homogeneous particles resulted from the temperature assisted synthesis. The particle sizes of doped SnO2 nanostructures were in the range of 40-100 nm. An enhanced sensitivity around 1783 was achieved with Cu doped SnO2 when compared with two other dopants i.e., Pt (1200) and Pd: SnO2(502). The high sensitivity of Cu: SnO2 is due to formation of CuO and its excellent association and dissociation in the presence of CO with adsorbed atmospheric oxygen at sensor operation temperatures resulted in high conductance. Cu: SnO2 may be an alternative and cost effective sensor for industrial applications.